Abstract
A new phosphorus-containing dicarbamates, di(N-carbomethoxylaminomethyl)benzyl phosphine oxide was first synthesized from tetrakis(hydroxymethyl)phosphonium sulfate (THPS) via five step reactions (Scheme ), and its structure was proved by FTIR, (1H, 13C, and 31P) NMR, and MS. A series of thermally stable phosphorus-containing polyureas were synthesized via trans-ureation polycondensation of the new monomer with aliphatic diamines, including poly(propylene glycol) bis(2-aminopropyl ether), respectively. All of the above polyureas were fully characterized by inherent viscosity, GPC, solubility tests, and thermogravimetric analysis. The thermal gravimetric analysis results showed that the initial breakdown temperature of all phosphorus-containing polyureas was observed at above 265 °C while complete weight loss occurred in the region of 406–465 °C, which shows all polyureas have good thermal stability. GPC results showed that the maximum number-average molecular weight of the synthesized polyureas reached 59,100 g mol−1. The solubility tests results demonstrated that incorporating the ether moieties into the polymer main chain can enhance the solubility of polyureas in organic solvents.
1. Introduction
Introduction of inorganic elements into the backbone of organic polymers can obtain a series of high-performance materials, the preparation of these materials is an area of current interest.[Citation1,2] Phosphorus is one of the most abundant elements on earth and the development of this fascinating non-metal element in the polymer chemistry is clearly of both fundamental and applied interest.[Citation3] Small amounts of phosphorus in a polymer can be greatly advantageous, imparting flame retardancy,[Citation4–6] adhesion to metals,[Citation7] ion-exchange characteristics [Citation8], and biodegradable and blood compatible characteristics.[Citation3] One of the most direct ways for the introduction of phosphorus into a polymer is direct phosphorylation, for example reaction of the polymer with PCl3 and POCl3.[Citation9–11] However, these materials are better considered to be modified organic polymers rather than organic/inorganic hybrid polymers. Polyphosphonates and polyphosphates are of commercial interest because of their flame-retarding characteristics and their potential as high-performance plastics.[Citation12,13] Although they have some attractive properties, such as good transparency and hardness, the obvious disadvantage is the lack of long-term hydrolytic stability, which limited their usage in many practical applications. This problem has been partially overcome by elimination of the phosphorus–oxygen bonds in the backbone of polymers. For the past years, phosphorus–carbon bonds containing monomers and polymers have been the subject of extensive research. A new type of polymers based on a phosphorus–carbon backbone is poly(methylenephosphines) of structure [–P(R)–]n, produced by the polymerization of phosphaalkenes (P(R)=
).[Citation14–17] An alternative synthetic route to incorporate phosphorus–carbon bonds into the backbones of polyolefins is copolymerization of olefinic monomers with small phosphorus heterocycle compounds.[Citation18] And via the polycondensation of monoalkyl and monoaryl phosphines with 1,4-diiodobenzene, a series of poly(p-phenylenephosphine)s were successfully synthesized.[Citation19] Recently, 4,4′-(phenylphosphoryl)dibenzoic acid [Citation20–22] was also used as phosphorus-containing component to synthesize a series of main-chain phosphorus–carbon bonds containing polymers with long-term hydrolytic stability.
Looking into present research results in phosphorus chemistry, most phosphorus-containing monomers and polymers were synthesized from white phosphorus,[Citation23] phosphine (PH3),[Citation19,24] PCl3, and POCl3.[Citation25,26] However, white phosphorus is toxic and pyrophoric, and in fact, it is not available through standard chemical suppliers and is typically converted on site into other compounds. Part of phosphorus-containing monomers and polymers rely on PCl3, such as DOPO and its derivatives,[Citation25–27] arylphosphoric dichloride [Citation20,28] and phosphorus-containing acylchloride.[Citation29–31] Since reactive phosphorus–chlorine bond is not necessary in most polymers, it is desirable to avoid the use of chlorine altogether. Moreover, PCl3 is so hazardous and unstable that its storage and use are extremely inconvenient.
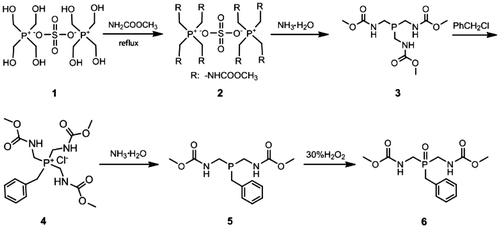
PH3 is mainly consumed as an intermediate in organophosphorus chemistry, even though it is a highly toxic and pyrophoric gas. However, PH3 can conveniently react with formaldehyde in sulfuric acid solution to give tetrakis(hydroxymethyl)phosphonium sulfate (THPS),[Citation32] which is a safe phosphorus-containing compound used as flame retardant and biocides. In fact, THPS is also a reactive intermediate for many fine phosphorus compounds. Tris(N-carbomethoxyl-aminomethyl)phosphine and its oxide were successfully synthesized from THPS by Frank.[Citation33] These compounds contain methyl carbamate functional groups, which can produce isocyanates through thermal decomposition method. This process is a promising ecologically safe method of obtaining isocyanates without using the toxic phosgene.[Citation34–36] As we all know, isocyanates are widely used in the preparation of the polyureas and polyurethanes.[Citation37] Furthermore, many efforts have been made to synthesize directly polyurethanes and polyureas via polycondensation dicarbamates monomers with diols and diamines.[Citation38–41] Inspired by these studies, we designed and synthesized a novel phosphorus-containing dicarbamates, di(N-carbomethoxyl-aminomethyl)benzyl phosphine oxide from THPS. Five phosphorus-containing polyureas were prepared by trans-ureation polycondensation of di-(N-carbomethoxylaminomethyl)benzyl phosphine oxide with five different diamines catalyzed by 1,5,7-triazabicyclododecene (TBD). In this study, the di(N-carbomethoxylaminomethyl)benzyl phosphine oxide was designed and synthesized as a new phosphorus–carbon bonds containing reactive monomer, which can expediently introduce the phosphorus element into the main chain of polyureas via an isocyanate-free strategy. The present work is the first attempt to synthesize phosphorus-containing monomers and polymers using the relatively stable and secure THPS as a start material. The results show that THPS is a viable alternative of PH3 and PCl3 in the synthesis of phosphorus–carbon bonds containing monomers and polymers.
