Abstract
The purpose of this paper is the expansion of new colored polymers based on lawsone. Polymers should be highly thermally stable in order to be colored materials. Also, their solubility and color intensity should be good enough. First, the hydroxyl group of lawsone was covalently linked to acryloyl chloride, abbreviated as LA. Free radical copolymerization of polymerizable derivative of lawsone with methacrylic acid, acrylic acid, and acrylonitrile was carried out using 2, 2′-azobisisobutyronitrile (AIBN) as initiator at the temperature 80 °C. The colored polymers were then characterized by the UV/vis, FT-IR, and 1H NMR spectroscopies; and DSC and TGA studies. These colored polymers exhibit a high degree of thermal stability.
Introduction
The concept of dyeing started in 415 BC with dyeing of wool by natural materials. In 1856, the first synthetic dye Mauve was discovered.[Citation1] The use of eco-friendly and non-toxic natural dyes has become a matter of significant importance because of increased environmental awareness in order to avoid some toxic of synthetic dyes.[Citation2] This issue has led to an increasing attitude towards the natural dyes as a substitute of synthetic ones in last few years, especially according to their useful effects on biological systems.[Citation3,4]
One of the most famous natural dyes is lawsone. The lawsone (2-hydroxyl- 1,4- naphthoquinone) is the main coloring element of henna that exists in dried leaves with a concentration of 1–1.5% w/w.[Citation5–7] The lawsone is a red-orange dye with optical absorption maximum of 452 nm in the UV–vis analysis. The properties of henna are connected with the presence of this natural compound. It is practically insoluble in water and soluble in methanol, dichloromethane, acetone, chloroform, ethyl acetate, isopropyl alcohol, diethyl ether, dimethylformamide (DMF), and dimethylsulfoxide (DMSO).[Citation8] The dyeing, antispasmodic, antibacterial, UV absorption, corrosion inhibitor properties have been attributed to the presence of lawsone.[Citation9] The solubility of these dyes is limited and this problem resolved as dye polymers. The polymeric dyes are chromophores that are attached to polymeric scaffolds. The dyes can either incorporate into polymer backbones or attached as side chains.[Citation10] They are classified as graft and block types according to their structures. Either of graft or block polymeric dyes offers the advantage of allowing a range of many physical properties, such as absorption, viscosity, solubility, and migratory, that are tunable. The range of color and polymer chemistry is actually endless.[Citation11,12] Now, they are being applied in hair dyes,[Citation13,14] fiber,[Citation15,16] jet-printing,[Citation17,18] solid-state polymeric dye lasers.[Citation19,20] The major classes of synthetic dyes are toxic or even carcinogenic with long turnover times.[Citation21] The natural colored polymers with acrylate scaffolds are biodegradable and their solubility is not restricted and color intensity is higher than others.[Citation22]
This paper deals with an effective preparation and characterization of the colored polymers lawsone. The structure of polymers was characterized with the FT-IR and 1H NMR spectroscopy methods. The λmax and color intensity of polymers were determined by a UV–vis spectrophotometer in DMSO solvent. The solubility of products was identified in various solvents. Thermal properties of polymers were characterized by DSC and TGA studies.
Experimental setup
Materials
The all of reagent and solvents were purchased from Merck. The acrylic acid, methacrylic acid, and acrylonitrile were distilled under reduced pressure to remove inhibitors, before use. The initiator α, α-azobis (isobutyronitrile) (AIBN) was purified by crystallization from methanol. All the solvents were distilled and stored over a drying agent. Tetrahydrofuran (THF) was dried by a standard method before use. Thin layer chromatography was carried out with silica gel 60 GF (Merck). Synthesis of monomer and copolymerization were carried out under dry argon to exclude oxygen and moisture from the reaction systems.
Measurements
Infrared spectra were recorded with a Shimadzu FT-IR-408 spectrophotometer as KBr pells. 1H NMR spectra were recorded on a Brucker 250 AC spectrometer in dimethyl sulfoxide (d6) as a solvent at room temperature. The DSC and TGA curves were obtained on a TGA/SDTA 851 calorimeter at heating and cooling rates of 10 °C/min under N2. The λmax and color intensity of products were determined on a Philips PU 8620 UV spectrophotometer in DMSO solvent using a 1-cm quartz cell.
