Abstract
Novel oligomer–metal complexes (4a–f) of the ligand 2,5-bis ((1H-1,2,4-triazol-3-yl) carbamoyl) terephthalic acid (3) were prepared using various metal salts viz. Mn(II), Fe(II), Co(II), Ni(II), Cu(II), and Zn(II) and characterized by physicochemical, thermogravimetric, and spectroscopic techniques. Electronic spectral analysis and magnetic measurement studies were taken into account for the geometry of oligomer–metal complexes. Polymeric properties like number average molecular weight () and degree of polymerization have also been carried out. Ligand (3) was synthesized using pyromellitic diahydride and 1H-1,2,4-triazol-3-amine. Ligand was duly characterized by physicochemical, spectroscopic, and thermogravimetric techniques. All novel synthesized compounds 3 and 4a–f were evaluated for their antibacterial activity against various Gram-positive and Gram-negative bacterial strains. Growth inhibition was compared with the standard drug ciprofloxacin. Antifungal activity was also carried out against different fungal strains. The antifungal drug, ketoconazole, was used as a positive control. The results showed significantly higher antibacterial and antifungal activity of oligomer–metal complexes compared to the ligand.
1. Introduction
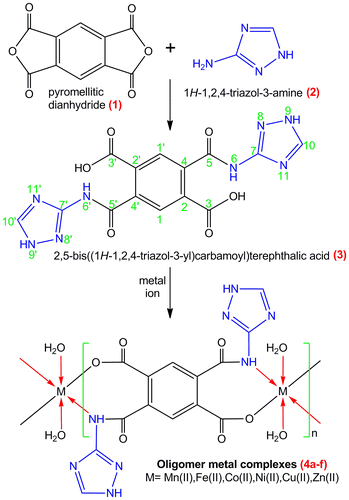
In recent years, the design and construction of polymer–metal complexes, often referred to as metal-organic frameworks (MOFs) have received great attention.[Citation1–4] Numerous polymer–metal complexes have been reported not only because of their intriguing variety of architectures and topologies [Citation5–7] but also for their properties of high porosity and enormous internal surface areas.[Citation8,9] These properties have potential applications in clean energy, such as storage media for methane, hydrogen, and acetylene [Citation10,11] or as absorbents or membrane fillers in separation and purifications of chemicals.[Citation12,13] Polymer–metal complexes can be used in catalysis, magnetism, luminosity, and chemical sensors.[Citation14,15] The structures of polymer–metal complexes are greatly depending upon the structure of the organic ligands, the coordinative geometry of metal ions, metal–ligand ratio, and other factors.[Citation16,17] Among various organic ligands, multicarboxylate ligands are often used to synthesized polymer–metal complexes e.g. 1,2,4,5-benzenetetracarboxylate, 3,3′,4,4′-biphenyltetracarboxylic acid,[Citation18,19] 1,1′-biphenyl-2,3′,3,4′-tetracarboxylic acid, [Citation20] and methylene diisophthalic acid [Citation21] have been extensively used for the synthesis of various polymer–metal complexes. On the other hand, the use of auxiliary N-containing ligands is also an effective method for the framework formation of polymer–metal complexes owing to the fact that they can satisfy and even mediate the coordination needs of the metal center and consequently generate more meaningful architectures.[Citation22,23] Patel and his co-workers have reported the studies on various polymer/oligomer–metal complexes.[Citation24–27] A more detailed exploration has been undertaken by us in the study of polymer/oligomer–metal complexes. With the aim of investigating the influence of auxiliary ligands on frameworks of oligomer–metal complexes, based on bisamic acid containing carboxylic and amide group, we carried out the study for the reaction of metal (II) salts with novel bisamic acid (3). The MOF based on bisamic acid of pyromellitic dianhydride has not attracted any attention. Hence, initial work in this direction has been reported by us recently.[Citation28–30] This prompted us to extend our work using other auxiliary ligand such as 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl) terephthalic acid (3). Figure summarize our synthetic approach to the various phases of this work, viz., (a) preparation of ligand (bisamic acid) from pyromellitic dianhydride and 1H-1,2,4-triazol-3-amine; (b) synthesis of oligomer–metal complexes using various metal (II) acetates, e.g. Mn(II), Fe(II), Co(II), Ni(II), Cu(II), and Zn(II) metal ions. Ligand when incorporated with transition metal ions would produce a broad spectrum of antimicrobial property. The details of these procedures and the results obtained are discussed below.