2. Experimental
2.1. Materials
Tetrakis(hydroxymethyl)phosphonium sulfate (THPS) was purchased from Hubei Xingfa Chemical Company, China; methyl carbamate, benzyl chloride, 1-methyl-2-pyrrolidinone (NMP), N,N-dimethylacetamide (DMAc), TBD, 1,6-hexamethylenediamine, 1,8-octamethylenediamine, 1,12-dodecamethylenediamine, poly(propylene glycol) bis(2-aminopropyl ether) (PPGda, with and 2000 g mol−1) were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. All chemicals were used as received without further purification.
2.2. Techniques
1H,13C, and 31P NMR spectra of the monomers and polymers were recorded using 400 MHz Brucker NMR spectrometer in DMSO-d6, CDCl3, or CF3COOD/CDCl3 containing small amount of TMS as internal standard.
Fourier transform infrared (FTIR) spectra were recorded with a Bruker Vertex 70 FTIR spectrometer. Solid samples were prepared as KBr pellets and recorded at room temperature with a Bruker Vertex 70 FTIR spectrometer in the region of 4000–400 cm−1 with a resolution of 2 cm−1. Samples (6d–e) for FTIR spectroscopy were prepared by CHCl3 solution casting onto KBr windows. The solvent CH3Cl was allowed to evaporate at room temperature under vacuum. Band intensities were assigned as weak (w), medium (m), shoulder (sh), strong (s), and broad (br).
Thermal gravimetric analysis (TGA) data for polymers were taken on a PerkinElmer Thermal Analysis under N2 atmosphere at a rate of 10 °C min−1 from room temperature up to 700 °C. Dynamic mechanical thermal analysis (DMTA) was performed on polymer films (with dimensions around 40 × 10 × 1.0 mm) with a PerkinElmer Instruments Diamand DMA analyzer, working in a tensile mode. The storage modulus, E′, loss modulus, E″, and the loss tangent, tanδ, of each sample were obtained as function of temperature over the range from −150 to 200 °C, at frequency of 1 Hz, and at a heating rate of 5 °C min−1. The polymer films for DMTA were prepared by compression molding at 140 °C for 5 min.
The molecular weight of polymer was determined by gel permeation chromatography (GPC) in N,N-Dimethylformamide (DMF) containing LiCl (0.5%, wt%) using polystyrene as standards. The flow rate of DMF (containing LiCl) was maintained as 1 mL/min. The polymer solution was prepared by dissolving 10 mg of the sample in 1 mL of DMF(containing LiCl), filtered and injected for recording the GPC chromatograms. The chromatograms were recorded using Waters 510 pump and Waters 410 differential RI detector. The intrinsic viscosities of 1.0 g dL−1 polyureas solution in 1.5% (w/v) LiCl/DMAc at 30 °C were measured using an Ubbelohde viscometer.
2.3. Monomer synthesis
2.3.1. Synthesis of octakis(N-carbomethoxylaminomethyl)diphosphonium sulfate 2
The compound 2 was synthesized according to reported method.[Citation33] A mixture of 75% aqueous 1 (135.4 g, 0.25 mol) and methyl carbamate (150.1 g, 2.00 mol) was heated to reflux with constant stirring, and held at 106–108 °C for 2 h, allowed to cool, stripped of water under reduced pressure giving 218.7 g (101.5%) of 2 as an almost colorless tacky glass.
2.3.2 Synthesis of tris(N-carbomethoxylaminomethyl)phosphine 3
The compound 3 was synthesized according to reported method.[Citation33] A solution of 2 (200.0 g, 0.23 mol) in distilled water (300 mL) was purged with argon, and treated with concentrated ammonium hydroxide (100 mL) over 15 min at 20–25 °C. Solids started to separate after a few minutes, and after 2 h another 150 mL of distilled water was added to facilitate stirring. After 4 h, the solid was collected on a filter, rinsed with water, and dried in vacuum at room temperature, giving 138.6 g (102.1%) of white solid powder 3, mp:112–119 °C.
2.3.3. Synthesis of benzyl tris(N-carbomethoxylaminomethyl) phosphonium chloride 4
The 3 (100 g, 0.34 mol) was heated to 140 °C in oil bath upon melted completely under argon atmosphere, 46.8 g (0.37 mol) benzyl chloride preheated to 140 °C was added rapidly under mechanical agitation, after 10 min, the products precipitated directly from the reaction mixtures. Allowed to cool, the precipitate was filtered, washed with hot ethanol, and dried in drying oven at 100 °C, giving 101.0 g (70.4%) of white solid powder 4, recrystallized from water (500 mL), giving 69.7 g of white crystalline 4, mp 209–210 °C. IR (KBr): 3313(m), 3199(m), 3061(m), 3006, 2957(m), 2907(m), 2880(m), 2845(w), 1723(s), 1697(s), 1533(s), 1458(m), 1392(m), 1268(s), 1195(m), 1151(m), 1015(m), 975(w), 883(m), 852(m), 770(m), 700(m)cm−1. 1H NMR(D2O,TMS) δ: 3.58(s, 9H), 3.79–3.83(d, 2H), 4.176–4.184(d, 6H), 7.29–7.36(m, 5H) ppm. 31PNMR(D2O) δ: 27.11 ppm.
2.3.4. Synthesis of di(N-carbomethoxylaminomethyl) benzyl phosphine 5
Concentrated ammonium hydroxide (30 mL) was added to a well-stirred slurry of 4 (42.18 g, 0.1 mol) in water (110 mL) over 15 min at 21–26 °C in an apparatus previously purged with argon. After 2 h, more water (90 mL) was added to facilitate stirring. The mixture was then stirred for 3 h and filtered, and the filter cake was washed with water and dried in a vacuum desiccator, giving 25.95 g (87.0%) of white solid, mp 62–65 °C.