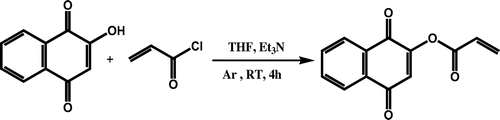
Synthesis of monomer from lawsone and acryloyl chloride: LA
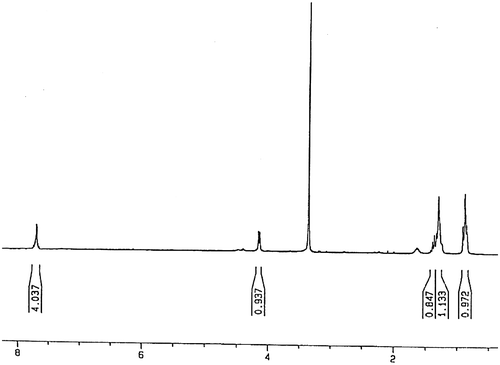
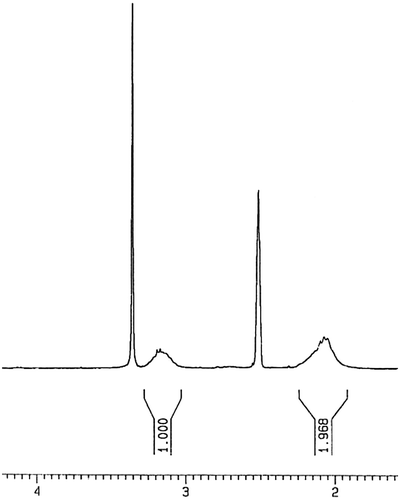
For preparing of monomer (LA), 0.4 g lawsone was dissolved in 10-mL dried THF in a two-necked flask. The flask was degassed under argon gas with stirring at room temperature. After 10 min, 0.12 mL acryloyl chloride was added to this solution under stirring, followed by 0.35-mL pure triethylamine. A deep red color appeared immediately. After 4 h, the solvent removed by rotary evaporation, then 30 mL of toluene was added to mixture followed the solution was washed with 10% sulfuric acid. For removing of remaining sulfuric acid on solution, the 20% NaHCO3 solution was used. After removing impurities from solution, toluene was removed by rotary evaporation. The product was chromatographed over silica-gel by CH2Cl2:CHCl3 in a ratio of 40:1. The dark red product (LA) was dried in an evacuated desiccator. The yield of final product was above 95% (Scheme ). 1H NMR (DMSO-d6, ppm): 0.86–1.4 (CH=CH2 of vinyl ester), 4.1 (–C=CH of lawsone), 7.7–7.8 (Ar–H) (Figure ). FT-IR (KBr, cm−1): 2964–3074 (aromatic C–H), 1754 (C=O), 1594–1681 (aromatic C=C), 1405–1458 (vinyl C=C), 1260 (C–O).
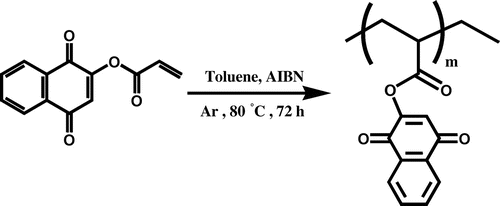
Polymerization of LA monomer: HLA
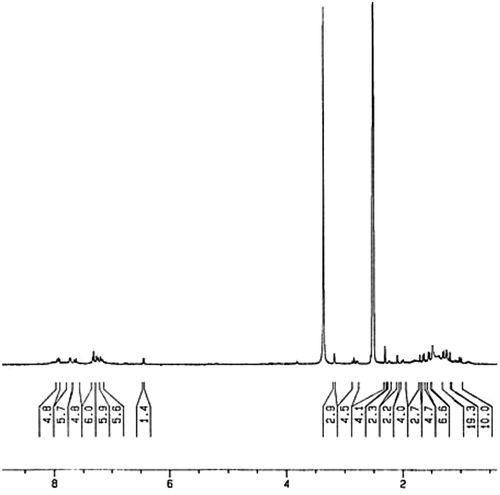
For preparing of homopolymer (HLA), the monomer LA was dissolved in 10 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed under argon gas, sealed under vacuum, and maintained at 80 ± 1 °C in a water bath, with stirring for about 72 h. The polymerization temperature was well controlled in a water bath. After reacting for 72 h, the ampoule was cooled rapidly to room temperature. Then the solutions were poured from ampoules into cooled methanol. The dark orange precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 90%) of HLA (Scheme ). 1H NMR (DMSO-d6, ppm): 1–2.3 (aliphatic C–H), 2.8–3.3 (–C=CH of lawsone), 6.45–8 (Ar–H) (Figure ). FT-IR (KBr, cm−1): 2961 (aromatic C–H), 2850–2919 (aliphatic C–H), 1688–1760 (C=O), 1538–1592 (aromatic C=C), 1462 (vinyl C=C), 1261 (C–O).
