Abstract
Poly(urethanamide) was synthesized from Balanites roxburgii oil (BRO). Initially, BRO was modified by epoxidation and subsequently the epoxy ring was hydrolyzed. The resulting polyol Balanites roxburgii oil (PBRO) was reacted with diethanolamine to form corresponding fatty amide polyol (FAP). This FAP was reacted with various isocyanates like hexamethylene diisocyanate (HDI), biuret of hexamethylene diisocyanate, and isophorone diisocyanate at NCO to OH ratio 0.75:1 and 1:1. The poly(urethanamide) films were evaluated for chemical resistance, optical and mechanical properties. Furthermore, the water absorption test, in vitro degradation, and thermogravimetric analysis of poly(urethanamide) films were studied. Epoxidized Balanites roxburgii oil and PBRO were characterized by FTIR and physico-chemical analysis. FAP was characterized by FTIR, 1H NMR, and physico-chemical analysis.
Introduction
In the present scenario, use and demand of renewable materials for industrial applications are increasing due to fast depletion of petroleum resources, environmental legislation, and cost.[Citation1–4] The natural materials available are carbohydrates, vegetable oil, rosins, lignin, tannin, etc.[Citation5,6] Among them, vegetable oils are easily available renewable materials. Vegetable oil contains triglyceride structure, which contains double bond, ester groups, etc. Triglycerides can be easily modified by simple chemical reactions and form valuable products such as polyurethanes, polyamides, surfactants, alkyd, epoxy resins, etc.[Citation7–13]
The growing importance of polymeric materials from renewable resources has put polyols and polyurethanes derived from vegetable oils, especially due to their simple preparation yet greatly promising applications.[Citation14–18] Polyurethanes are the most important polymeric materials which exhibit versatile properties suitable for use in many applications, such as footwear, machinery industry, coatings and paints, rigid insulations, elastic fibers, and medical devices. They posses wide range of mechanical strength, excellent abrasion resistance, toughness, and chemical resistance.[Citation19–21] Another important class of polymer is poly(urethanamide), which contains both amide and urethane linkage in the same polymer; hence, it possesses the properties of polyurethane and polyamide.[Citation22] There are several oils such as soyabean oil, linseed oil, Pongamia glabra seed oil, Albizia benth oil, neem seed oil, castor oil, nahar seed oil, etc. used for the synthesis of poly(urethanamide), poly(esteramide), and poly(ether amide) for coating applications.[Citation23–28]
Vegetable oil-dependent industries rely mostly on oils such as linseed oil and soybean oil which are very expensive. In India, a wide variety of wildly grown plants and herbs are available. Balanites roxburgii plant is abundant in different parts of this country, especially in the western region of India, and the seeds of which contain high oil content (32%). It holds considerable promise as a source of unsaturated oil and suitable for the production of industrial chemical feedstock. BRO is one of the main sources of triglyceride for the synthesis of polymer, surfactant, and lubricating products. According to the literature, B. roxburgii oil is not yet used in the coating field for polymer synthesis. The B. roxburgii seed is rich source of oil as compared to the soybean seed (18.21%), Prosopis Juliflora seed (6–8%), and neem seed (23–27%).[Citation29–31]
In the present research work, we used BRO for the synthesis of fatty amide polyol (FAP). It was reacted with HDI, biuret of hexamethylene diisocyanate (BUHDI), and isophorone diisocyanate (IPDI) with different NCO/OH ratio for cross-linked poly(urethanamide) synthesis. Furthermore, the coating properties, gel content, water absorption test, and bio-degradability of poly(urethanamide) were studied.
Experimental setup
Materials
B. roxburgii seeds were collected from western region of India. Hydrogen peroxide (50%), glacial acetic acid, sulfuric acid, sodium sulfate, diethyl ether, diethanolamine, sodium chloride, sodium methoxide, xylene, methyl ethyl ketone (MEK), sodium hydroxide, and hydrochloric acid were purchased from S.D. Fine-Chem. Ltd. Hexamethylene diisocyanate, BUHDI, and isophorane diisocyanate were obtained from Bayer Material Science. Dibutyltin dilaurate (DBTDL) was purchased from Otto-Chemie Pvt. Ltd. All the reagents were used without purification.
Extraction of Balanites roxburgii oil (BRO)
B. roxburgii seeds were air-dried, ground to a powder form, and then subjected for oil extraction by soxhlet method using petroleum ether (B.P. 60–80 °C) as a solvent. The oil was washed with 2 wt% aqueous NaOH solution. The oil content of seeds was 32 wt%. The fatty acid constituent of BRO is Palmitic acid (16:0) 17%, Palmitoleic acid (16:1) 4.3%, Stearic acid (18:0) 7.8%, Oleic acid (18:1) 32.4%, Linoleic acid (18:2) 31.3%, and Linolenic acid (18:3) 7.2%.[Citation32] The physico-chemical analysis of BRO is shown in Table .