2.3.5. Synthesis of di(N-carbomethoxylaminomethyl) benzyl phosphine oxide 6
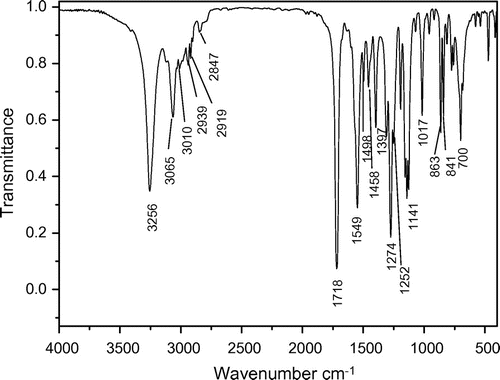
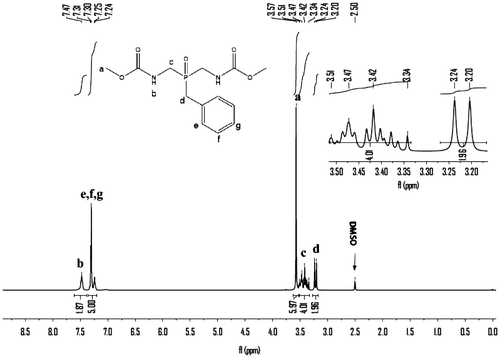
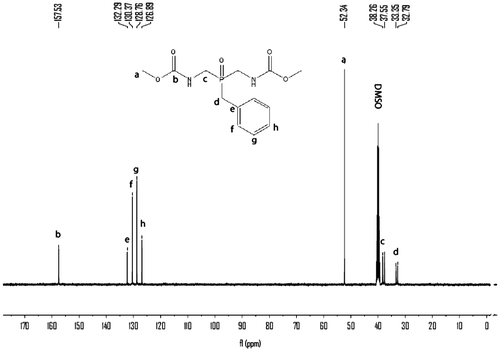
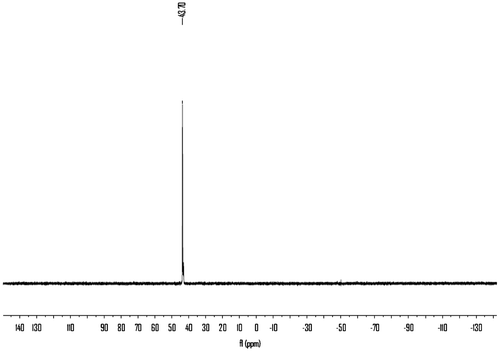
Hydrogen peroxide (30%, 9.07 g, 0.08 mol) was added dropwise to a well-stirred slurry of 5 (23.86 g, 0.08 mol) in water (100 mL) over a 30 min period, with ice-bath cooling applied to keep the reaction temperature below 30 °C. After 3 h, the white solids were collected on a filter, rinsed with water, and air-dried, recrystallized from water, giving 18.1 g (71.0%) of crystalline 6, mp: 181–182 °C. IR (KBr): 3255(s), 3065(m), 3010, 2939(w), 2919(w), 2903(w), 2847(w), 1718(s), 1549(s), 1497(m), 1458(m), 1397(m), 1275(s), 1252(m), 1194(m), 1156(m), 1141(m), 1127(s), 1017(m), 957(w), 863(m), 841(m), 774(w), 758(s), 700(m)cm−1. 1H NMR (400 MHz, DMSO-d6) δ: 7.45–7.48 (t, 2H,), 3.57 (s, 6H,), 3.36–3.52 (m, 4H,), 3.19–3.22 (d, 2H,), 7.23–7.32 (m, 5H,) ppm; 13C NMR (300 MHz, DMSO-d6) δ: 157. 51, 52.41, 126.76, 128.62, 130.12, 132.34, 37.46, 38.10 ppm; 31P NMR (400 MHz, DMSO-d6) δ: 43.70 ppm; EIMS m/z (%): 314(7), 226(80), 223(39), 191(61), 136(87), 91(100), 88(55), 59(12).
2.4. Polymer synthesis
2.4.1. Synthesis of polyureas 6a–c
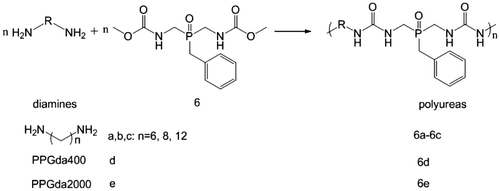
The polyureas 6a–c were synthesized by direct polycondensation reaction, as an example the preparation of polyurea 6a is explained as follows. The 6a was prepared from the reaction of di(N-carbomethoxylaminomethyl) benzyl phosphine oxide 6 with 1,6-hexamethylenediamine.
The mixtures of monomer 6 (3.14 g, 10 mmol), 1,6-hexamethylenediamine (1.16 g, 10 mmol), TBD (0.14 g, 1 mmol), and DMAc (8 mL) were added into a 50 mL three-necked round-bottom flask, which was equipped with an argon inlet, a Vigreux column connected to a Dean-Stark condenser, and a mechanical stirrer. The reaction mixture was heated at 140 °C on an oil bath for 20 h under argon flow with constant stirring. Then, the reaction mixture was poured into 100 mL hot ethanol and the precipitated polymer was collected by filtration and washed thoroughly with hot ethanol and dried at 70 °C for 12 h under vacuum to gain 3.00 g (82%) of white solid polymer 6a.
Polymer 6a. FTIR (KBr): 3326(s), 3058(w), 3030(w), 2933(s), 2855(m), 1642(s), 1622(s), 1565(s), 1496(w), 1479(w), 1457(w), 1396(m), 1252(s), 1154(s), 1068(w), 1044(w), 846(m), 700(m), 634(m)cm−1.
Polymer 6b. FTIR (KBr): 3339(s), 3061(w), 3033(w), 2929(s), 2852(m), 1642(s), 1620(s), 1565(s), 1496(w), 1458(w), 1388(m), 1300(w), 1244(m), 1154(m), 1068(w), 1042(w), 841(m), 700(m), 636(m)cm−1.
Polymer 6c. FTIR (KBr): 3341(s), 3060(w), 3032(w), 2923(s), 2850(m), 1640(s), 1569(s), 1498(w), 1462(w), 1386(m), 1236(m), 1157(m), 1068(w), 1047(w), 864(m), 844(m), 700(m), 643(m)cm−1.