2. Experimental
2.1. Materials and measurements
All common reagents and solvents were used of analytical grade and were used without further purification. Alumina-supported pre-coated silica gel 60 F254 thin layer chromatography (TLC) plates were purchased from the E. Merck (India) Limited, Mumbai and were used to check purity of compounds, and to study the progress of the reaction whereby TLC plates were illuminated under Ultraviolet light (254 nm), evaluated in I2 vapors, and visualized by spraying with Draggendorff’s reagent. Infrared spectra (FT–IR) were obtained from KBr pellets in the range of 4000–400 cm−1 with a Perkin Elmer spectrum GX spectrophotometer (FT–IR) instrument. 1H NMR and 13C NMR spectra were acquired at 400 MHz on a Bruker NMR spectrometer using DMSO-d6 (residual peak at δ ~2.5 or ~ 39.5 ppm, 300 °K) as a solvent as well as TMS an internal reference standard. Liquid chromatography – atmospheric pressure chemical ionization-mass spectrometry (LC/APCI/MS) in both positive and negative modes of operation was used for the determination of the compounds. The eluent was delivered by a liquid chromatograph model HP 1090 (Hewlett-Packard, CA). The mobile phases used for the elution of the analytes consisted of 40% acetonitrile, 60% water, and 0.3% acetic acid at a flow rate of 0.8 mL/min. This HPLC system was connected to a HP mass spectrometer, model HP1100, system equipped with an APCI probe. Micro analytical (C, N, H) data were obtained using a Perkin–Elmer 2400 CHN elemental analyzer. The solid diffuse electronic spectra were recorded on a Beckman DK–2A spectrophotometer with a solid reflectance attachment. MgO was employed as a reference. Magnetic moments [Citation31] were determined by the Gouy method with mercury tetra thiocyanetocobaltate (II), [HgCo(NCS)4] as calibrant (Xg = 1644 × 10−6 cgs units at 20 °C), by Citizen Balance (at room temperature). Molar susceptibilities were corrected using Pascal’s Constant.[Citation32] The thermogravimetric studies were carried out with a model Perkin Elmer thermogravimetry analyzer at a heating rate of 10 °C min−1 in the temperature range 50–700 °C under nitrogen. The metal content of the oligomer–metal complexes was carried by decomposing a weighed amount of each oligomer–metal complexes with HClO4, H2SO4, and HNO3 (1:1.5:2.5) mixture followed by standard EDTA titration method.[Citation33] Due to poor solubilty of oligomer–metal complexes in DMSO, number average molecular weight () of oligomer–metal complexes was determined by non-aqueous conductometric titration. It was carried out in pyridine solution against standard sodium methoxide in pyridine soloution as titrant. The number average molecular weight of each sample was calculated according to method reported in literature.[Citation34] The melting point was checked by the standard open capillary method. In order to facilitate the correct structural assessment, i.e. the coordination site, we have tried a lot to generate a crystal for single crystal X ray analysis but we did not succeed. Hasanzadeh et al. [Citation35] have reported this type of acid amide metal complex. So from the obtained data and reference article, interpretations become straightforward.
2.2. Synthesis of ligand 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl)terephthalic acid (3)
Ligand was synthesized by adding a solution of 1H-1,2,4-triazol-3-amine (16.816 g, 0.2 mol) in 30 mL THF dropwise to a stirred solution of pyromellitic dianhydride (21.813 g, 0.1 mol) in 45 mL THF and keeping the temperature of the medium close to 70–80 °C for 2 h, thus the obtained ensuing solution was poured into ice water in which the reaction product precipitated. The final light yellow precipitates were filtered, washed with dry THF followed by ethanol, and purified by column chromatography using ethyl acetate: hexane (1:1) solvent system and dried at room temperature. 1H NMR (DMSO-d6, δ ppm): 12.47 (d, 2H, H9,H9′), 10.89 (s, 2H, 2–COOH), 9.70 (s, 2H, 2–NH–), 8.63 (s, 2H, H1,H1′), 7.87 (d, 2H, H10,H10′); 13C NMR (DMSO–d6, δ ppm): 125.45, 133.63, 135.12, 142.98, 151.89, 166.80, 169.42.
2.3. Synthesis of oligomer–metal complexes (4a–f)
All oligomer–metal complexes were synthesized using equimolar amount of ligand and various metal (II) salts. A warm clear solution of ligand (5.045 g, 0.01 mol) in dimethylsulphoxide was neutralized by adding dropwise a solution of 0.1 M sodium hydroxide. The reaction medium was maintained at pH of about 7–8. A pasty mass was observed. It was diluted with water to make the solution clear. To the above solution, a solution of metal (II) acetate viz., Mn(II), Fe(II), Co(II), Ni(II), Cu(II), and Zn(II) acetate (0.01 mol) was added with constant stirring and the pH of the reaction mixture was adjusted to 6–7. The oligomer–metal complexes thus separated out in the form of a suspension was digested on a water bath for 1 h, filtered, washed, and dried in air at room temperature. These oligomer–metal complexes are insoluble in common organic solvents like methanol, ethanol, chloroform, acetone, and benzene, while partially soluble in DMSO. The results obtained were furnished in Table .