Table 1. Physico-chemical analysis of BRO, EBRO, PBRO, and FAP and poly(urethanamide).
Synthesis of epoxidized Balanites roxburgii oil (EBRO)
The reaction was carried out in a four-necked round-bottom flask, equipped with mechanical stirrer, condenser, and thermometer pocket. BRO (20 g), glacial acetic acid (2.64 g), and H2SO4 (2% of total weight of acetic acid and hydrogen peroxide) were charged in the flask. About 5.98 g of hydrogen peroxide (50%) was added dropwise for half an hour into the reaction mixture at room temperature. After the addition of hydrogen peroxide was complete, reaction temperature was gradually increased to 60 °C and stirring was continued for 7 h. The progress of reaction was monitored by measuring epoxy value at regular intervals. After desired conversion was achieved, reaction was stopped, product was washed with water, and organic phase was extracted in diethyl ether and dried over anhydrous sodium sulfate. The reaction was carried out according to the reference [Citation33]. The conversion of reaction was 74.32%.
FTIR (cm−1): 824.83 (C–O stretching of oxirane); 2927.62 and 2857.20 (stretching of CH2).
Synthesis of polyol Balanites roxburgii oil (PBRO)
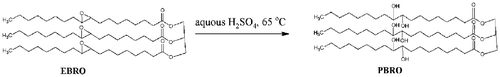
EBRO was reacted with aqueous H2SO4 to form polyhydroxyl oil. Synthesis of polyol by epoxy ring opening using water was done according to the reference [Citation34]. The reaction is shown in Figure .
FTIR (cm−1): 3438.23 (O–H stretching); 1167.77 (C–O stretching); 2927.17 and 2856.29 (stretching of CH2).
Synthesis of FAP
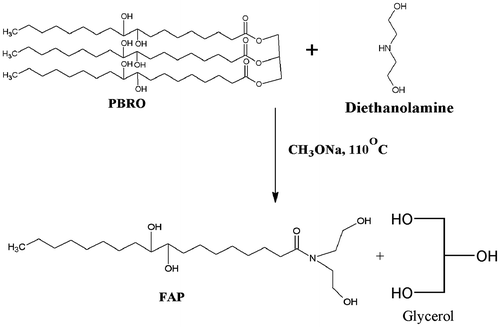
0.35 mol of diethanolamine and 0.007 mol of sodium methoxide were mixed in a four-necked round-bottom flask fitted with a mechanical stirrer, thermometer, and condenser. The contents were heated at 110 °C and PBRO (0.1 mol) was added dropwise over a period of 60 min. The progress of the reaction was monitored by thin-layer chromatography using ethyl acetate as mobile phase. As the reaction was completed, product was dissolved in diethyl ether, washed with 15% NaCl, and dried over anhydrous sodium sulfate. The etherel layer was filtered and evaporated in a vacuum evaporator to obtain FAP. The reaction yield was 96.7%. The reaction is shown in Figure .
FTIR (cm−1): 1622.15 (C=O stretching of amide); 1173 (C–O stretching); 3380.89 (O–H stretching); 2926.79 and 2856.02 (stretching of CH2); 1070 (C–N stretching).
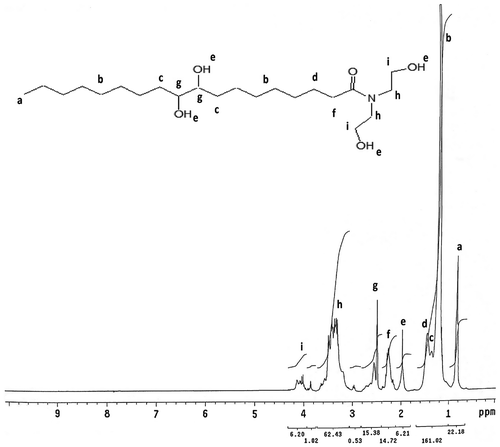
1H NMR (DMSO) δ: 0.9 t (CH3); 1.2 (CH2–CH2–CH2 alkyl chain); 1.35 q (–CH2–C–O–); 1.54 q (CH2–C–CO–N); 2.0 s (–OH); 2.3 t (–CH2–CO–N); 2.5 q (–CH–O); 3.4 t (–CH2–N–); 4.1 t (–CH2–O).