2.4.2. Synthesis of polyureas 6d–e
In a 50 mL three-necked round-bottom flask, which was equipped with an argon inlet, a Vigreux column connected to a Dean-Stark condenser, and a mechanical stirrer, were placed a mixture of di(N-carbomethoxylaminomethyl) benzyl phosphine oxide 6 (10 mmol), diamine (PPGda-400, or PPGda-2000) (10 mmol), and TBD (1 mmol). The mixture was heated at 140 °C for 11 h under argon flow with constant stirring. The resultant viscous mass was further condensed by applying high vacuum at 140 °C for 6 h. In this case, the catalyst TBD was removed by sublimation. Then it was cooled to room temperature, polyureas 6d and 6e were obtained as a light yellow transparent resins-like and a pale yellow rubber-like materials, respectively.
Polymer 6d. FTIR (KBr): 3323(s), 3065(w), 3034(w), 2974(s), 2926(s), 2874(m), 1650(s), 1558(s), 1499(w), 1454(w), 1375(m), 1299(w), 1248(m), 1157(m), 1107(s), 1014(w), 922(w), 845(w), 700(m), 648(w)cm−1.
Polymer 6e. FTIR (KBr): 3328(m), 3063(w), 3033(w), 2972(s), 2929(s), 2869(s), 1676(m), 1552(m), 1454(m), 1374(m), 1343(w), 1297(w), 1249(w), 1110(s), 1015(w), 929(w), 863(w), 700(m), 666(w)cm−1.
3. Results and discussion
3.1. Synthesis di(N-carbomethoxylaminomethyl) benzyl phosphine oxide 6
The synthesis route of the monomer 6 has been shown in Scheme . The synthesis of the compound 3 from THPS by way of the intermediate 2 has been reported by Frank.[Citation33] All of these operations were performed under argon, the product became hot and sticky when exposed to air for the oxidation reaction. Due to oxygen-sensitive, the compound 3 was used in next step directly without further purification and characterization.
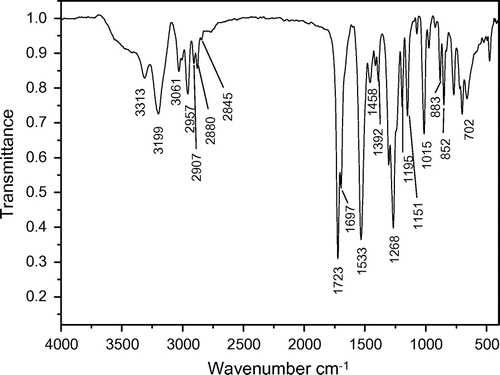
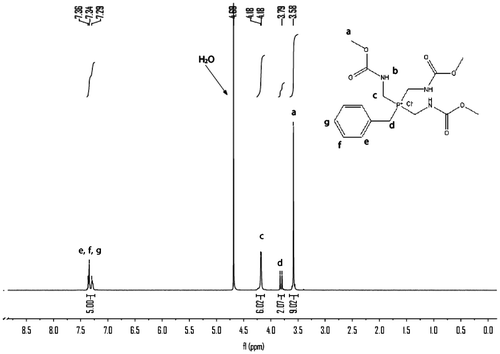
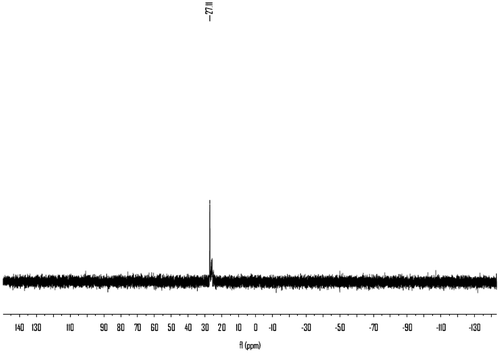
The dried 3 reacted with excess benzyl chloride without additional solvent. In order to shorten the reaction time and decrease the side reaction, the benzyl chloride was preheated to 140 °C before mixing with the molten 3. The chemical structure and purity of the key intermediate 4 were confirmed with FTIR, 1H NMR, and 31P NMR spectroscopy. The IR spectrum of 4 is shown in Figure . The compound exhibited characteristic absorption bands at 1697 and 1723 cm−1 for the carbonyl groups (C=O stretching vibration of amidic groups and ester groups, respectively) and 1392 cm−1 (C–N stretching vibration) and 1268 cm−1 (C–O stretching vibration). The absorption bands of amide groups appeared at 3313, 3199 (N–H stretching), and 1533 cm−1 (N–H bending vibration). The 1H NMR spectrum of 4 showed peaks that confirm its chemical structure, which is shown in Figure . A singlet peak at 3.58 ppm related to H(a) of methyl and peaks at 7.29–7.36 ppm were assigned to H(e), H(f), and H(g) related to aromatic protons. The double peaks at 4.18 and 3.79 ppm were assigned to H(c) and H(d) related to methylene protons. The 31P NMR spectrum of 4 shows a peak at 27.11 ppm related to the chemical shift of the phosphonium (Figure ).
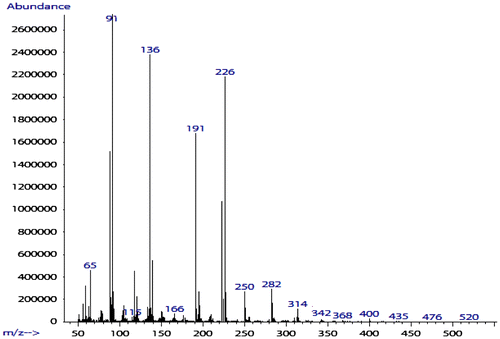
The monomer 6 was obtained by means of hydrolysis of 4 with ammonium hydroxide and then oxidation with 30% peroxide hydrogen. The structure of 6 was proved with MS, FTIR, 1H NMR, and 13C NMR, 31P NMR spectroscopy. The molecular ion peak (m/z: 314) and the characteristic fragments ion (m/z: 91, 136, 197, 226) of the expected compound 6 were observed from the MS spectrum as shown in Figure . The FTIR spectrum of 6 shows a strong peak at 3256 cm−1, which were assigned to the NH related to amide groups in this compound and strong peak at 1718 cm−1 related to carbonyl groups (Figure ). Also 1H NMR spectrum of 6 showed that a set of peaks at 7.24–7.31 ppm were assigned to the H(e), H(f), and H(g) related to aromatic protons, two peaks at 3.20 and 3.24 ppm and a set of multiplet peaks at 3.34–3.50 ppm were assigned to the H(d) and H(c) related to methylene protons, respectively. A singlet peak at 3.58 ppm was assigned to H(a) related to methyl protons and a broad singlet peak at 7.47 ppm was assigned to H(b) (Figure ). Also, the 13C NMR spectrum of 6 showed eight different carbon atoms (Figure ). Furthermore, the 31P NMR spectrum of the compound 6 shows a peak at 43.70 ppm related to the chemical shift of the phosphine oxide (Figure ).