Table 1. Physicochemical parameters of the ligand and its oligomer–metal complexes.
2.4. Biological activity
2.4.1. Antibacterial activity (in vitro)
Compounds (3 and 4a–f) were screened for in vitro antibacterial activity against Gram-positive bacterial strains (Bacillus subtilis [BS] and Staphylococcus aureus [SA]) and Gram-negative bacterial strains (Salmonella typhimurium [ST] and Escherichia coli [EC]) utilizing the agar diffusion assay.[Citation36,37] The wells were dug in the media with the help of a sterile metallic borer. Recommended concentration (100 μl) of the test sample (1 mg/mL in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, ciprofloxacin were served as negative and positive controls, respectively. The plates were incubated immediately at 37 °C for 24 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). Growth inhibition was compared with the standard drug. In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains.
2.4.2. Antifungal activity (in vitro)
Compounds (3 and 4a–f) were also examined for antifungal activity against different fungal strains, i.e. Penicillium expansum [PE], Botryodiplodia theobromae [BT], Nigrospora sp. [NS], and Trichothesium sp. [TS]. The antifungal drug, ketoconazole, was used as a positive control. Antifungal screening for compounds (3 and 4a–f) and positive control was performed at a recommended concentration. The fungal strains were grown and maintained on potato dextrose agar plates. The cultures of the fungi were purified by single spore isolation technique. Each compounds (3 and 4a–f) in DMSO solution was prepared for testing against spore germination of each fungus. The fungal culture plates were inoculated and incubated at 25 ± 2 °C for 48 h. The plates were then observed and the diameters of the zone of inhibition (in mm) were measured. The percentage inhibition for fungi was calculated after five days using the formula given below:
where X = Area of colony in control plate,
Y = Area of colony in test plate.
3. Results and discussion
3.1. Synthesis of ligand 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl)terephthalic acid
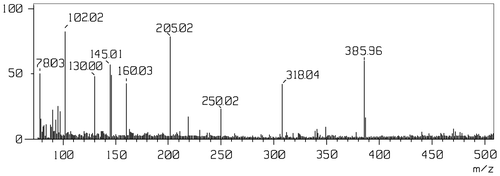
To the best of our knowledge, ligand (3) has not been reported previously. The characterization of the reaction product provided the first unambiguous proof of the successful synthesis of 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl)terephthalic acid. The FT–IR spectrum of ligand showed the most relevant peaks of the triazole ring and 1,2,4,5–tetra-substituted benzene ring, other than typical absorptions arising from the band at 3540 cm−1 and 1709 cm−1 for carboxylic acid and 3241 and 1685 cm−1 for O=C–NH group.[Citation38] In the 1H NMR spectrum, the signal at 12.47 ppm was ascribed to the protons of the triazol rings. The singlet at 10.89 ppm was ascribed to the protons of carboxylic -OH group and a singlet at 9.70 ppm was attributed to the –NH proton of amide group, which was further confirmed by 13C NMR value i.e. 169.42 and 166.80 were attributed to carboxylic carbon and amide carbon, respectively. The recorded LC-MS spectrum (Figure ) showed nine m/z peaks including molecular ion peak, which supports the structural formula of ligand as mentioned in the Figure . The highest m/z peak is observed at 385.96, which confirmed the molecule weight of ligand. In order to verify the attached carboxylic acid groups in ligand, thermal analysis was carried out. The ligand started to lose weight because of thermal degradation. Thermogram of product 3 indicated that the degradation occurred in two steps. First stage of degradation started from 150 to 300 °C, which might be attributed to decarboxylation of product 3. The value of wt. loss 22.6% observed during this temperature range is quite consistent with the theoretical value 22.78%. The second major stage at about 320–700 °C attributed to the monomer decomposition/pyrolysis. The 4–5% char residue remained at 700 °C. The expected structure was thus clearly verified by the spectroscopic and thermal analysis which indicated the absence of any detectable impurity, particularly of the two reagents used to prepare compound 3.
3.2. Synthesis of oligomer–metal complexes
3.2.1. Physical properties
Elemental analysis of all oligomer–metal complexes was in good agreement with proposed structures. All oligomer–metal complexes exhibited 1:1 metal to ligand stoichiometry. The structures of oligomer–metal complexes were consistent with the FTIR, electronic spectra and TGA. The geometry of the central metal ion was confirmed by electronic spectra and magnetic susceptibility measurements. The degrees of polymerization (DP) for all oligomer–metal complexes are in the range of 5–6 (Table ). All the data provide good evidence that the chelates are polymeric in nature. The suggested structure of the oligomer–metal complexes is shown in Figure .
