Abstract
Functionalization of bio-based oligomers is a relevant approach in order to allow these materials to replace more conventional and nonbiodegradable polymers in a wider range of applications. This article will report on the hydroxylation of isosorbide-based ethersulfone oligomers. The hydroxy end-group was controlled by the variation of polycondensation parameters. Comparative study was conducted to assess the effect of isosorbide excess and the monomer concentration on structural composition and molecular weights of produced oligomers. Depending on reaction conditions, various products were synthesized, mainly α,ω-dihydroxyethersulfone oligomers. The characterization of the synthesized polyethersulfone was demonstrated by MALDI–TOF, NMR spectroscopy, differential scanning calorimetry, and size exclusion chromatography.
Introduction
Recently, the syntheses of bifunctional oligomers based on renewable materials have been investigated as potential building blocks that could be used to produce materials with enhanced properties.[Citation1–4] Many studies reported the synthesis of functional monomers and oligomers based on isosorbide (IS), which belongs to 1,4;3,6-dianhydrohexitols,[Citation5–8] known as renewable and sustainable OH-functional monomers.[Citation9–12] The scope of this study is the synthesis of new bio-based dihydroxy functional oligomers of polyethersulfones (PES) based on IS as precursor of biomaterials. The choice of PES is owing to their good thermal and chemical stabilities, oxidation resistance, and remarkable mechanical characteristics.[Citation13–15] The hydroxy end-groups were controlled by the variation of the stoichiometric ratio [Citation16] between IS monomer issued from corn starch [Citation2–5] and 4,4’-difluorodiphenylsulfone (DFDPS). A comparative study was conducted to investigate the effect of both IS excess and concentration on structural composition and molecular weights of prepared oligomers.
Several oligoethersulfones with different percentage of end-groups were synthesized by finely tuning the experimental parameters. Optimized polycondensation conditions yielded over 94% of α,ω-dihydroxy linear oligomers (Figure ) with low molecular weights (6 kDa) and narrow polydispersity index (Ð = 1.11). The chemical characterization of the synthesized PES was carried out using different complementary analytical methods.[Citation17,18] Even though MALDI–TOF quantification may be hampered by the poor reproducibility of the signal intensity,[Citation19] it was very useful in this study in combination with 1H and 13C NMR spectroscopy, differential scanning calorimetry (DSC), and size-exclusion chromatography (SEC) to investigate the structural composition.
This work confirms that novel oligomers with tuned physicochemical properties can be designed in a simple and efficient step growth polymerization using stoichiometric adjustments, leading to promising precursors of biomaterials.
Experimental
Materials
IS was kindly supplied by Roquette Freres (Lestrel, France). It was recrystallized in acetone and used after drying over P4O10 in a dessicator. Anhydrous potassium carbonate and 4,4’difluorodiphenyl sulfone were purchased from Sigma Aldrich.
Polycondensations
The polymerization was performed by reacting 5.0 mmol of IS, 5.0 mmol of DFDPS, and 10.5 mmol of K2CO3 in 12.5, 25, or 50 mL of DMSO, depending on the DFDPS concentration, 0.3, 0.15, or 0.075 M, into a 100-mL cylindrical three-neck glass reactor, equipped with mechanical stirrer and distillation head. Five milliliter of toluene was added dropwise through a dropping funnel. The reaction mixture was heated until 140–145 °C, so that a slow azeotropic distillation of the toluene was observable. The loss of toluene was replaced continuously from the dropping funnel. After 12 h, the resulting viscous solution is slowly poured and precipitated into cold methanol. The resulting precipitate is isolated by filtration, washed with cold methanol, and then dried in vacuo.
Measurements
The inherent viscosity (η) was measured with Ubbelohde viscometer at 20 °C in DMSO containing 2 vol% of TFA.