3.2. Phosphorus-containing polymer synthesis
The synthesis of polyureas usually involves highly toxic isocyanates, which are derived from even more toxic phosgene.[Citation42–44] Herein, we synthesized five phosphorus-containing polyureas 6a–e by polycondensation of the monomer 6 with five diamines a–e (Scheme ). In the first polymerization experiment, equimolar amounts of the monomer 6 and aliphatic diamines a–c were used. Polycondensation was performed at 140 °C applying catalytic amounts (0.1 equiv.) of TBD as catalyst. Early solidification of the reaction mixtures and only partial removal of methanol did not allow for a complete conversion or high degrees of polymerization. To avoid early solidification of the polymerization mixture, high boiling solvents DMAc and NMP were used, the details are listed in Table . Also, two PPGda grades with different and 2000 g mol−1) were used in the polymerizations with 6 obtaining the polymer 6d and 6e, respectively. Since monomer 6 can be completely dissolved in the PPGda while heated to 100 °C, high boiling solvents were not used in the polymerization process. The polycondensation was performed in one-pot but in two stages: initially, the polymerization was proceeded by stirring the reactants at 140 °C for 11 h under argon purge, subsequently the viscous oligomers were condensed by applying high vacuum for 6 h.
Table 1. Results of the polymerizations of the monomer 6 with diamines a–e.
3.3. Polymer characterization
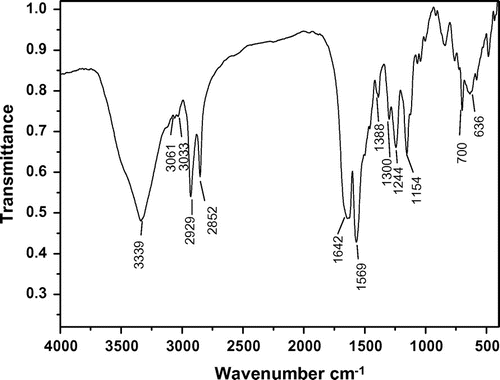
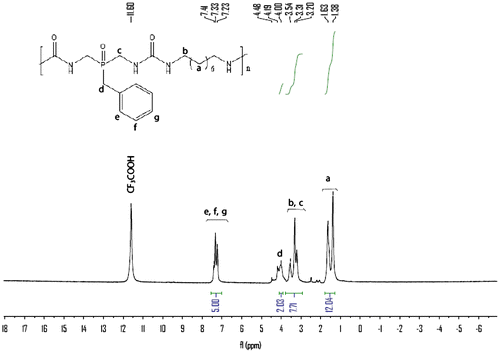
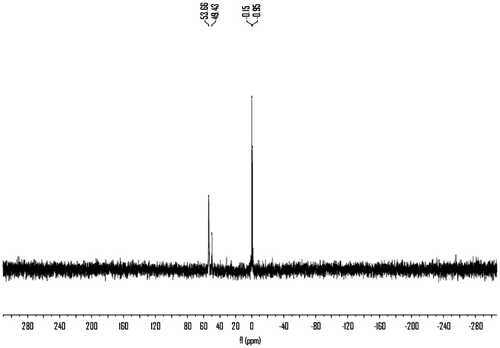
The structure of polymers 6a–c was confirmed as polyurea by means of IR spectroscopy, 1H NMR and 31P NMR. Also, the structure of polymers 6d–e was confirmed as polyurea by means of IR spectroscopy and 31P NMR. The representative IR spectrum of polymer 6b is shown in Figure . The polymer exhibited characteristic absorption bands at 1642 and 1569 cm−1 for the carbonyl groups (C=O stretching vibration of urea groups) and 1388 cm−1 (C–N stretching vibration). The absorption bands of amide groups appeared at 3339 cm−1 (N–H stretching). The IR spectrum of polyureas 6a, 6c–e is shown in Supporting Information, Figure S1–S4. The IR spectrum of 6d and 6e exhibited characteristic absorption bands at 1105 and 1010 cm−1 for the ether bonds. The 1H NMR spectrum of polymer 6b (Figure ) showed peaks that confirm its chemical structure, the aromatic protons H(e), H(f), and H(g) appeared in the region of 7.23–7.41 ppm and the peaks in the region of 1.38–1.63 ppm are assigned to H(a) related to methylene protons in the polymer backbone. The peaks in the region of 3.20–3.54 ppm are assigned to H(b) and H(c) related to methylene protons which connected to N–H group and 4.0 ppm is assigned to H(d) related to methylene protons which connected to benzene ring, 4.19–4.48 ppm is assigned for N–H urea groups in the polymer backbone. The 1H NMR spectrum of 6a and 6c are shown in Supporting Information, Figure S5–S6. The 31P NMR spectra of the polyureas 6a–c were recorded in the mixed deuterated reagents CF3COOD/CDCl3 while those of 6d and 6e were recorded in CDCl3. The 31P NMR spectrum of 6a–e (the 31P NMR spectrum of 6b is shown in Figure and of 6a, 6c–e are shown in Supporting Information, Figure S7–S10) also showed resonances at δ = 54.21, 53.66(49.43), 53.8(49.40, 31.26), 44.55(25.04), and 45.08 ppm, respectively.