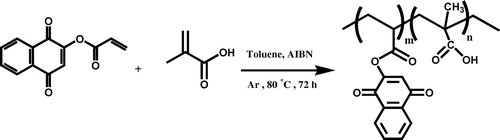
Copolymerization of LA monomer with methacrylic acid: PMLA
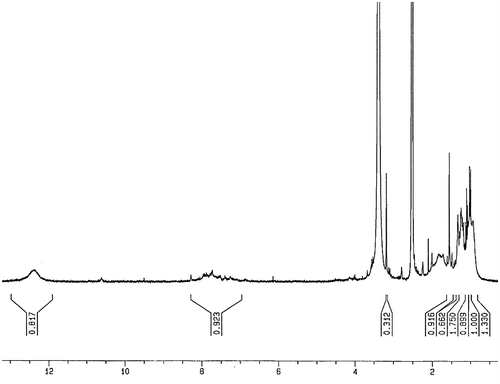
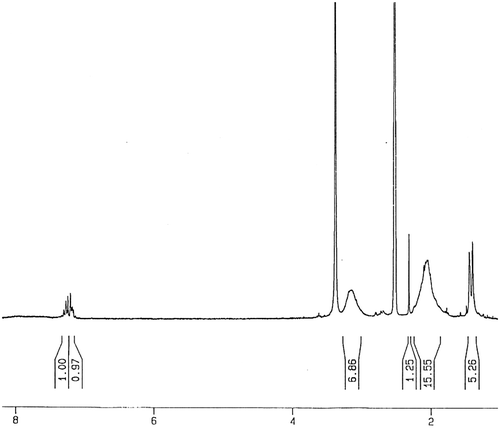
For preparing of copolymer (PMLA), a mixture of LA monomer and methacrylic acid with molar ratio of 1:1 was dissolved in 10 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed under argon gas, sealed under vacuum, and maintained at 80 ± 1 °C in a water bath, with stirring for about 72 h. After this time, the ampoule was cooled rapidly to room temperature. Then the solutions were poured into cooled methanol. The brown precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 95%) of copolymer (Scheme ). 1H NMR (DMSO-d6, ppm): 0.93–1.56 (aliphatic C–H), 3.18 (–C=CH of lawsone), 7.72–7.96 (Ar–H), 12.36 (–COOH) (Figure ). FT-IR (KBr, cm−1): 2600–3446 (–COOH), 2985 (aromatic C–H), 2875 (aliphatic C–H), 1700–1716 (C=O), 1637–1653 (aromatic C=C), 1473–1488 (vinyl C=C), 1180–1271 (C–O).
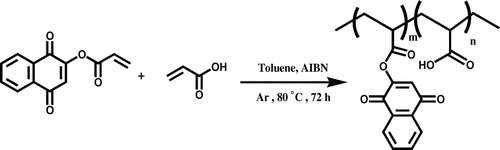
Copolymerization of LA monomer with acrylic acid: PALA
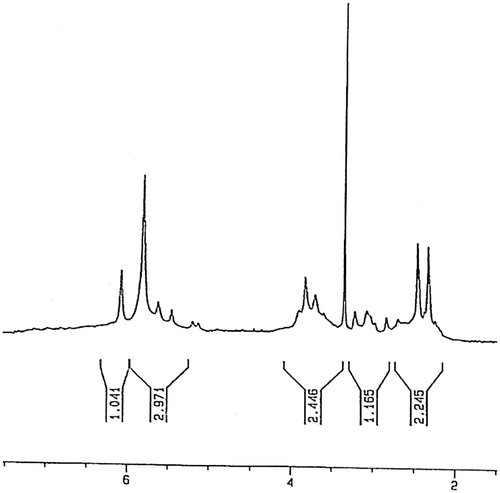
For preparing of copolymer (PALA), a mixture of LA monomer and acrylic acid with molar ratio of 1:1 was dissolved in 10 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed under argon gas, sealed under vacuum, and maintained at 80 ± 1 °C in a water bath, with stirring for about 72 h. After 72 h, the ampoule was cooled rapidly to room temperature. Then the solutions in ampoule were poured into cooled methanol. The red precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 45%) of copolymer (Scheme ). 1H NMR (DMSO-d6, ppm): 2.31– 3.24 (aliphatic C–H), 3.54–3.94 (–C=CH of lawsone), 5.5–6.1 (Ar–H) (Figure ). FT-IR (KBr, cm−1): 2450–3424 (–COOH), 2965 (aromatic C–H), 2880 (aliphatic C–H), 1654–1719 (C=O), 1542–1611 (aromatic C=C), 1457 (vinyl C=C), 1097–1261 (C–O).