Synthesis of poly(urethanamide)
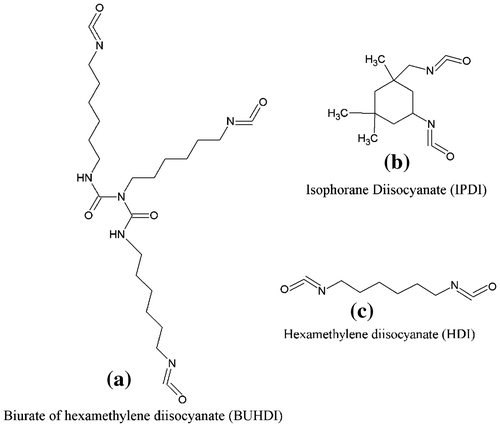
FAP (5 gm) and DBTDL (0.0005 g) were stirred with 10 ml xylene in a flask under nitrogen atmosphere. HDI (the amount HDI as per to maintain NCO/OH mole ratio 0.75) was then added dropwise. Then, the reaction mixture was heated at 50°C. The progress of reaction was monitored by the determination of hydroxyl values at regular intervals of time. After completion of reaction, material was removed from the reactor and applied onto the mild steel panel (3 × 5 inches). The same procedure was followed for HDI with NCO/OH mole ratio 1, for BUHDI with NCO/OH mole ratio 0.75 and 1, and for IPDI with NCO/OH mole ratio 0.75 and 1. The structures of BUHDI, IPDI, and HDI are shown in Figure .
Measurements and characterization
FTIR spectra (Shimadzo 8400 s, Japan) of EBRO, PBRO, and FAP films were recorded by using ATR technique. The range of spectra was 4000–600 cm−1.
1H NMR spectrum of FAP was recorded at 400 MHz using Bruker Biospin (Avance AV500WB, Germany) using deuterated dimethyl sulfoxide as solvent and tetramethylsilane (TMS) as an internal standard.
Thermal analysis of cured polymer was done by Thermogravimetric analysis instrument DSC/TGA (Q600 ((T.A. Instruments, US). Sample was taken in a sample pan and was kept in the thermal analysis under nitrogen atmosphere at heating rate of 10 °C/min. between the temperature ranges 30–500 °C.
Iodine value was determined according to Wijs method by using iodine monochloride solution in acetic and carbon tertrachloride, and excess halogen by the addition of KI solution and titration of the liberated iodine with a sodium thiosulphate was determined. (ASTM D 1959-97)
The percentage oxirane oxygen was determined by direct method using hydrobromic acid solution in glacial acetic acid. [Citation35] From the oxirane content values, the relative percentage conversion to oxirane was calculated using the following expression:
where OOexp (g/100 g sample) is the experimentally obtained oxirane oxygen and OOthe is the theoretically obtainable maximum oxirane oxygen, which was determined from the following expression:
where Ai (126.9) and Ao (16.0) are the atomic weights of iodine and oxygen, respectively, and IV0 is the initial iodine value of oil BRO.
Hydroxyl value was determined by using ASTM D 1957-86, by refluxing the sample with pyridine-acetic anhydride mixture, and then titrated with KOH in the presence of phenolphthalein indicator.
Saponification value of BRO was determined by refluxing the sample with alcoholic KOH and then titrated with 0.5 N HCl using phenolphthalein indicator.[Citation35]
Viscosity of BRO, EBRO, PBRO, and FAP was measured by bubble time method according to the ASTM D 1545-98. Viscosity of the sample was measured by comparing the viscosity with standard viscosity tubes filled with transparent liquids having predetermined viscosities in stokes.
Gloss of the cured films was measured on calibrated instrument at 60° angle of reflectance using a digital mini gloss meter (Rhopoint Instruments, ASTM D 523-99). This test method covers the measurement of the specular gloss of coating for glossmeter geometries of angle 60°.
The cured film was carefully peeled off from the Teflon sheet, in order to measure the gel content, and a known weight of the polymer film was extracted in xylene at room temperature for 24 h. The residues were dried at 70 °C in order to get a constant weight. The gel content of the cured film was then determined by the equation: . Where Wt is the weight after extraction and W0 is the weight before extraction.
Water absorption of poly(urethanamide) films was performed according to ASTM D-570. Poly(urethanamide) films were dried at 80 °C in a vacuum oven until a constant weight was attained. Then, they were immersed in water at room temperature for 24 h. Poly(urethanamide) films were removed, patted dry with a lint free cloth, and weighed. Percent water gain was determined by the equation:
where Wd and Ww denote initial weight of material prior to the exposure to water absorption and weight of material after exposure to water absorption, respectively.
Impact resistance was carried out on the both side of coated metal panels and analyzed in accordance to the standard ASTM D-2794. This test method covers a procedure for rapidly deforming, by impact, a coating film and its substrate and for evaluating the effect of such deformation.
The hardness of the coating which was applied onto metal panel was measured by pencil hardness test according to ASTM D 3363. This test method covers a procedure for rapid, inexpensive determination of the film hardness of an organic coating on a substrate in terms of pencil leads of known hardness.