The molecular weight of 6a–e was determined by gel permeation chromatography (GPC) in N,N-dimethylformamide (DMF) containing LiCl (0.5%, wt%) using polystyrene as standards and the number-average molecular weights () were 39,900, 48,500, 52,300, 58,800, and 59,100 g mol−1, with a polydispersity (PDI) of 1.19, 1.15, 1.23, 1.40, and 1.50, respectively. However, some of the polymers have a PDI lower than 1.50 (theoretical value for linear step-growth polymers is 2), which might be explained by the removal of part of the low molecular weight polymer during the purification which is required to remove the TBD. The intrinsic viscosities of 6a–e solution (1.0 g dL−1) in 1.5% (w/v) LiCl/DMAc at 30 °C were measured using an Ubbelohde viscometer and the intrinsic viscosities of the polyureas were obtained in the range of 0.18–0.33 as shown in Table .
3.4. Solubility of polymers
The solubility of polyureas 6a–e was investigated as 0.01 g of polymeric sample in 2 mL of solvent. Remarkably, as shown in Table , 6a–c showed high resistance to solvents, including polar solvents such as H2O, EtOH, acetone, DMF, NMP, 5% LiCl solution, non-polar solvents such as CCl4, cyclohexane. Polymers 6d–e were easily soluble at room temperature in polar organic solvents such as ethanol, methanol, NMP, DMF, DMAc, acetone, and CHCl3, and partially soluble in THF on heating, insoluble in solvents such as CCl4, cyclohexane, and H2O. All polyureas are soluble in TFA and 1.5%(w/v) DMF/LiCl. Added lithium chloride in DMF can destroy the hydrogen bonds of polyureas. The incorporation of monomers with flexible group, such as ether moieties in the polymer backbone, led to good solubility for polymers 6d–e in various solvents, especially polar organic solvents.
Table 2. Solubility of phosphorus-containing polyureas 6a–e.
3.5. Thermal properties
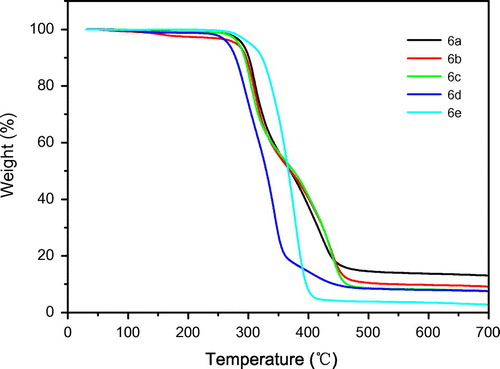
TGA at a rate of 10 °C min−1 under nitrogen atmosphere was utilized to examine the thermal properties of the polymers 6a–e, and the results are summarized in Table . Figure shows the TGA curves of polymers 6a–e. The thermal stability of the polymers was studied on the basis of 5 and 10% weight losses (T5 and T10, respectively) of the polymers and the residue at 700 °C (char yield). As given in Table , the T5 for 6a–e were 290, 280, 284, 265, and 303 °C, respectively, and the maximum decomposition temperatures (Tm) were 457, 465, 465, 441, and 406 °C, respectively. TGA data [Citation45] indicated that aromatic polyureas are inherently thermally unstable, since their initial breakdown was observed at 150–200 °C while complete weight loss occurred in the region of 400–500 °C. The TGA results of 6a–e showed that the initial breakdown was observed at above 265 °C while complete weight loss occurred in the region of 406–465 °C. The char yield for 6a–e at 700 °C was 13.12, 9.22, 7.68, 7.56, and 2.88%, respectively. The linear segmented polyureas are composed of alternating hard segments (HSs) and soft segments (SSs). In the polyureas 6a–e, the monomers 6 act as hard segments, and the low molecular diamines a–c or poly(propylene glycol) bis(2-aminopropyl ether) d, e form the soft segments. As shown in Table , the hard segment contents (HSCs) of 6a–e were 73.0, 68.5, 61.1, 44.0, and 15.6%. The char yield of 6a–e at 700 °C increased with the increasing of HSCs (in accord with P content) of the polymer. Overall, all polyureas 6a–e show a low char yield.
Table 3. Thermal behavior of polyureas 6a–e.
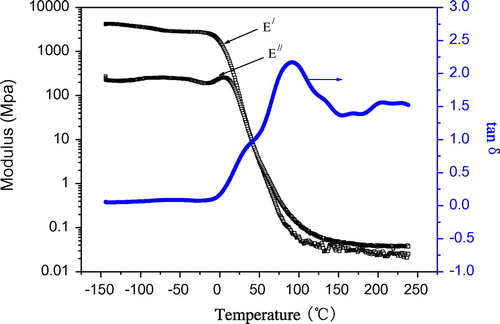
The elastomeric properties of polyureas are a result of the occurrence of phase separation between the HS and the SS, which is affected by the HS length distribution. As shown in Table , the hard segment contents (HSCs) of polyureas 6a–c are much higher (73.0, 68.5, and 61.1% for 6a, 6b, and 6c, respectively). The high HSCs of these polyureas result in a kind of toughened plastics, without real elastomeric properties. The HSC of polymer 6e (15.6%) is so low that it is impossible to form a film. Only polymer 6d (the HSC reaches 44.0%) can be compressed or cast into films and the thermo mechanical properties could be measured. The dynamic mechanical behavior of the 6d sample was analyzed at frequency of 1 Hz. The results for the storage modulus, E′, the loss modulus, E′, and tanδ are shown in Figure . As shown from curves of loss modulus, E″, and the loss tangent, tanδ, the wide secondary relaxation (named ß relaxation) can be clearly observed from −150 to −6 °C. It was related to subsize movement of molecular chain and there was only a slight decline in storage modulus E′. Moreover, when up to 6.5 °C, there was a peak in main relaxation (glass transition) in loss modulus and loss tangent, and there was a prominent decrease in storage modulus. Since Polymer 6d entered into melting process directly after its glass transition, the loss tangent increased sharply with the drastically decreased storage modulus. Within the scope of test temperature, the storage modulus decreased from 4000 (−150 °C) to 0.3 Mpa (200 °C), there were five orders of magnitude in the total changes.