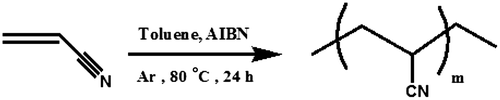
Polymerization of Acrylonitrile: PAN
For preparing of homoacrylonitrile (PAN), the acrylonitrile (1 mL) was dissolved in 10 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed under argon gas, sealed under vacuum, and maintained at 80 ± 1 °C in a water bath, with stirring for about 24 h. The polymerization temperature was well controlled in a water bath. After reacting for 24 h, the ampoule was cooled rapidly. Then the solutions were poured into cooled methanol. The light yellow precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 95%) of PAN (Scheme ). 1H NMR (DMSO-d6, ppm): 2.05–2.09 (CH2), 3.15–3.19 (C–H) (Figure ). FT-IR (KBr, cm−1): 2938 (aliphatic C–H), 2243 (CN), 1454 (bending C–H).
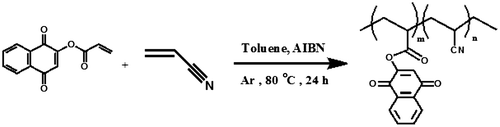
Copolymerization of LA monomer with acrylonitrile: PANLA
For preparing of copolymers (PANLA1 and PANLA2), a mixture of LA monomer and acrylonitrile with molar ratios of 1:10 and 1:20 was dissolved in 10 mL of toluene and was mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoules were degassed under argon gas, sealed under vacuum, and maintained at 80 ± 1 °C in a water bath, with stirring for about 24 h. After 24 h, the ampoules were cooled rapidly to room temperature. Then the solutions in ampoules were poured into cooled methanol. The brown precipitates were collected and washed with methanol and dried under vacuum to yield (approximately 95%) of copolymers (Scheme ). 1H NMR (DMSO-d6, ppm): 1.4–2.31 (aliphatic C–H), 3.06–3.18 (–C=CH of lawsone), 7.15–7.3 (Ar–H) (Figure ). FT-IR (KBr, cm−1): 2983 (aromatic C–H), 2939 (aliphatic C–H), 2243 (CN), 1716–1771 (C=O), 1558–1636 (aromatic C=C), 1455 (vinyl C=C), 1079–1210 (C–O).
Result and discussion
Preparation of colored polymers with natural dyes is very interesting in organic synthesis field. There are various methods for these compounds preparation. In the present work, first we synthesized monomer (LA) by treatment of lawsone with acryloyl chloride and triethylamine as a moderate base. The monomer is prepared by SN2 mechanism and is stable under heat and moisture. Then, the LA polymerized with various vinyl compounds by FRP (Free Radical Polymerization) mechanism. The structure of products is characterized with FT-IR and 1H NMR analyses.
Interestingly, in compound LA because of magnetic anisotropy effect, vinyl protons appear in the region below 2 ppm.
The molar compositions of LA and methacrylic acid in copolymer PMLA were calculated from the ratio integrated intensities of the peaks around 7.7–8 ppm, corresponding to four protons of benzene ring in LA units to the total area between 0.93 and 1.56 ppm, which were attributed to eight protons corresponding to three protons in LA and five protons in methacrylic acid. The molar compositions of LA and methacrylic acid were calculated from below two equations where m and n were the mole fractions of LA and methacrylic acid, respectively.
The molar compositions of LA and acrylic acid in copolymer PALA were calculated from the ratio-integrated intensities of the peaks around 5.5–6.1 ppm, corresponding to four protons of benzene ring in LA units to the total area between 2.31 and 3.24 ppm, which were attributed to six protons corresponding to three protons in LA and three protons in acrylic acid. The molar compositions of LA and acrylic acid were calculated from below two equations where m and n were the mole fractions of LA and acrylic acid, respectively.