3.2.2. Infrared spectra
IR spectral bands of the ligand and its oligomer–metal complexes suggest the formation of desired oligomer–metal complexes and support their structure. Spectral features provide valuable information regarding the nature of functional group attached to the metal atom. In order to study the bonding mode of ligand to the oligomer–metal complexes, the IR spectrum of free ligand was compared with the spectra of oligomer–metal complexes. Considerable differences to be expected were observed. The band at about 3509 cm−1 for carboxylic acid in ligand was virtually disappeared from the spectra of oligomer–metal complexes (Table ). Oligomer–metal complexes exhibit more broadened band in the region near 2970–2980 cm−1 indicating the presence of coordinated water molecules.[Citation39] The coordinated water in all the oligomeric metal (II) complexes presents different peaks at 980 cm−1 (rocking) and 760 cm−1 (wagging), whereas none of these vibrations appear in the spectra of uncoordinated ligands. A band at 1630 cm−1 in free schiff base is due to νC–N vibration. The shifting of this group to lower frequency (~1590 cm−1) in the oligomer–metal complexes when compared to free ligand suggests the coordination of metal ion through nitrogen atom of amide group,[Citation40] it is expected that coordination of nitrogen to the metal atom would reduce the electron density in the amide link and thus lower the absorption.[Citation41] A band at 1709 cm−1 is assigned to νC=O stretching frequency in the spectrum of free schiff base which is also shifted to lower frequency at about 1690 cm−1 in all the oligomer–metal complexes. This indicates the involvement of oxygen atom of hydroxy group of –COOH group in bonding with metal ions.[Citation42] New bands, which were not present in the spectrum of ligand, appeared in the spectra of oligomer–metal complexes, e.g. presence of sharp band in the region of 525–535 cm−1 can be assigned to νM–N,[Citation42] which indicated the involvement of nitrogen in coordination. The medium intensity bands for νM–O [Citation43] have been observed at 625–635 cm−1 due to M–O coordination. The appearance of νM–N and νM–O vibrations supports the involvement of N and O atoms in complexation with metal ions under investigation. These overall data suggest that the amide-N and carboxylate-O groups are involved in coordination with the metal (II) ion in oligomer–metal complexes. These features confirmed the proposed structure of oligomer–metal complexes as shown in Figure .
Table 2. FT-IR frequencies and electronic spectral data of the ligand and its oligomer–metal complexes.
3.2.3. Magnetic moments and electronic spectral data
The information regarding geometry of the oligomer–metal complexes were obtained from their electronic spectral data and magnetic moment values (Table ). The diffuse electronic spectrum of the [Cu–L–(H2O)2]n shows two broad bands around 15,943, and 22,739 cm−1 due to the 2T2g → 2Eg transition, while the second may be due to charge transfer, respectively. This suggests a distorted octahedral structure for the [Cu–L–(H2O)2]n oligomer, which was further confirmed by its μeff value 1.97 B.M. The [Ni–L–(H2O)2]n oligomer shows two absorption bands at 15,552 cm−1, 22,967 cm−1, and 9879 cm−1 due to 3A2g → 3T1g(F), 3A2g → 3T1g(P), and 3A2g → 3T2g, respectively. The [Co–L–(H2O)2]n polymer shows two absorption bands at 22,921 cm−1, 15,544 cm−1, and 9837 cm−1 corresponding to 4T1g(F) → 4T1g(P), 4T1g(F) → 4A2g(F), and 4T1g(F) → 4T2g(F) transitions, respectively, which indicated an octahedral configuration for the [Ni–L–(H2O)2]n and [Co–L–(H2O)2]n oligomers.[Citation44] This configuration was further confirmed by its μeff value 2.93 B.M. and 4.39 B.M. The spectrum of [Fe–L–(H2O)2]n show bands at 36,049 cm−1 and 19,073 cm−1 assigned to the transitions 5T2g(F) → 3T1g and 5T2g(F) → 3Eg, and its μeff 5.02 B.M. suggesting octahedral configuration. The spectrum of [Mn–L–(H2O)2]n show weak bands at 16,412, 17,743, and 23,169 cm−1 assigned to the transitions 6A1g → 4T1g(4G), 6A1g → 4T2g(4G), and 6A1g → 4A1g,4Eg, respectively, suggesting an octahedral structure for the [Mn–L–(H2O)2]n oligomer.[Citation45] This configuration was further confirmed by its μeff value 5.74 B.M. As the spectrum of the [Zn–L–(H2O)2]n oligomer is not well interpreted, but its μeff value shows that it is diamagnetic as expected. Magnetic moments μeff of all coordination oligomer revealed that all oligomers except Zn(II) metal ion oligomer are paramagnetic, while Zn(II) metal ion oligomer is diamagnetic.
