Abstract
New photocrosslinkable prepolymer, 2-hydroxy-3-[p-(1-{p-[2-hydroxy-3-(vinylcarbonyloxy)propoxy]phenol}-1-phenylethyl)phenoxy]propyl acrylate (HVPA) was synthesized from p-[1-(p-hydroxyphenyl)-1-phenylethyl]phenol (HPPP), 1, 1-bis{p-[(2-oxiranyl)methoxy]phenyl}-1-phenylethane with acrylic acid and was characterized by 1H NMR, 13C NMR and FT-IR spectroscopy. The photocrosslinking studies were carried out by irradiating the mixture containing the prepolymer, HVPA (75%), with the diluent, tri(ethyleneglycol)diacrylate (25%), in the presence of a photoinitiator, Irgacure 651 (DMPA), under medium pressure mercury vapour lamp at various time intervals and various DMPA concentrations. The kinetics of the photopolymerization, percent double bond conversion and the rate of polymerization of the photocrosslinked polymers were studied using FT-IR spectroscopy. The result showed that an increasing DMPA concentration increases percent double bond conversion. The effect of viscosity of system on kinetics of photopolymerization was studied. It was found that percent double bond conversion increased with decrease in the viscosity of the system. The percent gel content and hardness of the photocrosslinked polymers were studied, and it was observed that the percent gel content and hardness of the photocrosslinked polymers were increased with increasing irradiation time and DMPA concentration. The photopolymerization studies of the synthesized prepolymer, HVPA, were compared to the commercial prepolymer, bisphenol-a-glycerolate (1-glycerol/phenol) diacrylate (BISGA). The results showed that percent double bond conversion of synthesized prepolymer, HVPA, was higher than commercial prepolymer, BISGA. This showed that HVPA was more suitable for industrial applications than BISGA.
1. Introduction
An active research on ultraviolet (UV) radiation curing has been in progress since 1980s in India. The main components of UV-curable coating are prepolymer, diluent and a photoinitiator.[Citation1] The physical as well as chemical properties of the cured coatings will depend on the nature and the structure of the prepolymer.[Citation2] The design of new prepolymers, diluents and photoinitiators will presumably accompany the market growth. The pressure for the development of green technologies such as UV-curing will induce new efforts and new applications in the field of UV-curing by photocrosslinkable polymers.
A UV-curing technology is widely used today in varnishes, inks and paints, which are employed to improve the surface properties of all kinds of materials, such as metals, encapsulation of semiconductor devices, paper, matrix material for composites, wood and coating industry.[Citation3–8] In optics and electric laminates, a UV-curing technology is used for optical fibre coatings, protective coatings for compact discs, heat-resistant coatings, corrosion protection coatings and weather-resistant coatings,[Citation8] holographic relief image protection, insulation layers for printed circuits and organic polymeric conductive materials.[Citation9] The structures and formulations of prepolymers, and diluents may be varied suitably for a particular application and property requirement.[Citation9] Because of non-yellowing, epoxy acrylates are usually used in coating industries when compared to urethane acrylates, polyether acrylates and polyester acrylates.
A UV-curing process, the liquid oligomer containing more than one reactive group is converted into solid photocrosslinked polymer, which depends on percent double bond conversion and the rate of polymerization. Several parameters influence the kinetics of photopolymerization such as structure of prepolymer, photoinitiator concentration, viscosity, irradiation time and temperature.[Citation10–12] The FT-IR has the most valuable technique to follow the kinetics of photopolymerization such as percent double bond conversion, rate of polymerization, termination rate coefficient and maximum rate of polymerization.[Citation13–17]
The reactive prepolymer and efficient photoinitiator have been synthesized in large number.[Citation18,19] The epoxy acrylates are well known, and established prepolymer is used in UV-curing technology and has excellent characteristics of moisture, good electrical and adhesion properties to many substrates.[Citation8,20] An undiluted form of an epoxy acrylate prepolymer is highly viscous, but when it is dissolved in most diluents, the viscosity is suddenly decreased. The commercial epoxy diacrylates are used on the basis of phenol and cresol derivatives.[Citation21] The bisphenol-a-glycerolate (1-glycerol/phenol) diacrylate (BISGA) is commonly used as a surface coating material, they suffer from the lack of reactivity, flexibility and chemical resistance, which arises from the chains linked in aromatic rings. An improvement in flexibility can be obtained by modification of long chain compounds into the resin before curing.
