Abstract
In order to get durable antibacterial polymer materials, two series of novel polymerizable Gemini quaternary ammonium salts (QAS) were synthesized via a facile approach. The first series of monomers which have a completely symmetrical structure contain two unsaturated double bonds that allow it to polymerize as cross-linker. The second series of monomers are asymmetric Gemini QAS and contain only one polymerizable double bond. All the QAS monomers were characterized by 1H NMR and IR spectra. The six kinds of QAS monomers were bonded to dental restoration resin system. To evaluate the bactericidal activity of these compounds against Escherichia coli and Staphylococcus aureus (S. aureus), the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration were tested by using broth dilution test. Among the six monomers, four compounds were found to have MIC values (for S. aureus) less than 10 μg/mL. The asymmetric Gemini QAS which have a long alkyl chain length of 12 carbons on the nitrogen showed stronger antibacterial activity than other monomers. The inhibitory effect of polymeric resins incorporated with QAS against the growth of S. aureus was determined by counting the number of viable cells. The result showed that the antibacterial activities of cured resins were increased as the concentration of QAS monomers increased.
1. Introduction
With increasing concerns of micro-organism contaminations and infections, there is a growing interest in the development of new antimicrobial compounds.[Citation1–8] Quaternary ammonium salts (QAS) are well known and effective antibiotics due to their broad spectrum efficacy, low toxicity, non-irritation, and chemically stable properties.[Citation9–14] However, there are some irreconcilable disadvantages of conventional quaternary ammonium compounds, for example, more additive amount, not sensitive to Gram-negative bacteria and so on.[Citation15–18] Gemini QAS, which have been studied by many chemists worldwide, can avoid the disadvantage of conventional QAS compounds. These Gemini biocides possess at least two hydrophobic chains and two cationic groups, and exhibit more excellent antibacterial activities than comparable conventional ones.[Citation19–23]
Several reports have described experiments in which an antibacterial agent was incorporated into dental filling materials, in order to inhibit bacterial attachment and plaque accumulation on their surfaces. However, the antibacterial activity of these materials is dependent upon release of the agent, which is associated with some disadvantages including influence on mechanical properties, toxic effects, and disruption of microbial homeostasis.[Citation24–26] Meanwhile, QAS polymeric materials that can kill bacteria without releasing any low-molecular-weight biocides have been developed.
Introduction of biocide monomers during the process of polymerization is one of the promising approaches in the development of non-leaching biocide polymers.[Citation27–31] In this perspective, polymerizable Gemini monomers, with quaternary ammonium groups as polar heads and methyl acrylic functions as the polymerizable units, were synthesized to build antibacterial materials.[Citation32] Antimicrobial activity of these materials likely results from the positively charged ammonium group (N+) interaction with negatively charged bacterial cell membrane.[Citation6,10,13–17] Long alkyl-chained QAS can utilize additional antibacterial activity by inserting into the bacterial membrane.[Citation18–23] According to the action mechanism of antibacterial materials containing QAS, it can be proposed that the antibacterial activity of polymeric QAS materials can be increased by increasing the degree of freedom of N+ groups. For building dental resin with improved antibacterial activity, novel polymerizable Gemini monomers, with a long spacer between the polymerizable units and N+ groups, were specifically designed. In this article, we describe the synthesis of six polymerizable Gemini quaternary ammonium monomers: N,N,N′,N′-tetramethyl-dimethylene-bis(1-undecanoate-2-(2-methacryloyl-oxy) ethyl) bromide (MEBU-TMEDA-MEBU), N,N,N′,N′-tetramethyl-hexamthy-lene-bis(1-undecanoate-2-(2-methacryloyloxy) ethyl) bromide (MEBU-TMHDA-MEBU), N,N,N′,N′-tetramethyl-dimethylene-dodecyl-1-undecanoate-2-(2-methacry-loyloxy)ethyl) bromide (MEBU-TMEDA-C12), N,N,N′,N′-tetramethyl-dimethylene-tetradecyl-1-undecanoate-2-(2-methacry-loyloxy)ethyl) bromide (MEBU-TMEDA-C14), N,N,N′,N′-tetramethyl-hexamthylene-dodecyl-1-undecanoate-2-(2-methacryloyl-oxy)ethyl) bromide (MEBU-TMHDA-C12), and N,N,N′,N′-tetramethyl-hexamthylene-tetradecyl-1-undecanoate-2-(2-methacryloyloxy) ethyl) bromide (MEBU-TMHDA-C14). These monomers were investigated on the bactericidal efficacy against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).[Citation33] The six monomers were bonded to dental resin system by light curing method which is a common method of clinical dental restoration. The inhibitory effect of cured resin incorporated with QAS against the growth of S. aureus on its surface was determined by counting the number of viable cells. The effect of monomer structure on the minimal inhibitory concentration (MIC), minimum bactericidal concentration (MBC) values of monomers, and the inhibitory effect of antibacterial resin was also carefully discussed.