The scratch hardness was determined by the automatic scratch hardness tester as per ASTM D 7027. This test method covers hardness of organic coating on a substrate by increasing weight in automatic scratch hardness tester.
Solvent resistance of coating was determined by ASTM D 5402-93 using solvent rub technique for assessing the solvent resistance of an organic coating by rubbing the coating with a cloth saturated with the appropriate solvent.
Adhesion test was performed by crosscut adhesion tester used to check the adhesion of the coated film to metallic substrate, according to ASTM D 3359.
Alkali resistance of the coated metal panels was evaluated for film whitening and blistering according to ASTM D 1647, by dipping coated panel in 3% aqueous solution of sodium hydroxide for 24 h.
Acid resistance of panels was evaluated for peeling and corrosion of coated part according to ASTM D 3260, by dipping the coated panel in 10 volume percent solution of conc. hydrochloric acid (31.8% HCl) in distilled water for 6 h.
Water resistance was performed by immersing the coated panels in water, the panels were evaluated for color change of coating, blistering, and skinning according to ASTM D 870-02.
Flexibility of coated metal panel was determined by the conical mandrel according to ASTM D 522-93a. This test method covers the determination of the resistance to cracking (flexibility) of attached organic coatings on substrates of metal.
Results and discussion
Physico-chemical analysis
The physico-chemical analysis of the BRO, EBRO, PBRO, and FAP is depicted in Table . The iodine value was determined for the analysis of unsaturation in the BRO and EBRO. The epoxidation is evident from the iodine value and also from epoxy equivalent of EBRO. The iodine value of BRO and EBRO was 108.6 and 2.71 g of I2/100 g of sample, respectively. The iodine value decreased with the transformation of BRO to EBRO indicating that the double bonds were consumed. The modification of epoxidized oil with water by epoxy ring opening reaction was confirmed by hydroxyl value of PBRO and the further formation of FAP was confirmed by hydroxyl value of FAP which is 480.2 mg of KOH/gm. Therefore, the increase in hydroxyl value from PBRO to FAP shows transformation reaction of PBRO to FAP. The viscosity measurement results of BRO, EBRO, PBRO, and FAP show that the viscosity was changed after transformation of BRO to FAP from 0.65 to 46.3 stokes at 25 °C. The hydroxyl values of poly(urethanamide) show that the hydroxyl groups of FAP were consumed during reaction with isocyanate.
FTIR analysis of EBRO, PBRO, and FAP
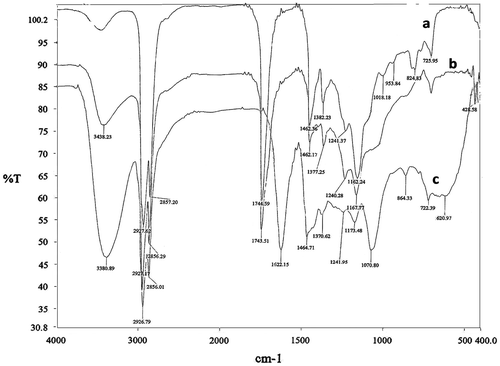
FTIR spectrum of EBRO, PBRO, and FAP was shown in Figure . The peak at 824.83 cm−1 in a spectrum exhibits C–O stretching (asymmetric stretch) bands for oxirane. The peak at 3438.23 cm−1 stretching corresponds to O–H group in b spectrum. The peak of epoxy stretching was absent in spectrum b, which shows formation of PBRO. It is evident that the epoxy ring was opened by water. The peak at 1622.15 cm−1 stretching corresponds to C=O of amide in spectrum c. The peak at 1167.77 and 1173.48 cm−1 stretching corresponds to C–O of hydroxyl in spectrum b and c, respectively. The peak at 3380.89 cm−1 showed the characteristic absorption band for alcoholic OH group in spectrum c. CH2 asymmetric and symmetric stretching peaks appear at 2926.79 and 2856.02 cm−1, respectively, in spectrum c. CH2 asymmetric and symmetric stretching peaks appear at 2927.17 and 2856.29 cm−1, respectively, in spectrum b. CH2 asymmetric and symmetric stretching peaks appear at 2927.62 and 2857.20 cm−1, respectively, in spectrum a. The C–N stretching peak of amide appears at 1070.80 cm−1 in spectrum c. The peak at 1462.36, 1462.17, and 1464.71 cm−1 corresponds to CH2 bending in spectrums a, b, and c, respectively. Thus, from the above FTIR data, it is concluded that EBRO, PBRO, and FAP were successfully synthesized from BRO.