3.2.4. Thermal analysis
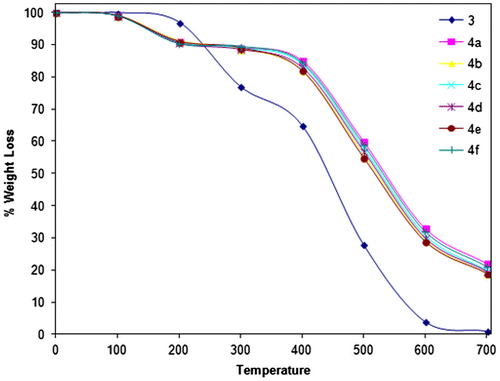
The thermal behavior was investigated by Perkin Elmer TGA analyzer at a heating rate of 10 °C min−1 in the temperature range 50–700 °C under nitrogen, which provides much information about the coordination compounds. In all the oligomer–metal complexes, there is no weight loss observed up to 100 °C. The decomposition occurred in two steps. First step occurred between 100 and 200 °C, which might be attributed to mass loss corresponding to water molecules (Figure ). The value of weight loss during this temperature became equal to weight loss of two water molecules. These value of wt. loss indicated that two water molecules were coordinated to the metal ion. Second step that occurred between 200 and 700 °C exhibits a mass loss corresponding to decomposition of ligand part in oligomer. The weight loss of oligomer–metal complexes was noticeable between 300 and 600 °C. The rate of degradation became maximum at a temperature between 400 and 600 °C. This may be due to accelerating by metal oxide which forms in situ. Each oligomer loses about 80% of its weight when heated up to 700 °C. On the basis of the relative decomposition (% weight loss) and the nature of thermogram, the oligomer–metal complexes may be arranged in order of their increasing stability as: Cu < Fe < Ni < Co < Zn < Mn.
3.3. Biological activity
3.3.1. Antibacterial activity
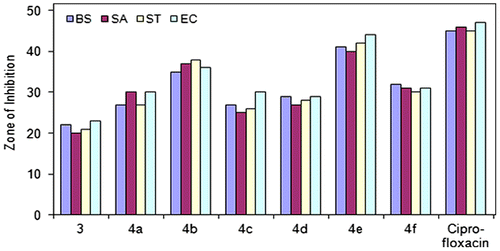
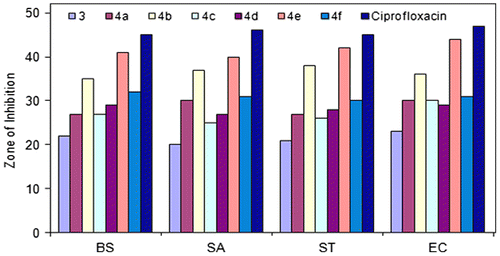
Based on the data from the antibacterial studies against both Gram-positive and Gram-negative bacterial strains, the following observations can be made. All compounds (3 and 4a-f) exhibited antibacterial activity against both Gram-positive and Gram-negative bacterial strains with zones of inhibition (ZOI) ranging from 20 to 44 mm (Figure ).
Ligand 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl)terephthalic acid (ZOI[BS] = 22 mm, ZOI[SA] = 20 mm, ZOI[ST] = 21 mm, ZOI[EC] = 23 mm) was found less active than its oligomer–metal complexes. Among the analogs 4a–f, compound 4e (ZOI[BS] = 41 mm, ZOI[SA] = 40 mm, ZOI[ST] = 42 mm, ZOI[EC] = 44 mm) was identified as a potent antibacterial agent against all Gram-positive and Gram-negative bacterial strains. Compound 4b (ZOI[BS] = 35 mm, ZOI[SA] = 37 mm, ZOI[ST] = 38 mm, ZOI[EC] = 36 mm) also had good antibacterial activity against bacterial strains. Compounds 4a, 4c, 4d, and 4f exhibited moderate antibacterial activity. Compounds 3 and 4a–f exhibited less antibacterial activity as compared to standard antibiotic drug, ciprofloxacin (ZOI[BS] = 45 mm, ZOI[SA] = 46 mm, ZOI[ST] = 45 mm, ZOI[EC] = 47 mm).
A comparative study of the growth inhibition zone values of schiff base and its oligomer–metal complexes indicated that the oligomer–metal complexes exhibited higher antibacterial activity than free schiff base (Figure ). Such increased activity of the oligomeric metal complexes can be explained on the basis of Overtone’s concept and Tweedy’s chelation theory.[Citation46] According to Overtone’s concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid soluble materials due to which liposolubility is considered to be an important factor that controls the antimicrobial activity. Chelation reduces the polarity [Citation47,48] of the metal ion mainly because of the partial sharing of its positive charge with the donor groups and possibly the π-electron delocalization within the whole chelate ring system thus formed during coordination. This process of chelation thus increases the lipophilic nature of the central metal atom, which in turn favors its permeation through the lipoid layer of the membrane. This in turn is responsible for increasing the hydrophobic character and liposolubility of the molecule in crossing cell membrane of the micro-organism, and hence enhances the biological utilization ratio and activity of the testing drug/compound. The biological activity of compounds also depends on the nature of the ligand, concentration, lipophilicity, nature of metal ion, coordinating sites, and geometry of the complex.