2. Experimental
2.1. Materials and bacterial strains
11-bromoundecanoic acid, 2-hydroxyethyl methacrylate, N,N,N′,N′-tetramethyle-thylenediamine, TMHDA, 1-bromododecane, 1-bromotetradecane, p-toluenesulfonic acid (PTSA), and hydroquinone were obtained from Aladdin Chemical Reagent CO. Ltd. Bisphenol glycidyl methacrylate (Bis-GMA) was obtained from Sigma-aldrich Reagent CO. Ltd. Triethylene glycol dimethacrylate (TEGDMA), camphorquinone (CQ), and N, N-dimethylaminoethyl methacrylate (DMAEMA) were obtained from Acros Organics Reagent CO. Ltd. Toluene, isopropanol, acetone, acetonitrile, and anhydrous diethyl ether were obtained from Shanghai Lingfeng Chemical Reagent CO. Ltd. All reagents were used without any purification. Yeast extract, peptone, agar were got from Sangon Biotech CO. Ltd and used as received. Bacterial strains used for antibacterial activity tests included S. aureus RN4220 and E. coli TOP10 strain.
2.2. Characterization
1H NMR spectra were recorded on a Varian Gemini 500 MHz spectrometer using tetramethylsilane as an internal standard in CDCl3 and DMSO-d6. Fourier-transform infrared (FT-IR) spectra were obtained with a Mattson 5000 FT-IR spectrometer using NaCl windows.
2.3. Synthesis of symmetrical Gemini quaternary ammonium monomers
2.3.1. Intermediates MEBU
Equimolar amounts of 2-hydroxyethyl methacrylate (6.5 g, 0.05 mol) and 11-bromoncanoic acid (13.25 g, 0.05 mol), 0.005 g hydroquinone, 0.077 g PTSA, and 40 mL toluene were added to a 100 mL round-bottom flask. The mixture was stirred for 24 h at 110 °C using magnetic stirrer. After evaporation of the solvent with a rotary evaporator under vacuum, the crude product was purified with column chromatography using ethyl acetate and petroleum ether as eluent. The pure product was isolated as clear, colorless oil.
1H NMR (CDCl3): δ = 1.29 (t, 10H, CH2), 1.43 (m, 2H, O=C–C–C–CH2), 1.63 (m, 2H, O=C–C–CH2), 1.86 (m, 2H, CH2–CBr), 1.96 (s, 3H, C=C–CH3), 2.32 (t, 2H, O=C–CH2), 3.43 (t, 2H, CH2Br), 4.35 (m, 4H, ester–CH2–CH2–ester), 5.60–6.14 (s, 2H, C=CH2) ppm.
FT-IR: σ = 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
2.3.2. Monomer MEBU-TMEDA-MEBU and MEBU-TMHDA-MEBU
The monomers MEBU-TMEDA-MEBU and MEBU-TMHDA-MEBU were made using the same procedure. About 0.01 mol TMEDA (or TMHDA), 0.02 mol MEBU, 0.001 g hydroquinone, and 16 mL isopropanol were added to a 50 mL round-bottom flask, and the mixture was stirred for 25 h at 95 °C using magnetic stirrer. After evaporation of the solvent, the crude product was dissolved by acetone. The supernatant was collected after centrifugation. After acetone was removed on a rotary evaporator, the crude product was purified with diethyletheranhydrous as eluent. All the final products were obtained as yellow viscous liquids.
MEBU-TMEDA-MEBU
1H NMR (DMSO-d6): δ = 1.24 (m, 24H, CH2), 1.5 (s, 4H, O=C–C–CH2), 1.72 (s, 4H, CH2–C–N), 1.87 (s, 6H, C=C–CH3), 2.39 (t, 4H,O=C–CH2), 3.15 (s, 12H, CH3–N–CH3), 3.36–3.37 (t, 8H, CH2–N–CH2), 4.30 (m, 8H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 4H, C=CH2) ppm.
FT-IR: σ = 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
MEBU-TMHDA-MEBU
1H NMR (DMSO-d6): δ = 1.24 (m, 28H, CH2), 1.5 (s, 4H, O=C–C–CH2), 1.72 (s, 8H, H2C–C–N), 1.87 (s, 6H, C=C–CH3), 2.39 (t, 4H, O=C–CH2), 3.15 (s, 12H, CH3–N–CH3), 3.36–3.37 (t, 8H, CH2–N–CH2), 4.30 (m, 8H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 4H, C=CH2) ppm.