The 500 MHz NMR spectra were recorded on a Bruker ‘UltraShield Plus 500’ spectrometer in 5 mm i.d. sample glass tubes. DMSO-D6 containing 2 vol% of TFA served as solvent. MALDI–TOF experiments were performed on an UltraflexIII (BrukerDaltonics) mass spectrometer including a Time-Of-Flight analyzer. This instrument was equipped with a smart beam laser. The instrument was operated in reflectron mode and positive ionization mode. The different voltages are 25 kV for ion source 1, 21 kV for ion source 2, and 1.625 kV for reflector detector voltage. The power laser used was a part of the total power: 19%. Its frequency was 66.7 Hz. With these experimental conditions, the full width at half maximum resolution was 15,696 for a peak at a ratio m/z that equals to 1023 Da. All spectra were obtained in autoXecute mode from the sum of 4800 single shoots. After each 200 single shoots, laser was moved on the target surface. All data were reprocessed using the Flex Analysis software from Bruker Daltonics®. The target solutions were prepared in DMSO/TFA solution at 2 g L−1 with dithranol as a matrix and NaI as dopant.
Differential Scanning Calorimetric data were obtained using DSC-Q20 (TA Intruments, USA) in flowing argon (50 mL min−1) at a heating rate of 10 °C min−1 from −50 °C to 320 °C.
Average molecular weights measurements were obtained from size exclusion chromatography on Agilent Technologies 1200 series system equipped with a refractometer, Waters 2414. The column is a PL Gel Mixed-D, 5 μm and the calibration is calculated with polystyrene standards in DMF.
Representative NMR DATA
1H NMR (DMSO-D6, δ): 7.87–7.81 (m, 4H, H8), 7.18–7.19 (d, 2H, J = 7.9, H7), 7.11–7.13 (d, 2H, J = 7.9, H7), 5.00 (brs, 3H, H3 and H2 and H5), 4.53 (s, 1H, H4), 3.94–3.86 (m, 2H, H1 and H6). 13C NMR (DMSO-D6, δ): 161.9–160.8 (C9), 134.4–134.1 (C10), 130.0–129.6 (C8), 116.4–116.2 (C7), 88.1 (C4), 81.6 (C3), 76.1 (C2), 75.6 (C5), 72.6 (C1), 71.2 (C6).
Results and discussion
Potential products of polycondensation reaction of IS with DFDPS
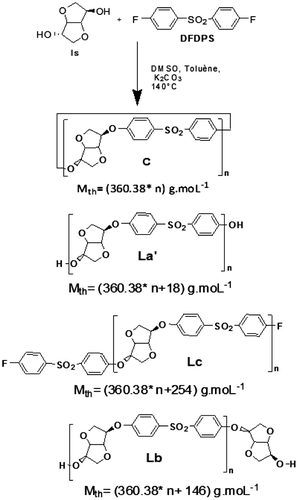
The structural composition of produced PES was followed by interpretation of MALDI–ToF spectra through the comparison between the calculated and measured peak intensities related to each of the possible produced structures La’, Lb, Lc, and C as shown in Figure .
The production of several linear and cyclic structures La’, Lb, Lc, and C was previously reported in numerous studies.[Citation20,21] The La’ structure resulted from the hydrolysis of C–F bond.
The corresponding calculated masses of each of the produced structures illustrated in Figure are summarized in Table .
Table 1. Calculated masses (including Na+ doping) of potential structures (cyclic C; linear La’, Lb, Lc).
Effect of the excess of IS at 0.3 mol L−1
In an attempt to determine the impact of the variation of IS excess on the structural composition and yields of produced dihydroxy PES at 0.3 mol L−1, we conducted a first series of experiments with 10, 20, and 30% of IS excess relative to the amount of DFDPS. The inherent viscosity, DSC, and SEC measurements were recorded.
The different experimental data and the structural composition obtained from the MALDI–ToF spectra of PES-4, 5, and 6 are presented in Table and are compared with the structural composition of PES-1 (0% excess of IS).[Citation21]
Table 2. Synthesis of PES with variation of IS excess at a concentration of 0.3 mol L−1.