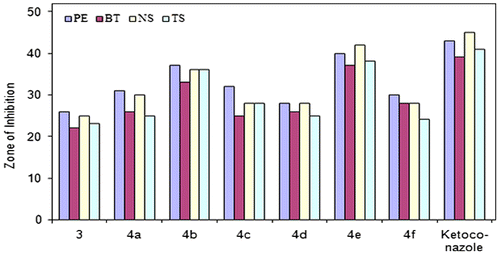
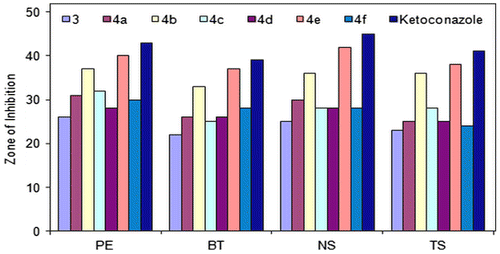
3.3.2. Antifungal activity
Based on the screening data from the antifungal studies, the following observations can be made. All compounds (3 and 4a–f) exhibited antifungal activity against different fungal strains (Figure ). Ligand 2,5-bis((1H-1,2,4-triazol-3-yl)carbamoyl)terephthalic acid (ZOI[PE] = 43 mm, ZOI[BT] = 39 mm, ZOI[NS] = 45 mm, ZOI[TS] = 41 mm) was found less active than its oligomer–metal complexes (Figure ). Compound 4e (ZOI[PE] = 40 mm, ZOI[BT] = 37 mm, ZOI[NS] = 42 mm, ZOI[TS] = 38 mm) was identified as a more active against all fungal strains. Compound 4b (ZOI[PE] = 37 mm, ZOI[BT] = 33 mm, ZOI[NS] = 36 mm, ZOI[TS] = 36 mm) also had good antifungal activity against fungal strains. Compounds 4a, 4c, 4d, and 4f exhibited moderate antifungal activity. Compounds 3 and 4a–f exhibited less antifungal activity as compare to standard antibiotic drug, ketoconazole (ZOI[PE] = 43 mm, ZOI[BT] = 39 mm, ZOI[NS] = 45 mm, ZOI[TS] = 41 mm).
4. Conclusions
New ligand and its oligomer–metal complexes were prepared in good amount of yield and were duly characterized. In the oligomer–metal complexes, ligand coordinates with one central metal atom at four coordination sites along with two water molecules. The structure of ligand and its oligomer–metal complexes is consistent with the elemental, spectral, and thermal analysis. The geometry of the central metal ion was confirmed by electronic spectra and magnetic susceptibility measurements. All the data provide good evidence that the chelate are polymeric in nature. All these oligomers do not melt up to 400 °C. The oligomers have moderate thermal stability and shows good biological activity.
Acknowledgments
Author is greatly thankful to the Head, Department of Chemistry, Sardar Patel University, Vallabh Vidyanagar for providing practical facility. Author is also expressing his sincere thanks to the Principal, Government Science College-Gandhinagar for giving necessary facility for this work.
References
- Hijikata Y, Horike S, Sugimoto M, Inukai M, Fukushima T, Kitagawa S. Pore design of two-dimensional coordination polymers toward selective adsorption. Inorg. Chem. 2013;52:3634–3642.10.1021/ic302006x
- Dikhtiarenko A, Khainakov SA, dePedro I, Blanco JA, García JR, Gimeno J. Series of 2D heterometallic coordination polymers based on ruthenium(III) oxalate building units: Synthesis, structure, and catalytic and magnetic properties. Inorg. Chem. 2013;52:3933–3941.10.1021/ic302725v
- Zheng B, Luo J, Wang F, Peng Y, Li G, Huo Q, Liu Y. Construction of six coordination polymers based on a 5,5′-(1,2-ethynyl)bis-1,3-benzenedicarboxylic ligand: Synthesis, structure, gas sorption, and magnetic properties. Cryst. Growth Des. 2013;13:1033–1044.10.1021/cg301224z
- Liu YY, Liu HY, Ma JF, Yang Y, Yang J. Syntheses, structures and photoluminescent properties of Zn(II) and Cd(II) coordination polymers with flexible tripodal triazole-containing ligands. Cryst. Eng. Comm. 2013;15:1897–1907.10.1039/c2ce26958a
- Kan WQ, Liu B, Yang J, Liu YY, Ma JF. A series of highly connected metal–organic frameworks based on triangular ligands and d10 metals: Syntheses, structures, photoluminescence, and photocatalysis. Cryst. Growth Des. 2012;12:2288–2298.10.1021/cg2015644
- Wu H, Yang J, Su ZM, Batten SR, Ma JF. An exceptional 54-fold interpenetrated coordination polymer with 103-srs network topology. J. Am. Chem. Soc. 2011;133:11406–11409.10.1021/ja202303b