FT-IR: σ = 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
2.4. Synthesis of asymmetric Gemini quaternary ammonium monomers
2.4.1. Intermediates MEBU-TMEDA and MEBU-TMHDA
The intermediates MEBU-TMEDA and MEBU-TMHDA were made with the same process. In 50 mL round bottom flask, 0.02 mol TMEDA (or TMHDA) was reacted with 0.02 mol MEBU. After 0.001 g hydroquinone was added, the reaction was conducted in 15 mL acetone for 25 h at 70 °C with magnetic stirring. The supernatant was collected after centrifugation. After acetone was evaporated with a rotary evaporator under vacuum, the intermediates were obtained as yellow viscous liquids.
MEBU-TMEDA
1H NMR (DMSO-d6): δ:1.24 (m, 12H, CH2), 1.5 (m, 2H, O=C–C–CH2), 1.68 (m, 2H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.2 (s, 6H, C–N(CH3)2), 2.39 (t, 2H, O=C–CH2), 2.61 (t, 2H, N–CH2), 3.15 (s, 6H, CH3–N+–CH3), 3.36–3.46 (t, 4H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2).
FT-IR: σ: 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
MEBU-TMHDA
1H NMR (DMSO-D6): δ:1.24 (m, 16H, CH2), 1.4 (m, 2H, CH2–C–N) 1.5 (m, 2H, O=C–C–CH2), 1.68 (m, 4H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.08 (t, 2H, O=C–CH2), 2.15 (s, 6H, C–N(CH3)2), 2.27 (m, 2H, CH2–N), 3.02 (s, 6H, CH3–N+–CH3), 3.36 (t, 4H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2).
FT-IR: σ: 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
2.4.2. Monomer MEBU-TMEDA-C12 and MEBU-TMEDA-C14
About 0.01 mol MEBU-TMEDA, 0.01 mol 1-bromododecane (or 1-bromotetradecane), 0.001 g hydroquinone, and 15 mL acetonitrile were added to 50 mL round-bottom flask, and the mixture was heated for 25 h at 95 °C with magnetic stirring. After evaporation of the solvent, the crude product was dissolved by acetone, and the supernatant was collected after centrifugation. Acetone was removed using rotary evaporator. Then the products were purified with anhydrous diethyl ether. The pure products were obtained as yellow viscous liquids.
MEBU-TMEDA-C12
1H NMR (DMSO-d6): δ = 0.9 (t, 3H, C–C–CH3), 1.24 (m, 30H, CH2), 1.5 (s, 2H, O=C–C–CH2), 1.72 (m, 4H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.39 (t, 2H, O=C–CH2), 3.15 (s, 12H, CH3–N+–CH3), 3.36–3.37 (t, 8H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2) ppm.
FT-IR: σ: 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
MEBU-TMEDA-C14
1H NMR (DMSO-d6): δ = 0.9 (t, 3H, C–C–CH3), 1.24 (m, 34H, CH2), 1.5 (s, 2H, O=C–C–CH2), 1.72 (m, 4H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.39 (t, 2H, O=C–CH2), 3.15 (s, 12H, CH3–N+–CH3), 3.36–3.37 (t, 8H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2) ppm.
FT-IR: σ: 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
2.4.3. Monomer MEBU-TMHDA-C12 and MEBU-TMHDA-C14
Via a procedure similar to that for monomer MEBU-TMEDA-C12 and MEBU-TMEDA-C14, 0.01 mol MEBU-TMHDA, 0.01 mol 1-bromododecane (or 1-bromotetradecane), 0.001 g hydroquinone, and 16 mL acetonitrile were heated for 25 h at 95 °C in 50 mL round-bottom flask. The products were purified with anhydrous diethyl ether. MEBU-TMHDA-C12 and MEBU-TMHDA-C14 were obtained as yellow viscous liquids.
MEBU-TMHDA-C12
1H NMR (DMSO-d6): δ = 0.9 (t, 3H, C–C-CH3), 1.24 (m, 34H, CH2), 1.5 (s, 2H, O=C–C–CH2), 1.72 (m, 8H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.39 (t, 2H, O=C–CH2), 3.15 (s, 12H, CH3–N+–CH3), 3.36–3.37 (t, 8H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2) ppm.