The same approach, the molar compositions of LA and acrylonitrile in copolymer PANLA1 were calculated from the ratio-integrated intensities of the peaks around 7.15–7.3 ppm, corresponding to four protons of benzene ring in LA units to the total area between 1.4 and 2.31 ppm, which were attributed to six protons corresponding to three protons in LA and three protons in acrylonitrile. The molar compositions of LA and acrylonitrile were calculated from below two equations where m and n were the mole fractions of LA and acrylonitrile, respectively.
A similar method was used to calculate the molar compositions of monomers in copolymer PANLA2.
The compositions of all copolymers are presented in Table .
Table 1. Molar composition of copolymers.
The products solubility was checked in eight solvents inclusive of water, ethyl acetate, toluene, diethyl ether, methanol, DMF, DMSO, and acetonitrile. The products solubility results are presented in Table . These results showed all compounds are insoluble in water and soluble in two solvents DMF and DMSO.
Table 2. Solubility study of colored compounds.
Molecular absorption of colored polymers
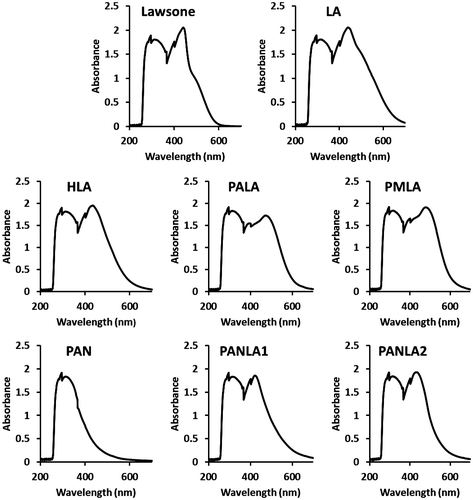
Absorption of photons in the ultraviolet/visible range (UV/vis) is a result of excitation of ground state valence or bonding electrons into higher energy orbitals. UV/vis spectrophotometry takes advantage of these electronic transitions to identify and quantify chemical substances. Chemical identification is possible by recording a spectrum. The UV/vis/NIR spectral range on the Spectra extends from 200 to 1000 nm (UV 200–400 nm, VIS 400–650 nm, NIR = near infrared 650–1000 nm). For this reason, absorbance spectra were obtained from 200 to 700 nm with 2 nm intervals. The all colored compounds (Figure ) absorption was measured with 4.9 g/l concentration in DMSO solvent (Figure ). The commonly observed transitions are n–π* or π–π*. We saw conjugation causes absorption signatures shift to longer wavelengths because the π–π* transitions are more intense than n–π* transitions. The wavelengths of maximum absorption of compounds are showed in Table .
Table 3. Molecular absorption study of colored compounds.
Thermal properties of the polymers
The thermal properties of the polymers were summarized in Table , including the initial decomposition temperature of the polymer (IDT), temperature of 50% weight loss of the polymer (PDT) and the temperature at which the maximum decomposition rate occurred for the polymer (PDTmax). Comparison of the polymers shows that the PANLA copolymers are more stable than other polymers. Comparing PANLA copolymers with PAN or PALA and PMLA copolymers with HLA has shown the homopolymers to the copolymers are stable. All polymers decompose completely by 900 °C leaving no residue at 950 °C.
Table 4. Thermal properties of colored polymers.
Conclusions
The colored polymers were synthesized in two steps. In step 1, LA monomer was prepared by the reaction of lawsone and acryloyl chloride with good yield. In subsequent step, the LA was polymerized with itself and other monomers. All products were soluble in DMF and DMSO. The UV/vis spectrophotometry study has shown the conjugation causes of absorption signatures shift to longer wavelengths. Study of the compounds’ thermal behavior showed that the PANLA copolymers are stable and suitable for applications as colored materials.
References
- Singh R, Jain A, Panwar S, Gupta D, Khare SK. Antimicrobial activity of some natural dyes. Dyes Pigm. 2005;66:99–102.10.1016/j.dyepig.2004.09.005
- Hao S, Wu J, Huang Y, Lin J. Natural dyes as photosensitizers for dye-sensitized solar cell. Sol. Energy. 2006;80:209–214.10.1016/j.solener.2005.05.009
- Aruona OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003;9–20:523–524.