1H NMR analysis of FAP
The 1H NMR spectra of FAP were shown in Figure . From figure of 1H NMR (DMSO) of FAP, FAP shows characteristics peaks at δ 0.9 t assign to terminal CH3, the peak at δ 1.2 corresponds to –CH2–CH2–CH2–, the peak at δ 1.35 q corresponds to –CH2–C–O–, the peak at δ 1.54 q assigns to –CH2–C–CO–N, the peak at δ 2.0 s corresponds to –OH, the peak at δ 2.3 t assigns to –CH2–CO–N, the peak at δ 2.5 q assigns to –CH–O, the peak at δ 3.4 t corresponds to –CH2–N–, and the peak δ 4.1 t assigns to –CH2–O. Thus, from the 1H NMR spectral data, it is concluded that FAP was successfully synthesized.
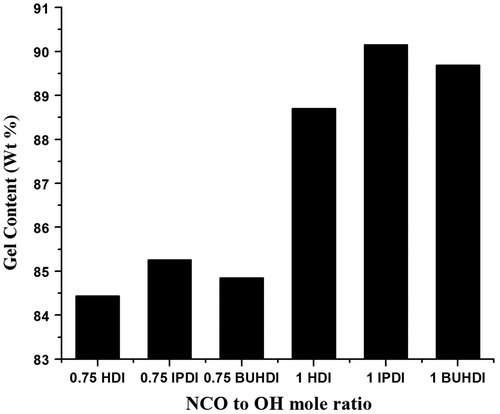
Gel content measurement (%)
The extent of cross-linking of FAP with HDI, IPDI, and BUHDI (NCO to OH mole ratio 0.75 and 1) was measured by extraction in terms of the gel content. Figure shows the change in gel content as a function of cross-linking of NCO and OH. It was found that with increase in NCO/OH mole ratio, the cross-linking of polymer was also increased indicating that the cross-linking of polymer is directly proportional to the gel content. At lower NCO to OH mole ratio, the gel content of polymers was 84–85% and at 1.00 mol ratio, the gel contents were enhanced up to 88–90%.
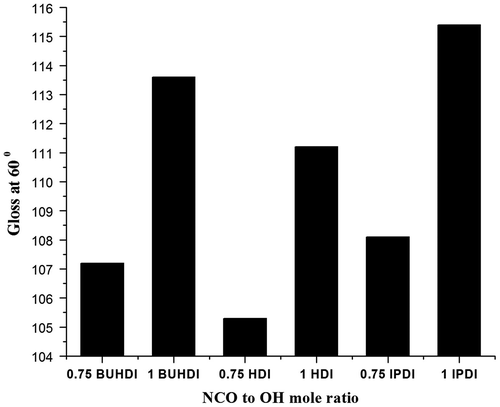
Gloss measurement
The effect of NCO on coatings was measured using a digital gloss meter at reflectance of 60°, as shown in Figure . It is observed that as the concentration of NCO increased, gloss of the coating also increases due to increase in cross-linking of polymer. Among BUHDI, HDI, and IPDI, the polymer of IPDI has high gloss, due to more surface smoothness property. However, BUHDI-based poly(urethanamide) forms more compact cross-linking network due to presence of three NCO functional group in BUHDI, which increase the gloss of BUHDI-based poly(urethanamide) coating than HDI-based poly(urethanamide).
Water absorption study of poly(urethanamide) films
Table shows the effect of NCO concentration on water uptake of the poly(urethanamide) film after subject to water absorption test. At 0.75 (NCO to OH mole ratio) concentration of NCO, the water absorption capacity of poly(urethanamide) film was greater than at 1.00 (NCO to OH mole ratio) concentration. It was observed that water absorption capacity of poly(urethanamide) film is inversely proportional to the NCO concentration. The increase in amount of cross-linking in cured poly(urethanamide) film would reduce the penetration of water molecule into the polymer network structure. This is owing to the fact that the intense cross-link network would hinder the water molecules to diffuse into the free volume followed by residing in the volume available in the polyurethane film. The polymeric material with higher cross-link density will possess lower free volume and lower water uptake.[Citation36]
Table 2. Percent water absorption by poly(urethanamide) films of different isocyanate at 0.75 and 1 NCO to OH mole ratio.
Water uptake of cured poly(urethanamide) film is also attributed to the presence of free volume and hydrophilic functional groups such as hydroxyl groups in their backbone structure. The hydroxyl groups in poly(urethanamide) film [poly(urethanamide) synthesized from 0.75 NCO to OH ratio] tend to attract water molecules which are polar in nature. The water molecules absorbed by polymer film may exist as bound water or unbounded cluster.[Citation37] The water absorption behavior is highly dependent on the free volume and chemical nature of polymer material. The high NCO concentration would give rise to an increase in cross-linking and a decrease in free volume. Therefore, the water uptake of poly(urethanamide) film decreases with increasing NCO concentration.