FT-IR: σ = 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
MEBU-TMHDA-C14
1H NMR (DMSO-d6): δ = 0.9 (t, 3H, C–C–CH3), 1.24 (m, 38H, CH2), 1.5 (s, 2H, O=C–C–CH2), 1.72 (m, 8H, H2C–C–N+), 1.87 (s, 3H, C=C–CH3), 2.39 (t, 2H, O=C–CH2), 3.15 (s, 12H, CH3–N+–CH3), 3.36–3.37 (t, 8H, CH2–N+–CH2), 4.30 (m, 4H, O=C–CH2–CH2–C=O), 5.71–6.02 (s, 2H, C=CH2) ppm.
FT-IR: σ = 3388 cm−1(ν C–N), 2921 cm−1(ν C–H), 1718 cm−1(ν C=O), 1632 cm−1(ν C=C), 1161 cm−1(ν (O=)C–O), 1328 and 940 cm−1(ν (C=)C–H).
2.5. Synthesis of QAS polymeric materials
Bis-GMA, TEGDMA, CQ, DMAEMA, and monomers (Table , for example, the resin incorporated with MEBU-TMEDA-MEBU) were added to a 5 mL beaker, stirred, and bubbles were removed by ultrasound. The above solutions were added dropwise to a diameter of 10 mm, a height of 2 mm mold, then cured for 100S with a dental curing light. Ultrasonic cleaning of the resin sheet to remove the unpolymerized monomer was done and it was air dried.
Table 1. The composition of polymeric materials.
2.6. Antibacterial assessment
The antibacterial assessments of polymerizable Gemini monomers were carried out using the MIC and the MBC method. Two micro-organisms were used including S. aureus and E. coli, which represent Gram-positive and gram-negative bacteria, respectively.[Citation34] The MIC is defined as the lowest concentration of an antimicrobial agent that will inhibit the growth of a micro-organism after incubation. The MIC values were determined by the broth dilution method using geometric twofold dilutions in LB. Solutions of biocide monomers in the series were mixed with 107 CFU/mL of the organism in test tubes. The test tubes were incubated at 37 °C for 24 h. The MIC value is the concentration of the first clear test tube.[Citation35,36] The MBC is defined as the lowest concentration of antimicrobial agent that will kill micro-organims and consequently will prevent growth after subculture onto antibiotic-free media. The MBC values were determined by agar dilution method.[Citation37] The inoculating needle was put into the clear test tubes with a little amount of solution, then lines were drawn on the agar plates. These plates were incubated at 37 °C for 24 h. The MBC value is the minimum concentration of the clear solution where plate has no colonies. The inhibitory effect of resin incorporated with QAS against the growth of S. aureus on its surface was determined by counting the number of viable cells.[Citation1–4] The cured resins were placed in test tubes with 104 CFU/mL of initial S. aureus suspension in PBS. These tubes were shaken in incubators at 37 °C for 24 h. About 0.2 mL bacteria suspensions were pipetted out from the tubes, and plated onto the triplicate solid agar using the spread plate method. After incubating for 24 h, the number of viable bacteria was counted.
3. Results and discussion
3.1. Synthesis of Gemini biocide monomers
Two series of novel polymerizable Gemini QAS were synthesized via a facile method. Gemini biocide monomers contain polymerizable moieties which can polymerize during the synthesis; we used mild reaction temperature and appropriate solvent to get high yield and avoid any polymerization reaction.
3.1.1. Symmetrical monomer synthesis
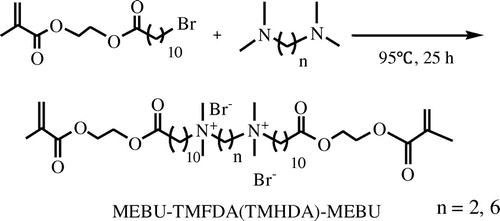
The symmetrical Gemini quaternary ammonium monomers were synthesized via a two-step route (Schemes and ). The synthesis of intermediate MEBU was carried out via esterification reaction according to the JP56156237 [Citation38] in an 80% yield. The synthesis route is shown in Scheme . MEBU, as a bromo compound, is known as very efficient quaternization agents leading very easily to the quaternary ammonium compounds when reacting with a tertiary amine. In this work, monomers MEBU-TMEDA-MEBU and MEBU-TMHDA-MEBU were prepared, in a 70% yield, via quaternization reaction of MEBU with TMEDA or TMHDA with isopropanol as solvent. Scheme outlines the general synthesis of symmetrical biocide monomers. In this reaction procedure, we controlled the proportion of MEBU and diamine (2:1) and used isopropanol as solvent.