- Polo AS, Murakami Iha NY. Blue sensitizers for solar cells: natural dyes from Calafate and Jaboticaba. Sol. Energy Mater. Sol. Cells. 2006;90:1936–1944.10.1016/j.solmat.2006.02.006
- Jain VC, Shah DP, Sonani NG, Dhakara S, Patel NM. Pharmacognostical and preliminary phytochemical investigation of Lawsonia inermis L. Leaf. Rom. J. Biol. Plant Biol. 2010;55:127–133.
- Chaudhary G, Goyal S, Poonia P. Lawsonia inermis linnaeus: a phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010;2:91–98.
- Muhammad HS, Muhammad S. The use of Lawsonia inermis Linn. (henna) in the management of burn wound infections. Afr. J. Biotechnol. 2005;4:934–937.
- Borade AS, Kale BN, Shete RV. A phyto pharmacological review on lawsonia inermis (Linn.). Int. J. Pharm. Life Sci. 2011;2:536–541.
- Chang H, Suzuka SE. Lawsone (2-OH-1,4-naphthoquinone) derived from the henna plant increases the oxygen affinity of sickle cell blood. Biochem. Biophys. Res. Commun. 1982;107:602–608.10.1016/0006-291X(82)91534-0
- Nicolescu FA, Jerca VV, Stancu IC, Vasilescu DS, Vuluga DM. New organic–inorganic hybrids with azo-dye content. Des. Monomers Polym. 2010;13:437–444.
- Hernández S, Beristain MF, Ogawa T. Diacetylene-containing polymers XII. Synthesis and characterization of dye-containing poly(hexa-2,4-butadiynylenoxydibenzoates). Des. Monomers Polym. 2002;5:125–139.10.1163/156855502760151634
- Armenta-Villegas L, Perez-Martinez AL, Navarro RE, Ogawa T. Synthesis, characterization and some optical properties of poly(hexa-2,4-diynylene-1,6-dioxy)dinaphthoates containing polar azo dye. Des. Monomers Polym. 2009;12:533–541.10.1163/138577209X12478291548944
- García T, Carreón-Castro MDP, Gelover-Santiago A, Ponce P, Romero M, Rivera E. Synthesis and characterization of novel amphiphilic azo-polymers bearing well-defined oligo(ethylene glycol) spacers. Des. Monomers Polym. 2012;15:159–174.10.1163/156855511X615047
- Mahkam M, Assadi MG, Zahedifar R, Allahverdipoor M, Doostie L, Djozan J. Synthesis and evaluation of new linear azo-polymers for colonic targeting. Des. Monomers Polym. 2004;7:351–359.10.1163/1568555041475301
- Mahkam M, Nabati M, Latifpour A, Aboudi J. Synthesis and characterization of new nitrogen-rich polymers as candidates for energetic applications. Des. Monomers Polym. 2014;17:453–457.10.1080/15685551.2013.867569
- Inceoglu S, Olugebefola SC, Acar MH, Mayes AM. Atom transfer radical polymerization using poly(vinylidene fluoride) as macroinitiator. Des. Monomers Polym. 2004;7:181–189.10.1163/156855504322890133
- Koseva NS, Novakov CP, Rydz J, Kurcok P, Kowalczuk M. Synthesis of aPHB-PEG brush co-polymers through ATRP in a macroinitiator–macromonomer feed system and their characterization. Des. Monomers Polym. 2010;13:579–595.10.1163/138577210X530675
- Feldman D. Polymer history. Des. Monomers Polym. 2008;11:1–15.10.1163/156855508X292383
- Pillai CKS. Challenges for natural monomers and polymers: novel design strategies and engineering to develop advanced polymers. Des. Monomers Polym. 2010;13:87–121.10.1163/138577210X12634696333190
- Beristain MF, Nakamura M, Nagai K, Ogaw T. Synthesis and characterization of poly[propargyl(3-methoxy-4-propargyloxy)cinnamate]: a polymer from a natural product. Des. Monomers Polym. 2009;12:257–263.10.1163/156855509X436076
- Han W, Lin B, Yang H, Zhang X. Synthesis of novel poly(ester amine) dendrimers by Michael addition and acrylate esterification. Des. Monomers Polym. 2013;16:67–71.10.1080/15685551.2012.705491
- Srivastava S. Co-polymerization of acrylates. Des. Monomers Polym. 2009;12:1–18.10.1163/156855508X391103