According to Table , the excess of IS was followed by a notable decrease in inherent viscosity values from 0.65 dL g−1 for PES-1 obtained in stoichiometric conditions to 0.09 dL g−1 for PES-6 obtained with 30% excess of IS. These results were accompanied by a slight decrease in Tg from 245 to 213 °C and a decrease in molecular weights (Mn) as assessed by SEC measurements.
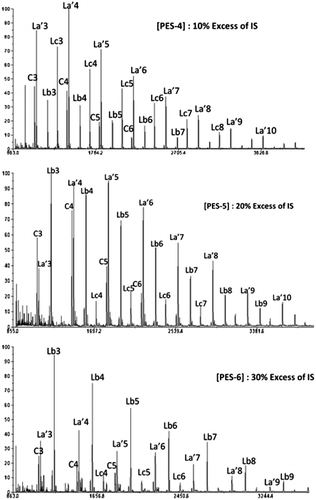
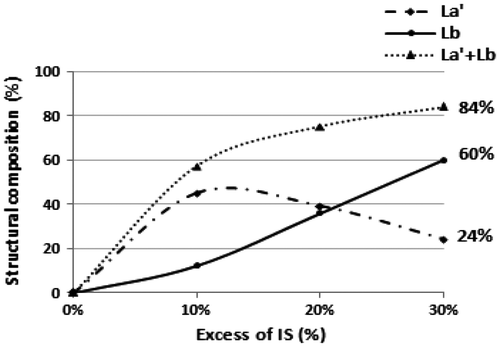
Investigations by MALDI–ToF analyses (Figure ) revealed a strong attenuation of cyclic form against a considerable increase in signals related to dihydroxy linear structures (Lb and La’).
As expected, the results listed in Table clearly prove that the excess of IS has an enormous influence on the structural composition. PES-6 shows 84% of dihydroxylated linear oligomers split between 60% of Lb form and 24% of La’ form (Figure ).
Figure 4. Effect of the variation of IS excess in the end group structure of produced oligomers at a concentration of 0. 3 mol L−1.
The proton NMR spectra of PES-4, 5, and 6 (Figure ) displayed signals of protons related to the various PES structures.
Figure 5. 1H NMR spectra [3.5–8.1 ppm] of oligoethersulfones at 0.15 mol L−1 [DMSO-D6, 500 MHz, 20 °C].
![Figure 5. 1H NMR spectra [3.5–8.1 ppm] of oligoethersulfones at 0.15 mol L−1 [DMSO-D6, 500 MHz, 20 °C].](/cms/asset/9b205f95-3c0f-4714-ae1a-475c60f8c2ee/tdmp_a_947554_f0005_b.gif)
The Lb structure was evidenced by signals corresponding, respectively, to proton 2* that related to hydroxyl group in α position of IS (4.25 ppm) and protons 1*, 3*, 4*, 5*, 6*.[Citation22] As we increase the excess of IS, the intensity of the identified signals goes in the same trend but with a strong attenuation of signals related to Lc structure, mainly protons a” at 7.22 ppm and b” at 7.89 ppm linked to carbons in ortho and meta position of fluorine. Emergence of a’ signal close to the aromatic zone could be assigned to La’ phenolic core. The NMR analyses clearly confirm the results previously obtained by MALDI–ToF analysis.
Effect of the variation in IS excess and monomer concentration
The previous experiments conducted at monomer concentration of 0.3 mol L−1 have shown encouraging results especially for PES-6. In order to confirm and improve in advance these results, we conducted a second series of reactions with lower concentrations. The first reactions were conducted at 0.15 mol L−1 with IS excess up to 100%.
According to the results presented in Table , Polycondensations yielded nearly quantitative amounts of PES (over 90%). Inherent viscosity values showed a decrease from 0.27 dL g−1 (equimolar mixture) to 0.06 dL g−1 (PES-10), demonstrating a decrease in molecular weights. These results were verified by SEC measurements where the oligomers presented a rather lower molecular weights (5640 < Mn < 9682 g mol−1) and a narrow polydispersity index (Ð) close to the unit.