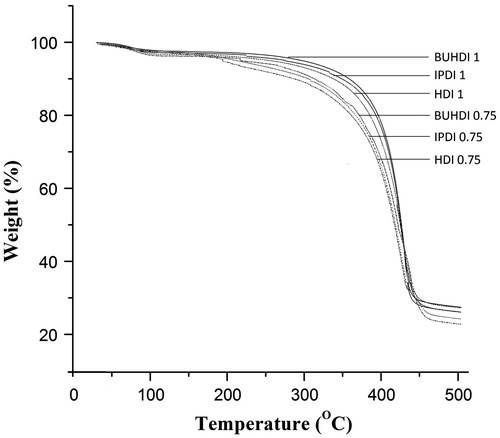
Thermogravimetric analysis
The thermal stability of cross-linked poly(urethanamide) containing different isocyanate (BUHDI, IPDI, and HDI) with different NCO to OH mole ratio (0.75 and 1.00) is shown in Figure . The initial degradation ascribed to the presence of aliphatic segments and final degradation associated with the decomposition of thermally stable amide linkages in the polymeric backbone.[Citation38] The disruption of urethane bonds which causes decomposition of hard segments corresponds to the temperature range 280–320 °C.[Citation39] The thermal stability of BUHDI-based polymer is higher than IPDI- and HDI-based polymer due to the higher cross-linking of three NCO group of BUHDI with polyol than IPDI and HDI. Also, thermal stability of IPDI-based polymer is higher than HDI-based polymer which may be due to the presence of cyclic moiety of IPDI.
In vitro degradation of BRO-based poly(urethanamide)
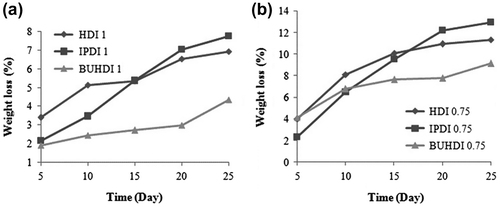
In vitro degradation of BRO-based poly(urethanamide) of IPDI, BUHDI, and HDI (NCO: OH mole ratio 0.75 and 1) was carried out in phosphate buffer solution (PBS, pH 7.4, 0.1 M), 1.0 mg proteinase k, and 1.0 mg sodium azide (preventing bacterial development) at 37 °C. The buffer solutions were refreshed every 48 h. The polymer films were removed from buffer solution at an interval of 5 days from a period of 5 to 25 days and washed thrice with distilled water and dried at 65 °C for 8 h. The degradation rate was evaluated by the weight loss of the polymers after 5, 10, 15, 20, and 25 days. It was observed that poly(urethanamide) of 0.75 mol ratio of NCO to OH degrades greater than mole ratio 1.00 mole ratio of NCO to OH. Furthermore, it was observed that as the NCO concentration is increased, the rate of degradation decreases because of the concentration of NCO which is directly proportional to the cross-linking of polymer. Cross-linking retards the degradation of poly(urethanamide). The weight of polymers decreases due to the enzymatic hydrolysis of amide linkage.[Citation40] In vitro degradation of poly(urethanamide) of 0.75 and 1.00 (NCO to OH mole ratio) with respect of weight loss is shown in Figure .
Mechanical testing of coating
The effect of concentration of HDI, BUHDI, and IPDI on performance of coating was determined by mechanical properties and it is shown in Table . The HDI- and BUHDI-based polymer have excellent flexibility as conferred by fatty amide chains and also the alkyl chain of HDI and BUHDI. But IPDI-based polymer looses flexibility and imparts rigidity in the polymer, due to the presence of cyclic ring in IPDI. The –NH groups of urethane linkages form hydrogen bonds with the substrate, which enhanced scratch hardness, impact resistance, adhesion, and pencil hardness of coatings.[Citation21] At 0.75 and 1.00 NCO to OH mole ratio, the cross-link poly(urethanamide) exhibits excellent mechanical properties such as hardness, adhesion, and impact resistance due to the long alkyl chain, urethane, and amide linkage.
Table 3. Mechanical properties and chemical resistance of poly(urethanamide) coatings.
Chemical resistance of coatings
Chemical resistance of coatings was evaluated by acid, alkali; water, and solvent rub according to the ASTM standards. The acid, alkali, and water resistance was evaluated by immersion method and was observed that all coatings show excellent chemical resistance. The solvent rub test carried out for 500 cycles using MEK shows excellent results without any defects in the coatings such as loss in gloss and dissolution of coatings films. Due to the presence of urethane linkage in the poly(urethanamide), it possess high solvent, acid, and alkali resistance. At NCO to OH mole ratio 0.75 and 1.00, coatings exhibit excellent chemical and solvent resistance, due to the cross-linked network in the resulting polymer. The solvent and chemical resistance of coatings is shown in Table .