3.1.2. Asymmetric monomer synthesis
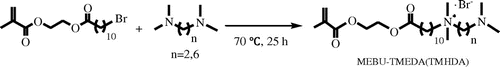
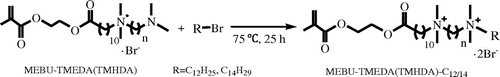
Unlike the symmetrical Gemini monomers, the other four polymerizable Gemini QAS which have asymmetric structure were synthesized via a three-step route (Schemes and ). MEBU was also prepared by reaction as shown in Scheme . Then we used MEBU as the first alkyl bromide to react with TMEDA or TMHDA, and got intermediate MEBU-TMEDA or MEBU-TMHDA in about 75% yield. In this quaternization reaction procedure, we controlled the proportion of MEBU and diamine at equimolar ratio and used isopropanol as solvent. The general route of synthesis is shown in Scheme . As the second alkyl bromide, 1-bromododecanes (1-bromotetradecanes) were added to MEBU-TMEDA or MEBU-TMHDA at equimolar ratio and refluxed in acetonitrile. At last, we got monomers MEBU-TMEDA-C12, MEBU-TMEDA-C14, MEBU-TMHDA-C12, and MEBU-TMEDA-C14 in about 70% yield, the general synthesis route is shown in Scheme .
3.2. Characterization of monomers
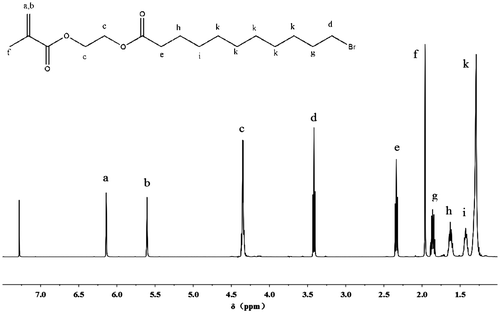
All the intermediates and Gemini QAS monomers were recorded using NMR and FT-IR to confirm their structures. Figure shows the 1H NMR spectrum of intermediate MEBU. The peaks at 5.60–6.14 ppm are assigned to the methacrylate double bond protons, and the chemical shift of methyl is at 1.96 ppm. Peak at 4.35 ppm is ascribed to methylenes between the two ester groups. Besides, the peaks at 1.29, 1.43, 1.63, 1.86, 2.32, and 3.43 ppm are from the methylenes of 11-Bromo-ncanoic acid. From the FT-IR spectra, the vibration of C=O appears at 1718 cm−1. The C=C appears at 1632 cm−1. The vibration of C–O of ester group appears at about 1162 cm−1. The C–H of alkene appears at 1328 and 940 cm−1. The 1H NMR and FT-IR results indicated that intermediate MEBU was successfully prepared.
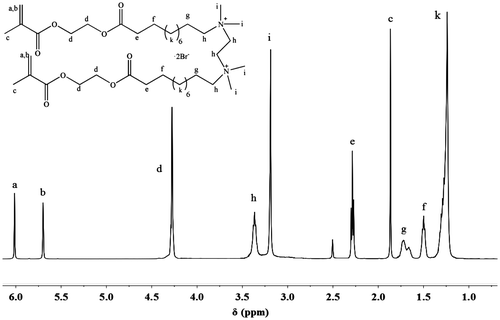
Figure shows the 1H NMR spectrum of symmetrical monomer MEBU-TMEDA-MEBU. Methylenes between the two N+ ionic groups appear at about 4.30 ppm, and the signal at 3.15 ppm is assigned to methyls attached to the N+, which means the MEBU has already reacted with TMEDA to form the quaternary ammonium structure. Absorption peaks around 1720 and 1640 cm−1 in FT-IR spectra, chemical shifts at 5.71–6.02(C=CH2), 1.87(C=C–CH3), and 4.30(O=C–CH2–CH2–C=O) ppm revealed that there were still methacrylate groups in the products. Similarly, MEBU-TMHDA-MEBU was also successfully synthesized, as demonstrated by1H NMR and FT-IR.
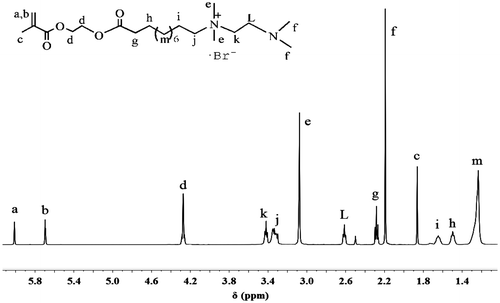
Intermediate MEBU-TMEDA was prepared via quaternization reaction of MEBU and TMEDA. All characteristic peaks of MEBU-TMEDA could be observed from Figure . The protons of alkene appear at 5.71–6.02 ppm. Methyls attached to the N atom and N+ ionic group appear at 2.2 and 3.15 ppm, respectively. The peaks at 3.36–3.46 ppm is ascribed to methylenes attached to the N+ ionic group, and the chemical shift of methylene attached to the N atom is at 2.61 ppm. The results revealed that MEBU-TMEDA was successfully synthesized. Similarly, intermediate MEBU-TMEDA was also prepared and demonstrated.