- Wang MM, Wang H, Gan GQ, Qu Y, Chen H, Lin ZD. Study on 1D chain coordination polymer of cinnamaldehyde salicyl hydrazone cadmium complex. J. Macromol. Sci. Part A Pure Appl. Chem. 2012;49:355–360.10.1080/10601325.2012.662080
- Zhang YB, Zhang WX, Feng FY, Zhang JP, Chen XM. A highly connected porous coordination polymer with unusual channel structure and sorption properties. Angew. Chem. Int. Ed. 2009;48:5287–5290.10.1002/anie.200901964
- Xie ZG, Ma LQ, deKrafft KE, Jin A, Lin WB. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010;132:922–923.10.1021/ja909629f
- Lan JH, Cao DP, Wang WC. High uptakes of methane in Li-doped 3D covalent organic frameworks. Langmuir. 2010;26:220–226.10.1021/la9020383
- Getman RB, Bae YS, Wilmer CE, Snurr RQ. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012;112:703–723.10.1021/cr200217c
- Bétard A, Fischer RA. Metal–organic framework thin films: from fundamentals to applications. Chem. Rev. 2012;112:1055–1083.10.1021/cr200167v
- Agarwal RA, Aijaz A, Ahmad M, Sañudo EC, Xu Q, Bharadwaj PK. Two new coordination polymers with Co(II) and Mn(II): selective gas adsorption and magnetic studies. Cryst. Growth Des. 2012;12:2999–3005.10.1021/cg300217v
- Yao RX, Xu X, Zhang XM. Magnetic modulation and cation-exchange in a series of isostructural (4,8)-connected metal–organic frameworks with butterfly-like [M4(OH)2(RCO2)8] building units. Chem. Mater. 2012;24:303–310.10.1021/cm202876r
- Cui YJ, Yue YF, Qian GD, Chen BL. Luminescent functional metal–organic frameworks. Chem. Rev. 2012;112:1126–1162.10.1021/cr200101d
- Tong ML, Hu S, Wang J, Kitagawa S, Ng SW. Supramolecular isomerism in cadmium hydroxide phases. Temperature-dependent synthesis and structure of photoluminescent coordination polymers of ∞- and β-cd2(oh)2(2,4-pyda). Cryst. Growth Des. 2005;5:837–839.10.1021/cg049610r
- Lu WG, Jiang L, Lu TB. Lanthanide contraction and temperature-dependent structures of lanthanide coordination polymers with imidazole-4,5-dicarboxylate and oxalate. Cryst. Growth Des. 2010;10:4310–4318.10.1021/cg100196j
- Yang GP, Wang YY, Ma LF, Liu JQ, Wu YP, Wu WP, Shi QZ. Hydrothermal syntheses and characterizations of three coordination polymers based on mixed organic ligands. Eur. J. Inorg. Chem. 2007;24:3892–3898.10.1002/(ISSN)1099-0682
- Wang JJ, Gou L, Hu HM, Han ZX, Li DS, Xue GL, Yang ML, Shi QZ. Ligand and pH-controlled Zn(II) bilayer coordination polymers based on biphenyl-3,3′,4,4′-tetracarboxylate. Cryst. Growth Des. 2007;7:1514–1521.10.1021/cg0703240
- Zang SQ, Su Y, Li YZ, Ni ZP, Zhu HZ, Meng QJ. Interweaving of triple-helical and extended metal−o−metal single-helical chains with the same helix axis in a 3d metal−organic framework. Inorg. Chem. 2006;45:3855–3857.10.1021/ic060286u
- Duan X, Lin J, Li Y, Zhu C, Meng Q. Syntheses, structures and properties of a series of organic–inorganic complexes based on methylenediisophthalic acid (H4MDIP). Cryst. Eng. Comm. 2008;10:207–216.10.1039/b709972j
- Mei CZ, Shan WW, Liu BT. Synthesis, crystal structure and luminescent properties of one 3D Cd(II) coordination polymer [Cd(H3BPTC)2(bpy)]n (H4BPTC=1,1’-biphenyl-2,2’,6,6’-tetracarboxylic acid, bpy = 4,4’-bipyridine). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011;81:764–768.
- Mei CZ, Wang JX, Shan WW. Synthesis and crystal structure of an infinite sandwich-type Cu(i) coordination polymer:{[Cu(abpy)2](H3bptc)·(H2O)}n constructed by a tetracarboxylic acid. Chinese J. Struct. Chem. 2011;8:1194–1198.
- Patel HS, Patel DJ. Coordination polymers of indole based bis-ligand: thermal, spectral, and antimicrobial aspects. Synth. React. Inorg. Met.-Org. Nano-Metal Chem. 2011;41:72–80.