Table 3. Synthesis of PES with variation of IS excess and monomer concentration.
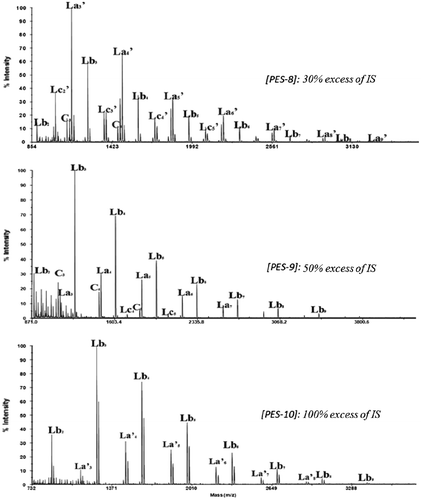
The examination of structural composition of produced PES by MALDI–ToF spectra given in Figure shows a notable structural change in the sense of producing more of dihydroxylated linear oligomers as shown in spectrum monitored for PES-10 (72% of Lb and 28% of La’ form).
Interestingly, increasing the excess of IS gave rise to Lb species at the expense of La’ to become predominant for 100% excess of IS.
To highlight the changes on structural composition of PES, we overlaid 13C-NMR spectra (Figure ) of PES-2, PES-9, and PES-10.
Figure 7. 13C-NMR spectra [68–180 ppm] of oligo-ethersulfones at 0.15 mol L−1 [DMSO-D6, 500 MHz, 20 °C].
![Figure 7. 13C-NMR spectra [68–180 ppm] of oligo-ethersulfones at 0.15 mol L−1 [DMSO-D6, 500 MHz, 20 °C].](/cms/asset/59b48d63-2720-43bf-8242-e01905c4ef92/tdmp_a_947554_f0007_b.gif)
We found out that PES-2 (equimolar ratios) gave a simplified spectrum of 13C-NMR, which shows only signals related to repetitive units of cyclic structure. However, when we increase the IS excess to 100%, new signals appear, mainly carbons 1*, 2*, 3*, 4*, 5*, 6* of IS [Citation23] as pending moiety in Lb oligomers and a, a’, b, and b’, which characterize the La’ terminals.
Moreover, the doublet located at 160.3 ppm in the 13C NMR spectrum corresponding to carbon (C9) of DFDPS can be explained by the endo and exo effect of IS hydroxyl group. Broadening of the area of this doublet showed additional signals owing to species (La’).
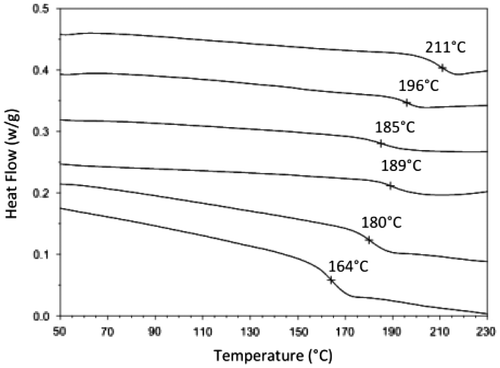
Thermal stability of reaction products was investigated by DSC analyses (Figure ) and it could be seen that the Tg shows a decrease from 237 PES-2 to 164 for PES-12, depending on the molecular weight and the structure composition allowed by the side chains.