Conclusion
From this study, it was revealed that the renewable B. roxburgii seed is one of the useful sources of oil, containing about 32 wt% of oil and can be utilized successfully for the synthesis of the cross-link poly(urethanamide) for coating applications. These coatings have good adhesion, gloss, hardness, impact, and chemical resistance properties due to the cross-link structure of poly(urethanamide). The polymer exhibits excellent water resistance and thermal stability. The poly(urethanamide) of various isocyanate degrades about 4–14% in 25 days. Due to good adhesion between coating and substrate, the BRO-based cross-linked poly(urethanamide) is useful in applications where high degree of chemical resistance is required.
Acknowledgments
We wish to thank UGC Green Tech. for providing financial support and also to Bayer Material Science Pvt. Ltd. for providing IPDI, HDI, and BUHDI.
References
- Gupta AP, Ahmad S, Dev A. Modification of novel bio-based resin-epoxidized soybean oil by conventional epoxy resin. Polym. Eng. Sci. 2011;51:1087–1091.10.1002/pen.21791
- Velayutham TS, Abd Majid WH, Ng BK, Gan SN. Effect of oleic acid content and chemical crosslinking on the properties of palm oil-based polyurethane coatings. J. Appl. Polym. Sci. 2013;129:415–421.10.1002/app.v129.1
- Kyu WL, Cheng H, Jin Y, Young WK, Keun WC. Modification of soybean oil for intermediates by epoxidation, alcoholysis and amidation. Korean J. Chem. Eng. 2008;25:474–482.
- Michael ARM, Jurgen OM, Ulrich SS. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007;36:1788–1802.
- Mohamed NB, Alessandro G. Monomers, polymers and composites from renewable resources. Great Britain: Elsevier; 2008.
- Bai-Liang X, Jia-Long W, Feng X, Run-Cang S. Polyols production by chemical modification of autocatalyzed ethanol-water lignin from Betula alnoides. J. Appl. Polym. Sci. 2013;129:434–442.
- Kalita H, Karak N. Epoxy modified bio-based hyperbranched polyurethane thermosets. Des. Monomers Polym. 2013;16:447–455.10.1080/15685551.2012.747163
- Eram S, Syed MA, Sharif A. Epoxidation, hydroxylation, acrylation and urethanation of Linum usitatissimum seed oil and its derivatives. Eur. J. Lipid Sci. Technol. 2007;109:134–146.
- Johannes TPD, Cuperus FP, Peter K. Renewable resources in coatings technology: a review. Prog. Org. Coat. 1996;27:45–53.
- Trevino AS, Trumbo DL. Acetoacetylated castor oil in coatings applications. Prog. Org. Coat. 2002;44:49–54.10.1016/S0300-9440(01)00223-5
- Stemmelen M, Pessel F, Lapinte V, Caillol S, Habas JP, Robin JJ. A fully biobased epoxy resin from vegetable oils: from the synthesis of the precursors by thiol-ene reaction to the study of the final material. J. Polym. Sci. Part A: Polym. Chem. 2011;49:2434–2444.10.1002/pola.v49.11
- David SB, Sathiyalekshmi K, Allen GRG. Studies on acrylated epoxydised triglyceride resin-co-butyl methacrylate towards the development of biodegradable pressure sensitive adhesives. J. Mater. Sci. Mater. Med. 2009;20:S61–S70.10.1007/s10856-008-3482-6
- Mohammad K, Eram S, Fahmina Z, Sharif A. Synthesis and characterization of ricinoleamide-based polyurethane. J. Am. Oil Chem. Soc. 2011;88:1989–1996.
- Zuleica L, Galen JS, Yuan-Chan T, Fu-Hung H. Soy-based polyols from oxirane ring opening by alcoholysis reaction. J. Appl. Polym. Sci. 2009;113:2552–2560.
- Honghai D, Liting Y, Bo L, Chengshuang W, Guang S. Synthesis and characterization of the different soy-based polyols by ring opening of epoxidized soybean oil with methanol, 1,2-ethanediol and 1,2-propanediol. J. Am. Oil Chem. Soc. 2009;86:261–267.