4. Conclusions
In this article, we have successfully synthesized a new phosphorus-containing dicarbamates 6 from THPS which is a relatively stable and secure raw material. Also a series of new phosphorus-containing polyureas 6a–e with good thermal properties were prepared by the trans-ureation polycondensation of aliphatic diamines and diamino-terminated poly(propylene glycol) (PPGda) using catalyst TBD. Due to incorporation of phosphorus atoms into the polymer main chain, the synthesized polyureas showed exceptional properties, such as high resistance to solvents and excellent thermal stability. The results presented herein also clearly demonstrate that incorporating the ether moieties into the polymer main chain can enhance the solubility of polyureas 6d–e in organic solvents. This study shows that THPS not only have favorable safety, solubility, and stability properties but also display excellent synthetic versatility in the synthesis of phosphorus-containing monomers and polymers.
Supplemental data
Supplemental data for this article can be accessed here http://dx.doi.org/10.1080/15685551.2014.918015.
TDMP_A_918015_Suppl.doc
Download MS Word (687.5 KB)Funding
The authors thank the NSF of China [grant number 21274049] for financial support. All authors give sincerely thanks to Analysis and Testing Center of HUST for NMR,TGA and DMA test.
References
- Manners I. Polymers and the periodic table: recent developments in inorganic polymer science. Angew. Chem. Int. Ed. 1996;35:1602–1621.10.1002/(ISSN)1521-3773
- Thoms C, Marquardt C, Timoshkin AY, Bodensteiner M, Scheer M. Die oligomerisierung von phosphanylboran durch titankomplexe [The oligomerization of phosphanylborane titanium complexes]. Angew. Chem. 2013;125:5254–5259.10.1002/ange.v125.19
- Monge S, Canniccioni B, Graillot A, Robin JJ. Phosphorus-containing polymers: a great opportunity for the biomedical field. Biomacromolecules. 2011;12:1973–1982.10.1021/bm2004803
- Canadell J, Hunt BJ, Cook AG, Mantecón A, Cádiz V. Flame retardance and shrinkage reduction of polystyrene modified with acrylate-containing phosphorus and crosslinkable spiro-orthoester moieties. Polym. Degrad. Stab. 2007;92:1482–1490.10.1016/j.polymdegradsTab.2007.05.017
- Chang S, Sachinvala ND, Sawhney P, Parikh DV, Jarrett W, Grimm C. Epoxy phosphonate crosslinkers for providing flame resistance to cotton textiles. Polym. Adv. Technol. 2007;18:611–619.10.1002/(ISSN)1099-1581
- Singh H, Jain AK. Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: a comprehensive review. J. Appl. Polym. Sci. 2009;111:1115–1143.
- Essahli M, Colomines G, Monge S, Robin JJ, Collet A, Boutevin B. Synthesis and characterization of ionomers based on telechelic phosphonic polyether or aromatic polyesters. Polymer. 2008;49:4510–4518.10.1016/j.polymer.2008.08.016
- Spiro DA, Darrell WC, Donna RQ. Development of bifunctional polymers for metal ion separations: ionic recognition with polymer-supported reagents. Ind. Eng. Chem. Res. 1991;30:772–778.
- Souza MAV. Evaluation of the biocide activity of phosphorylated and sulfophosphorylated resins. Mater. Lett. 2012;74:121–124.10.1016/j.matlet.2012.01.093
- Marcelo AVS, Luiz Claudio de SM, Marcos ASC, Wang SH, Luciana CC, Hiram CAF, Sandro CA. Synthesis, characterization and evaluation of phosphorylated resins in the removal of Pb2+ from aqueous solutions. Polym. Bull. 2011;67:237–249.
- Babu HV, Muralidharan K. Polyethers with phosphate pendant groups by monomer activated anionic ring opening polymerization: syntheses, characterization and their lithium-ion conductivities. Polymer. 2014;55:83–94.10.1016/j.polymer.2013.12.005
- Zhang K, Wu K, Zhang YK, Liu HF, Shen MM, Hu WG. Flammability characteristics and performance of flame-retarded epoxy composite based on melamine cyanurate and ammonium polyphosphate. Polym. Plast. Technol. Eng. 2013;52:525–532.10.1080/03602559.2012.762520
- Minegishi S, Komatsu S, Kameyama A, Nishikubo T. Novel synthesis of polyphosphonates by the polyaddition of bis(epoxide) with diaryl phosphonates. J. Polym. Sci., Part A: Polym. Chem. 1999;37:959–965.10.1002/(ISSN)1099-0518
- Noonan KJT, Gates DP. Ambient-temperature living anionic polymerization of phosphaalkenes: homopolymers and block copolymers with controlled chain lengths. Angew. Chem. Int. Ed. 2006;45:7271–7274.10.1002/(ISSN)1521-3773
- Noonan KJT, Gillon BH, Cappello V, Gates DP. Phosphorus-containing block copolymer templates can control the size and shape of gold nanostructures. J. Am. Chem. Soc. 2008;130:12876–12877.10.1021/ja805076y
- Siu PW, Serin SC, Krummenacher I, Hey TW, Gates DP. Isomerization polymerization of the Phosphaalkene MesP=CPh2: an alternative microstructure for poly(methylenephosphine)s. Angew. Chem. Int. Ed. 2013;52:6967–6970.10.1002/anie.201301881
- Dugal-Tessier J, Serin SC, Castillo-Contreras EB, Conrad ED, Dake GR, Gates DP. Enantiomerically pure phosphaalkene-oxazolines (PhAk-Ox): synthesis, scope and copolymerization with Styrene. Chem. Eur. J. 2012;18:6349–6359.10.1002/chem.201103887
- Hodgson JL, Coote ML. Propagation mechanisms in ring-opening polymerization of small phosphorus heterocycles: toward free-radical polymerization of phosphines? Macromolecules. 2005;38:8902–8910.10.1021/ma051691s
- Lucht BL, Onge NOSt. Synthesis and characterization of poly(p-phenylenephosphine)s. Chem. Commun. 2000;21:2097–2098.10.1039/b003989f