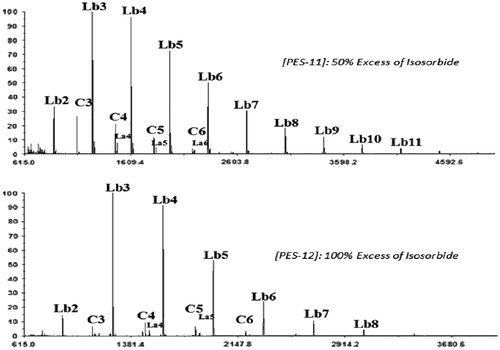
In order to increase the ratio of Lb species toward La’, two additional experiments at a concentration of 0.075 mol L−1 were conducted. First, MALDI–ToF MS observations (Figure ) showed that the obtained products were nearly majoritarily Lb species (around 95%). Interestingly, Tg recorded for PES-11 and PES-12, respectively, and obtained with 50% and 100% excess of IS decreased until 164 °C keeping average molecular weights between 6900 and 6050 g mol−1as the former concentration of 0.15 mol L−1. This could be explained by both the decrease in cyclic and La’ rigid form and the increase in Lb structure, which allows a higher molecular mobility.
Figure shows predominance of signals related to IS protons at the end of chain with a strong attenuation of proton signals of other structures. The structural composition of PES-12 was confirmed by NMR.
Conclusions
Functional oligomers of PES were produced by varying the IS excess and concentration toward DFDPS monomer. The combination of two powerful analytical techniques, MALDI–ToF and NMR spectroscopy, together with the measure of the viscosity was useful to characterize and to follow the evolution of structural composition. As a first result, a mixture of dihydroxy structures, namely La’ and Lb forms, was obtained. Significant increase in linear bifunctional Lb materials composition (94%) was observed when the excess of IS was set at 100% at 0.075 mol L−1. Relatively low molecular weights with narrow polydispersity were obtained, which indicates a good homogeneity. The increasing in dihydroxylated structures was accompanied by a decreasing in glass transition temperatures.
Finally, it should be mentioned that the present work could serve as a background for polycondensation of copolymers with enhanced properties or new bio-based materials such as polycarbonates and thermoplastic elastomers.
References
- Casarano R, Petri DFS, Jaffe M, Catalani LH. Synthesis and structural characterization of block and random low molecular weight copolymers composed of l-lactic acid and isosorbide succinate moieties. J. Braz. Chem. Soc. 2009;20:1414–1424.
- Medimagh R, Mghirbi S, Saadaoui A, Fildier A, Desloir-Bonjour M, Raffin G, Kricheldorf HR, Chatti S. Synthesis of biosourced polyether-amides from 1,4-3,6-dianhydrohexitols: characterization by NMR and MALDI–ToF mass spectrometry. C. R. Chimie. 2013;16:1127–1139.10.1016/j.crci.2013.05.004
- Besse V, Auvergne R, Carlotti S, Boutevin G, Otazaghine B, Caillol S, Pascault JP, Boutevin B. Synthesis of isosorbide based polyurethanes: an isocyanate free method. React. Funct. Polym. 2013;73:588–594.
- Paspirgelyte R, Grazulevicius JV, Grigalevicius S, Jankauskas V. N,N′-Diphenyl-1,4-phenylenediamine-based enamines containing reactive functional groups as building blocks for electro-active polymers. Des. Monomers Polym. 2009;12:579–587.10.1163/138577209X12519685854626
- Fenouillot F, Rousseau A, Colomines G, Saint-Loup R, Pascault J-P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): a review. Progr. Polym. Sci. 2010;35: 578–622.10.1016/j.progpolymsci.2009.10.001
- Beldi M, Medimagh R, Chatti S, Marque S, Prim D, Loupy A, Delolme F. Characterization of cyclic and non-cyclic poly-(ether-urethane)s bio-based sugar diols by a combination of MALDI–TOF and NMR. Eur. Polym. J. 2007;43:3415–3433.10.1016/j.eurpolymj.2007.06.003
- Chatti S, Bortolussi M, Loupy A. Synthesis of new diols derived from dianhydrohexitols ethers under microwave-assisted phase transfer catalysis. Tetrahedron Lett. 2000;56:5877–5883.