- Desroches M, Escouvois M, Auvergne R, Caillol S, Boutevin B. From vegetable oils to polyurethanes: synthetic routes to polyols and main industrial products. Polym. Rev. 2012;52:38–79.10.1080/15583724.2011.640443
- Ismail E, Motawie A, Sadek E. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt. J. Pet. 2011;20:1–8.10.1016/j.ejpe.2011.06.009
- Lligadas G, Ronda JC, Galià M, Cádiz V. Plant oils as platform chemicals for polyurethane synthesis: current state-of-the-art. Biomacromolecules. 2010;11:2825–2835.10.1021/bm100839x
- Petrovic ZS. Polyurethanes from vegetable oils. Polym. Rev. 2008;48:109–155.10.1080/15583720701834224
- Zlatanic A, Petrovic Z, Dusek K. Structure and properties of triolein-based polyurethane networks. Biomacromolecules. 2002;3:1048–1056.10.1021/bm020046f
- Cecilia OA, Emmanuel TA, Thomas Z. Studies on newly developed urethane modified polyetheramide coatings from Albizia benth oil. Prog. Org. Coat. 2011;71:89–97.
- Mohammad K, Fahmina Z, Ahmad S. Pongamia glabra seed oil based poly(urethane–fatty amide). J. Appl. Polym. Sci. 2010;117:1245–1251.
- Alam M, Sharmin E, Ashraf SM, Ahmad S. Newly developed urethane modified polyetheramide-based anticorrosive coatings from a sustainable resource. Prog. Org. Coat. 2004;50:224–230.10.1016/j.porgcoat.2004.02.007
- Yadav S, Zafar F, Hasnat A, Ahmad S. Poly (urethane fatty amide) resin from linseed oil – a renewable resource. Prog. Org. Coat. 2009;64:27–32.10.1016/j.porgcoat.2008.07.006
- Ahmad S, Ashraf SM, Naqvi F, Yadav S, Hasnat AA. Polyesteramide from Pongamia glabra oil for biologically safe anticorrosive coating. Prog. Org. Coat. 2003;47:95–102.10.1016/S0300-9440(03)00015-8
- Toliwal SD, Patel K. Modified neem (Azadirachta indica) oil based curing of acid functional acrylic copolymer resin for anticorrosive coating. J. Sci. Ind. Res. 2006;65:590–593.
- Pramanik S, Konwarh R, Sagar K, Konwar BK, Karak N. Bio-degradable vegetable oil based hyperbranched poly(ester amide) as an advanced surface coating material. Prog. Org. Coat. 2013;76:689–697.10.1016/j.porgcoat.2012.12.011
- Dutta S, Karak N. Synthesis, characterization of poly(urethane amide) resins from Nahar seed oil for surface coating applications. Prog. Org. Coat. 2005;53:147–152.10.1016/j.porgcoat.2005.02.003
- Lee H, Cho B, Kim MS, Lee W, Tewari J, Bae H, Sohn S, Chi H. Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens. Actuators, B. 2013;185:694–700.10.1016/j.snb.2013.04.103
- Marangoni A, Alli I. Composition and properties of seeds and pods of the tree legume Prosopis juliflora (DC). J. Sci. Food Agric. 1988;44:99–110.
- Kaura SK, Gupta SK, Chowdhury JB. Morphological and oil content variation in seeds of Azadirachta indica A. Juss.(Neem) from northern and western provenances of India. Plant Food Human Nutr. 1998;52:293–298.10.1023/A:1008013424150
- Azam MM, Waris A, Nahar NM. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy. 2005;29:293–302.
- Dinda S, Patwardhan AV, Goud VV, Pradhan NC. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008;99:3737–3744.10.1016/j.biortech.2007.07.015
- Campanella A, Baltanas MA. Degradation of the oxirane ring of epoxidized vegetable oils in liquid-liquid systems: I. Hydrolysis and attack by H2O2. Lat. Am. Appl. Res. 2005;35:205–210.
- Paquot C. Standard methods for the analysis of oil, fats and derivatives. Paris: Pergamon press; 1979.
- Saijun D, Nakason C, Kaesaman A, Klinpituksa P. Water absorption and mechanical properties of water swellable natural rubber. Songkl. J. Sci. Technol. 2009;31:561–565.
- Tan SG, Chow WS. Thermal properties, curing characteristics and water absorption of soybean oil-based thermoset. eXPRE. Polym. Lett. 2011;5:480–492.10.3144/expresspolymlett.2011.47
- Pramanik S, Konwarh R, Sagar K, Konwar BK, Karak N. Bio-degradable vegetable oil based hyperbranched poly(ester amide) as an advanced surface coating material. Prog. Org. Coat. 2013;76:689–697.10.1016/j.porgcoat.2012.12.011
- Wei Y, Cheng F, Li H, Yu J. Thermal properties and micromorphology of polyurethane resins based on liquefied benzylated wood. J. Sci. Ind. Res. 2005;64:435–439.
- Ghaffar A, Draaisma GJJ, Mihov G, Dias AA, Schoenmakers PJ, Vanderwal S. Monitoring the in vitro enzyme-mediated degradation of degradable poly(ester amide) for controlled drug delivery by LC-ToF-MS. Biomacromolecules. 2011;12:3243–3251.10.1021/bm200709r