- Yang R, Chen L, Jin R, Wang YZ. Main-chain liquid crystalline copolyesters with a phosphorus-containing non-coplanar moiety. Polym. Chem. 2013;4:329–336.10.1039/c2py20579c
- Yang XF, Li QL, Chen ZP, Han HL. Fabrication and thermal stability studies of polyamide 66 containing triaryl phosphine oxide. Bull. Mater. Sci. 2009;32:375–380.10.1007/s12034-009-0054-4
- Yang XF, Li QL, Chen ZP, Zhang L, Zhou YJ. Mechanism studies of thermolysis process in copolyamide 66 containing triaryl phosphine oxide. J. Therm. Anal. Calorim. 2013;112:567–571.10.1007/s10973-012-2579-9
- Tarasova NP, Smetannikov YV. Radiation chemical synthesis of modified phosphorus-containing polymers. Dokl. Chem. 2011;437:53–56.10.1134/S0012500811030049
- Guterman R, Berven BM, Chris Corkery T, Nie HY, Idacavage M, Gillies ER, Ragogna PJ. Fluorinated polymerizable phosphonium salts from PH3: surface properties of photopolymerized films. J. Polym. Sci., Part A: Polym. Chem. 2013;51:2782–2792.10.1002/pola.26692
- Lin CH, Hsu CK, Wang MW, Dai SA, Juang TY. Phosphinated phenols from acid-fragmentation of bisphenols and their unsymmetrical diamine derivatives for copolyimides. J. Polym. Sci., Part A: Polym. Chem. 2014;52:390–400.10.1002/pola.27012
- Wang PJ, Lin CH, Chang SL, Shih SJ. Facile, efficient synthesis of a phosphinated hydroxyl diamine and properties of its high-performance poly(hydroxyl imides) and polyimide-SiO2 hybrids. Polym. Chem. 2012;3:2867–2874.10.1039/c2py20156a
- Xie XX, Wang Z, Zhang K, Xu J. Novel phosphorous–nitrogen containing poly(aryl ether ketone)s: synthesis, characterization, and thermal properties. Des. Monomers Polym. 2013;16:38–46.10.1080/15685551.2012.705488
- Hussein MA, Asiri AM. Organometallic ferrocene- and phosphorus-containing polymers: synthesis and characterization. Des. Monomers Polym. 2012;15:207–251.10.1163/156855511X615650
- Senthil S, Kannan P. Novel thermotropic liquid crystalline polyphosphonates. Polymer. 2004;45:3609–3614.10.1016/j.polymer.2004.03.060
- Senthil S, Kannan P. Studies on the mesomorphic properties of ferrocenylene-based organophosphorous liquid-crystalline polymers containing phenyl and biphenyl pendant units. J. Appl. Polym. Sci. 2002;85:831–841.10.1002/(ISSN)1097-4628
- Rameshbabu K, Kannan P. Synthesis and characterization of thermotropic liquid crystalline polyphosphates containing photoreactive moieties. Liq. Cryst. 2004;31:843–851.10.1080/02678290410001703127
- Hoffman AJ. The action of hydrogen phosphide on formaldehyde. J. Am. Chem. Soc. 1921;43:1684–1688.10.1021/ja01440a035
- Frank AW. Synthesis of tris(aminomethyl)phosphine oxide and its carbon dioxide adduct from tetrakis(hydroxymethyl)phosphonium salts via their methyl carbamate derivatives. Can. J. Chem. 1981;59:27–33.10.1139/v81-005
- Wang YJ, Zhao XQ, Li F, Wang SF, Zhang JY. Catalytic synthesis of toluene-2,4-diisocyanate from dimethyl carbonate. J. Chem. Technol. Biotechnol. 2001;76:857–861.10.1002/(ISSN)1097-4660
- Uriz P, Serra M, Salagre P, Castillon S, Claver C, Fernandez E. A new and efficient catalytic method for synthesizing isocyanates from carbamates. Tetrahedron Lett. 2002;43:1673–1676.10.1016/S0040-4039(02)00094-1
- Dai YS, Wang Y, Yao J, Wang QY, Liu LM, Chu W, Wang GY. Phosgene-free synthesis of phenyl isocyanate by catalytic decomposition of methyl N-phenyl carbamate over Bi2O3 catalyst. Catal. Lett. 2008;123:307–316.10.1007/s10562-008-9424-6
- Hedaoo RK, Tatiya PD, Mahulikar PP, Gite VV. Fabrication of dendritic 0 G PAMAM-based novel polyurea microcapsules for encapsulation of herbicide and release rate from polymer shell in different environment. Des. Monomers Polym. 2014;17:111–125.10.1080/15685551.2013.840474
- Maike U, Oliver K, Alexander P, Michael ARM. Renewable Non-isocyanate based thermoplastic polyurethanes via polycondensation of dimethyl carbamate monomers with diols. Macromol. Rapid Commun. 2013;34:1569–1574.
- Chen HY, Pan WC, Lin CH, Huang CY, Dai SA. Synthesis and trans-ureation of N, N′-diphenyl-4,4′-methylenediphenylene biscarbamate with diamines: a non-isocyanate route (NIR) to polyureas. J. Polym. Res. 2012;19:1–11.
- Tang D, Mulder D-J, Noordover BAJ, Koning CE. Well-defined biobased segmented polyureas synthesis via a TBD-catalyzed isocyanate-free route. Macromol. Rapid Commun. 2011;32:1379–1385.10.1002/marc.v32.17
- Maulidan F, Michael ARM. Renewable polyamides and polyurethanes derived from limonene. Green Chem. 2013;15:370–380.
- Holzworth K, Jia Z, Amirkhizi AV, Qiao J, Nemat-Nasser S. Effect of isocyanate content on thermal and mechanical properties of polyurea. Polymer. 2013;54:3079–3085.10.1016/j.polymer.2013.03.067
- Lorenzini RG, Kline WM, Wang CC, Ramprasad R, Sotzing GA. The rational design of polyurea & polyurethane dielectric materials. Polymer. 2013;54:3529–3533.10.1016/j.polymer.2013.05.003
- Joshi M, Jauhari S, Desai KR. Synthesis, characterization and dyeing application of heterocyclic polymers in nylon and polyester. Polym. Plast. Technol. Eng. 2012;51:761–765.10.1080/03602559.2012.663851
- Mikroyannidis JA. Synthesis and thermal characteristics of phosphorus-containing polyureas and copolyureas based on 1-[(dialkoxyphosphinyl)methyl]-2,4- and -2,6-diaminobenzenes. J. Polym. Sci. Polym. Chem. Ed. 1984;22:3423–3437.10.1002/pol.1984.170221158