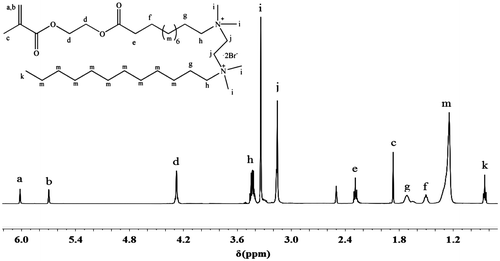
1H NMR spectra of asymmetric monomer MEBU-TMEDA-C12 are shown in Figure . The protons of alkene appear at 5.71–6.02 ppm. Methyls attached to the N+ ionic groups appear at 3.15 ppm. The peaks at 3.36–3.46 ppm are ascribed to methylenes attached to the N+ ionic group. Besides, the peaks at 0.90, 1.24, and 1.72 ppm are from the methylenes of 1-Bromododecane alkyl chain. The other asymmetric monomer MEBU-TMEDA-C14, MEBU-TMHDA-C12, and MEBU-TMEDA-C14 were also characterized with the similar methods.
3.3. Antibacterial properties of six monomers
The antibacterial activities of these Gemini monomers were investigated against S. aureus and E. coli, which are representative of Gram-negative and Gram-positive bacteria. The MIC values and the MBC values of biocide monomers were tested by using a broth dilution test.
As can be seen from MIC and MBC values presented in Table , all the synthesized Gemini monomers have excellent antibacterial activities for both Gram-negative and Gram-positive bacteria. Among the six compounds, four compounds were found to have MIC values (for S. aureus) less than 10 μg/mL. The asymmetric Gemini quaternary ammonium monomers have a lower MIC values than the monomers which have a completely symmetrical structure. Among the asymmetric Gemini QAS, the monomers which have a long alkyl chain length of 12 carbon atoms have better antibacterial activity. The spacer length between the two N+ ionic groups seems having teeny effect on the antibacterial ability for asymmetric Gemini quaternary ammonium monomers. On the contrary, it is an important factor that affects the MIC values and MBC values of the symmetrical Gemini QAS.
Table 2. The MIC values and MBC values of QAS monomers.
3.4. Antibacterial properties of QAS polymeric materials
The antibacterial properties of polymeric materials were investigated against S. aureus and determined by counting the number of viable cells.
The data of Table presented that the concertration of bacteria on the surface of antibacrerial modification composite resin was less than the control group. It showed that the resin incorporated with monomers have antibacterial activities, and the activities increased with the concentration of QAS monomers increased. The antibacterial activities of resin incorporated with asymmetric Gemini quaternary ammonium monomers (MEBU-TMEDA-C12, MEBU-TMEDA-C14, MEBU-TMHDA-C12, MEBU-TMHDA-C14) were better than the resin incorporated with symmetric Gemini quaternary ammonium monomers (MEBU-TMHDA-MEBU, MEBU-TMHDA-MEBU). The reason of poor peformance of the latter is that the symmetric Gemini QAS monomers have two unsaturated double bonds. When monomers were bonded to resin, both ends of the molecule were fixed, which reduced the freed degree of N+. The resin incorporated with MEBU-TMEDA-C12 and MEBU-TMEDA-C14 showed better antibacterial activities than the resin incorporated with MEBU-TMHDA-C12 and MEBU-TMHDA-C14. The long spacer between the two N+ of MEBU-TMHDA-C12 and MEBU-TMHDA-C14 reduced the charge density. The resin incorporated with MEBU-TMEDA-C12 had the best antibacterial activities.
Table 3. Antibacterial properties of QAS polymeric materials.
4. Conclusion
Two series of novel polymerizable Gemini quaternary ammonium monomers were synthesized with a facile method and characterized by 1H NMR and IR to confirm their expected structures. The antibacterial activities of monomers were investigated against S. aureus and E. coli bacteria. All novel biocide monomers came out as broad spectrum biocides against both Gram positive and negative bacteria. The six kinds of QAS monomers were bonded to the resin system by light-curing method to get QAS polymeric materials for dental resin. The inhibitory effect of resin against the growth of S. aureus was determined. The result showed that the resin incorporated with the monomers had antibacterial activities, and the antibacterial activities were increased with increasing the concentration of QAS monomers.
Funding
This research was supported by the grant from the Natural Science Foundation of China [grant number 81130078]; NUST Research Funding [grant number 2011YBXM54].