- Chatti S, Bortolussi M, Loupy A. Synthesis of diethers derived from dianhydrohexitols by phase transfer catalysis under microwave. Tetrahedron Lett. 2000;41:3367–3370.10.1016/S0040-4039(00)00416-0
- Lligadas G, Ronda JC, Galià M, Cádiz V. Monomers and polymers from plant oils via click chemistry reactions. J. Polym. Sci., Part A: Polym. Chem. 2013;51:2111–2124.10.1002/pola.26620
- David I, Habeych PBJ, Pleiss J, Vanegas D, Eggink G, Boeriu CG. Biocatalytic synthesis of polyesters from sugar-based building blocks using immobilized Candida antarctica lipase B. J. Mol. Catal. B: Enzym. 2011;71:1–9.
- Noordover BAJ, Haveman D, Duchateau R, van Benthem RATM, Koning CE. Chemistry, functionality, and coating performance of biobased copolycarbonates from 1,4:3,6-dianhydrohexitols. J. Appl. Polym. Sci. 2011;121:1450–1463.10.1002/app.33660
- Noordover BAJ, editor. Biobased step-growth polymers; chemistry, functionality and applicability. North Brabant: Technische Universiteit Eindhoven; 2007.
- Feldman D. Polymer history. Des. Monomers Polym. 2008;11:1–15.10.1163/156855508X292383
- Lecouvet B, Sclavons M, Bourbigot S, Bailly C. Thermal and flammability properties of polyethersulfone/halloysite nanocomposites prepared by melt compounding. Polym. Degrad. Stab. 2013;98:1993–2004.10.1016/j.polymdegradstab.2013.07.013
- Zhao W, Mou Q, Zhang X, Shi J, Sun S, Zhao C. Preparation and characterization of sulfonated polyethersulfone membranes by a facile approach. Eur. Polym. J. 2013;49:738–751.10.1016/j.eurpolymj.2012.11.018
- Ristic IS, Vukic N, Cakic S, Simendic V, Ristic O, Budinski-Simendic J. Synthesis and characterisation of polyester based on isosorbide and butanedioic acid. J. Polym. Environ. 2012;20:519–527.
- Ben Abderrazak H, Fildier A, Marque S, Prim D, Ben Romdhane H, Kricheldorf HR, Chatti S. Cyclic and non cyclic aliphatic–aromatic polyesters derived from biomass: Study of structures by MALDI–ToF and NMR. Eur. Polym. J. 2011;47:2097–2110.10.1016/j.eurpolymj.2011.07.009
- Sahre K, TH, Pospiech D, Eichhorn KJ, Fischer D, Voit B. Monitoring of the polycondensation reaction of bisphenol A and 4,4′-dichlorodiphenylsulfone towards polysulfone (PSU) by real-time ATR–FTIR spectroscopy. Eur. Polym. J. 2006;42:2292–2301.10.1016/j.eurpolymj.2006.05.025
- Walterová Z, J Horský. Quantification in MALDI–TOF mass spectrometry of modified polymers. Anal. Chim. Acta. 2011;693:82–88.10.1016/j.aca.2011.03.019
- Hani MA, Chatti S, Kricheldorf HR, Zarrouk H. Polycondensation of isosorbide and various diols by means of diphosgene characterization by a combination of MALDI and NMR. Recent Res. Devel. Organic Chem. 2007;11:1–11.
- Chatti S, H M, Bornhorst K, Kricheldorf HR. Poly(ether sulfone) of isosorbide, isomannide and isoidide. High Perform. Polym. 2007;21:105–118.
- Jeol S. Stratégies de modifications physico-chimiques des polyesters semi-cristallins. Application à la fabrication de bouteilles en poly (éthylène téréphtalate) [Ph.D. thesis] [Physicochemical modification approachs of semicristalline polyesters. Application to the manufacture of bottles based on Polyethylene terephthalate]. Lyon: INSA de Lyon; 2006.
- Varkey EC, Sreekumar K. Isosorbide based chiral polyurethanes: optical and thermal studies. J. Mater. Sci. 2010;45:1912–1920.10.1007/s10853-009-4177-1