- Patel HS, Patel KD. Synthesis, characterization and in vitro antibacterial activity of coordination polymers based on bis bidentate ligand. Int. J. Polym. Mater. 2011;60:518–528.10.1080/00914037.2010.531819
- Patel DJ, Patel HS. Polymeric coordination compounds derived from transition metal (II) with bis-dentate ligand: synthesis, spectroscopic, magnetic and thermal studies. Int. J. Polym. Mater. 2012;61:276–287.10.1080/00914037.2011.584222
- Patel HS, Patel KD. Arabian J. Chem. Forthcoming 2013. doi:10.1016/j.arabjc.2013.03.019.
- Patel YS, Patel HS, Srinivasulu B. Synthesis, spectral, magnetic, thermal and biological aspects of pyromellitic dianhydride based co-ordination polymers. Int. J. Plast. Technol. 2012;16:117–124.10.1007/s12588-012-9035-3
- Patel YS, Patel KD, Patel HS. J. Saudi Chem. Soc. Forthcoming 2013. Available from: http://dx.doi.org/10.1016/j.jscs.2012.11.008.
- Patel YS, Dixit RB, Patel HS. Synthesis, characterization, and biological activity of coordination polymers derived from pyromellitic dianhydride. Turk. J. Chem. 2013;37:978–986.10.3906/kim-1206-22
- Vanparia SF, Patel TS, Sojitra NA, Jagani CL, Dixit BC, Patel PS, Dixit RB. Synthesis, characterization and antimicrobial study of novel 4-{[(8-hydroxyquinolin-5-yl)methyl] amino} benzenesulfonamide and its oxinates. Acta Chim. Slov. 2010;57:660–667.
- Vogel AI. A textbook of quantitative inorganic analysis. 3rd ed. London: Longman; 1961.
- Jeffery GH, Bassett J, Mentham J, Denney RC. Vogel’s textbook of quantitative inorganic analysis. 5th ed. Harlow: Longman; 1989.
- Chatterjee SK, Gupta ND. Effects of structure and composition on the titration curves of some synthetic copolymers in nonaqueous media. J. Polym Sci. 1973;11:1261–1270.10.1002/pol.1973.170110613
- Hasanzadeh R, Najafi Moghadam P, Samadi N. Synthesis and application of modified poly(styrene-alt-maleic anhydride) networks as a nano chelating resin for uptake of heavy metal ions. Polym. Adv. Technol. 2013;24:34–41.10.1002/pat.3046
- Alam S. Synthesis, antibacterial and antifungal activity of some derivatives of 2-phenyl-chromen- 4-one. J. Chem. Sci. 2004;116:325–332.10.1007/BF02711433
- Pelzar MJ, Chan ECS, Krieg NR. Antibiotics and other chemotherapeutic agents in microbiology. 5th ed. New York, NY: Blackwell Science; 1998.
- Silverstein RM, Webste FX. Spectrometric identification of organic compounds. 6th ed. New York, NY: Wiley; 2004.
- Panchal PK, Pansuriya PB, Patel MN. In-vitro biological evaluation of some ONS and NS donor Schiff’s bases and their metal complexes. J. Enzyme Inhib. Med. Chem. 2006;21:453–458.10.1080/14756360600628551
- Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 3rd ed. New York, NY: Wiley; 1978.
- Lever ABP. Inorganic electronic spectroscopy. 2nd ed. Amsterdam: Elsevier; 1984.
- Malik A, Parveen S, Ahmad T, Alshehri SM, Singh PK, Nishat N. Coordination polymer: Synthesis, spectral characterization and thermal behaviour of starch-urea based biodegradable polymer and its polymer metal complexes. Bioinorg. Chem. Appl. 2010;19:848130.
- Soliman EM, El-Shabasy M. Synthesis, characterization and electrical conductivity properties of homo- and hetero-di and trimetallic complexes of mixed azo dyes. J. Mat. Sci. 1994;29:4505–4509.10.1007/BF00376273
- Lewis J, Wilkins RS. Modern coordination chemistry. New York, NY: Interscience; 1960.
- Pappalardo R. Note on the optical absorption of MnCl2 and MnBr2. J. Chem. Phys. 1960;33:613–914.10.1063/1.1731199
- Tweedy BG. Plant extracts with metal ions as potential antimicrobial agents. Phytopathology. 1964;55:910–914.
- Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT. In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J. Enzyme Inhib. Med. Chem. 2006;21:95–103.10.1080/14756360500456806
- Chohan ZH, Supuran CT. Organometallic compounds with biologically active molecules: In vitro antibacterial and antifungal activity of some 1,1’-(dicarbohydrazono) ferrocenes and their cobalt(II), copper(II), nickel(II) and zinc(II) complexes. Appl. Organomet. Chem. 2005;19:1207–1214.10.1002/(ISSN)1099-0739