References
- He JW, Söderling E, Vallittu PK, Lassila LVJ. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J. Biomater. Sci., Polym. Ed. 2013;5:565–573.10.1080/09205063.2012.699709
- Pupo YM, Farago PV, Nadal JM, Esmerino LA, Maluf DF, Zawadzki SF, Michél MD, Santos FA, Gomes OMM, Gomes JC. An innovative quaternary ammonium methacrylatepolymer can provide improved antimicrobial properties for a dental adhesive system. J. Biomater. Sci., Polym. Ed. 2013;24:1443–1458.10.1080/09205063.2013.766784
- Zhang Y, Ding MM, Zhou LJ, Tan H, Li JH, Xiao HN, Li JS, Snow J. Synthesis and antibacterial characterization of gemini surfactantmonomers and copolymers. Polym. Chem. 2012;3:907–913.10.1039/c2py00558a
- Ayfer B, Dizman B, Elasri MO, Mathias LJ, Avci D. Synthesis and antibacterial activities of new quaternary ammonium monomers. Des. Monomers Polym. 2005;8:437–451.10.1163/1568555054937935
- Dizman B, Elasri MO, Mathias LJ. Synthesis and antimicrobial activities of new water-soluble bis-quaternary ammonium methacrylate polymers. J. Appl. Polym. Sci. 2004;94:635–642.10.1002/(ISSN)1097-4628
- Mathias LJ, Shemper BS, Alirol M, Morizur JF. Synthesis of new hydroxylated monomers based on methacrylate, dimethacrylate, and tetramethacrylate michael adducts and photopolymerization kinetics of bulk cross-linkers. Macromolecules. 2004;37:3231–3238.10.1021/ma035260q
- Gao CL, Wu XE, Lv GH, Ding MX, Gao LX. Syntheses and properties of soluble biphenyl-based polyimides from asymmetric bis(chlorophthalimide)s. Macromolecules. 2004;37:2754–2761.10.1021/ma0350720
- Chern YT, Tsai JY. Low dielectric constant and high organosolubility of novel polyimide derived from unsymmetric 1,4-Bis(4-aminophenoxy)-2,6-di-tert-butylbenzene. Macromolecules. 2008;41:9556–9564.10.1021/ma802305q
- Yang CP, Su YY, Wu KL. Synthesis and properties of new aromatic polyimides based on 2,5-bis(4-amino-2-trifluoromethylphenoxy)-tert-butylbenzene and various aromatic dianhydrides. J. Polym. Sci., Part A: Polym. Chem. 2004;42:5424–5438.10.1002/(ISSN)1099-0518
- Xue Y, Guan Y, Zheng A, Wang H, Xiao H. Synthesis and characterization of ciprofloxacin pendant antibacterial cationic polymers. J. Biomater. Sci., Polym. Ed. 2012;23:1115–1128.10.1163/092050611X576639
- Kusama M, Matsumoto T, Kurosaki T. Soluble polyimides with polyalicyclic structure.3. polyimides from (4arH,8acH)-decahydro-1t,4t:5c,8c-dimethanonaphthalene- 2t,3t,6c,7c-tetracarboxylic 2,3:6,7-dianhydride. Macromolecules. 1994;27:1117–1123.10.1021/ma00083a008
- Li WM, Li SH, Zhang QY, Zhang SB. Synthesis of bis(amine anhydride)s for novel High Tgs and organosoluble poly(amine imide)s by palladium-catalyzed amination of 4-chlorophthalide anhydride. Macromolecules. 2007;40:8205–8211.10.1021/ma071213c
- Koohmareh GA. New organo-soluble polyimides based on a new dianhydride: 4-(4-t-butyl-phenyl)-2,6-bis(3,4-phenyl dicarboxylic acid anhydride)pyridine. Des. Monomers Polym. 2007;10:517–525.10.1163/156855507782401169
- Liaw DJ, Hsu PN, Chen WH, Lin SL. High glass transitions of new polyamides, polyimides, and poly(amide−imide)s containing a triphenylamine group: synthesis and characterization. Macromolecules. 2002;35:4669–4676.10.1021/ma001523u
- Kim HS, Kim YH, Ahn SK, Kwon SK. Synthesis and characterization of highly soluble and oxygen permeable new polyimides bearing a noncoplanar twisted biphenyl unit Containing tert-butylphenyl or trimethylsilyl phenyl groups. Macromolecules. 2003;36:2327–2332.10.1021/ma0214557
- Patel HS, Patel VC. New monomers for polyimides. Des. Monomers Polym. 2000;3:191–197.10.1163/156855500300142889
- Mikroyannidis JA, Spiliopoulos IK. Soluble phenyl- or alkoxyphenyl-substituted rigid-rod polyamides and polyimides containing m-Terphenyls in the main chain. Macromolecules. 1998;31:1236–1245.
- Andrzejewska E, Marcinkowska A. New aspects of viscosity effects on the photopolymerization kinetics of the 2,2-bis[4-(2-hydroxymethacryloxypropoxy)phenyl]propane/triethylene glycol dimethacrylate monomer system. J. Appl. Polym. Sci. 2008;110:2780–2786.10.1002/app.v110:5
- Dickens SH, Stansbury JW, Choi KM, Floyd CJE. Photopolymerization kinetics of methacrylate dental resins. Macromolecules. 2003;36:6043–6053.10.1021/ma021675 k
- Abbanat D, Macielag M, Bush K. Novel antibacterial agents for the treatment of serious Gram-positive infection. Expert Opin. Invest. Drugs. 2003;12:379–399.10.1517/eid.2003.12.issue-3
- Kourai H, Yabuhara T, Shirai A, Maeda T, Nagamune H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur. J. Med. Chem. 2006;41:437–444.10.1016/j.ejmech.2005.10.021
- Pérez L, Torres JL, Manresa A, Solans C, Infante MR. Synthesis, aggregation, and biological properties of a new class of gemini cationic amphiphilic compounds from arginine, bis(Args). Langmuir. 1996;12:5296–5301.10.1021/la960301f
- Fisicaro E, Compari C, Duce E, Donofrio G, Rozycka-Roszak B, Wozniak E. Biologically active bisquaternary ammonium chlorides: physico–chemical properties of long chain amphiphiles and their evaluation as non-viral vectors for gene delivery. Biochim. Biophys. Acta. 2005;1722:224–233.10.1016/j.bbagen.2004.12.013
- Hoque J, Akkapeddi P, Yarlagadda V, Uppu DS, Kumar P, Haldar J. Cleavable cationic antibacterial amphiphiles: synthesis, mechanism of action, and cytotoxicities. Langmuir. 2012;28:12225–12234.10.1021/la302303d
- Obłak E, Piecuch A, Krasowska A, Luczyński J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013;3:9–638.
- Brycki B. Gemini alkylammonium salts as biodeterioration inhibitors. J. Microbiol. 2010;59:227–231.
- Zana R. Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002;248:203–220.10.1006/jcis.2001.8104
- Lee SB, Koepsel RR, Morley SW, Matyjaszewski K, Sun Y, Russell AJ. Permanent, nonleaching antibacterial surfaces. 1. synthesis by atom transfer radical polymerization. Biomacromolecules. 2004;5:877–882.10.1021/bm034352 k
- Kanazawa A, Ikeda T, Endo T. Novel polycationic biocides: synthesis and antibacterial activity of polymeric phosphonium salts. J. Polym. Sci., Part A: Polym. Chem. 1993;31:335–343.10.1002/pola.1993.080310205
- Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465.10.1021/bm034130 m
- Inchausti I, Sasia PM, Katime I. Copolymerization of dimethylaminoethylacrylate-methyl chloride and acrylamide in inverse emulsion. J. Mater. Sci. 2005;40:4833–4838.10.1007/s10853-005-2003-y
- Cakmak I, Ulukanli Z, Tuzcu M, Karabuga S, Genctav K. Synthesis and characterization of novel antimicrobial cationic polyelectrolytes. Eur. Polym. J. 2004;40:2373–2379.10.1016/j.eurpolymj.2004.06.004
- Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002.10.1016/j.biomaterials.2006.03.003
- Kourai H, Yabuhara T, Shirai A, Maeda T, Nagamune H. Syntheses and antimicrobial activities of a series of new bis-quaternary ammonium compounds. Eur. J. Med. Chem. 2006;41:437–444.10.1016/j.ejmech.2005.10.021
- Dizman B, Elasri MO, Mathias LJ. Synthesis and characterization of antibacterial and temperature responsive methacrylamide polymers. Macromolecules. 2006;39:5738–5746.10.1021/ma0607620
- Dizman B, Elasri MO, Mathias LJ. Synthesis, characterization, and antibacterial activities of novel methacrylate polymers containing norfloxacin. Biomacromol. 2005;6:514–520.10.1021/bm049383+
- Yuan SL, Cai ZT, Xu GY, Jiang YS. Mesoscopic simulation study on the interaction between polymer and C12NBr or C(9) phNBr in aqueous solution. Colloid Polym. Sci. 2003;281:1069–1075.10.1007/s00396-003-0881-6
- Kokai TK. (Bromoacetoxy) alkyl methacrylates[P]. Japan JP 56156237. 1981.