In many of the research works, commercial prepolymer, BISGA is used to study the kinetics of photopolymerization, but not known the kinetics of photopolymerization are carried out by a newly synthesized prepolymer, 2-hydroxy-3-[p-(1-{p-[2-hydroxy-3-(vinylcarbonyloxy)propoxy]phenol}-1-phenylethyl)phenoxy]propyl acrylate (HVPA) with increased reactivity, when compared to the commercially available prepolymer. In BISGA, bisphenol is the base group and the ethyl group is present in between aromatic rings of bisphenol epoxy acrylate. The curing speed is one of the very important parameters in kinetics of photopolymerization. The main aim of the present work is to study the synthesis of a new prepolymer, HVPA in which bisphenol is the base group and the phenyl ethyl group is introduced in between aromatic rings of bisphenol epoxy acrylates to improve the reactivity, adhesion, flexibility and chemical resistance in HVPA, when compared to the commercially available prepolymer, BISGA. The shelf life of BISGA is very short (3–4 months), does not allow enough time to import on demand. Finally a new prepolymer, HVPA, was produced with a shelf time of 9–12 months. This is the main advantage of this study. In this paper, HVPA was synthesized, characterized and its photopolymerization kinetics were studied by FT-IR. The effect of photoinitiator concentration and viscosity of system on the kinetics of photopolymerization was studied. The percent double bond conversion of the photocrosslinked polymers was compared to the commercial prepolymer, BISGA, by FT-IR.
2. Experimental
2.1. Materials
Phenol, acetophenone, acetone, epichlorohydrin, acetic acid, hydrochloric acid (HCl), sulphuric acid (H2SO4), isopropanol, chloroform, methanol, 1,4-dioxane, benzene, sodium hydroxide (NaOH), potassium hydroxide (KOH) and hydroquinone were purchased from SRL (India) and used without further purification. Acrylic acid (≥99.0, SRL, India) was used after removal of inhibitor for acrylation reactions. The reagent, triethylamine (SRL, India) was heated under reflux over sodium wire for 8 h, distilled and finally collected over fresh sodium wire. Ethyl alcohol was refluxed with calcium oxide for 6 h, allowed to stand overnight and distilled. The photoinitiator, Irgacure 651 (DMPA), prepolymer, bisphenol-a-glycerolate(1-glycerol/phenol)diacrylate (BISGA) and difunctional diluent, tri(ethyleneglycol)diacrylate (TEGDA) were purchased from Aldrich (USA) and used without further purification.
2.2. Synthesis of p-[1-(p-hydroxyphenyl)-1-phenylethyl]phenol
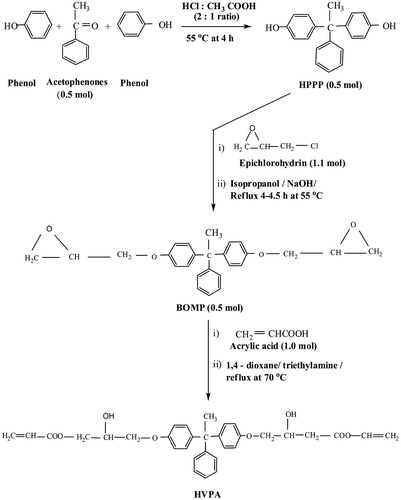
One mole of phenol was mixed with the solution containing 200 mL of acetic acid in 400 mL of concentrated HCl into a 1000 mL five-necked round bottom flask fitted with a heating mantle, funnel, thermometer, mechanical stirrer and reflux condenser. The contents of the flask were stirred for a period of 15 min, and then the solution was heated to 55 °C, 0.5 mol acetophenone was introduced dropwise to the solution for about 4 h. Then, the mixture was poured into 75 mL boiling water containing 2 N KOH. The solid product was separated and acidified with dilute H2SO4, then washed with distilled water and dried at 50 °C for 12 h. Recrystallization from benzene and methanol-water system gave light yellow crystals. The synthesis of the HPPP is shown in Figure . Yield 90%.
2.3. Synthesis of 1, 1-bis{p-[(2-oxiranyl)methoxy]phenyl}-1-phenylethane
A 500 mL five-necked round bottom flask was fitted with a heating mantle, funnel, thermometer, reflux condenser and mechanical stirrer. Then 0.5 mol of HPPP, 1.1 mol of epichlorohydrin and 300 mL of isopropanol were added to the flask. After thoroughly mixed, then the contents were heated at 55 °C for 15 min. Subsequently, a solution of 1.25 mol of NaOH in 500 mL water was added dropwise to the solution and then stirred continuously at 55 °C for 4–4.5 h. The temperature of the system was maintained at 55 °C for 4–4.5 h. The clear aqueous upper layer was carefully removed and the resin was washed with water. Finally, the product in the flask was washed several times with deionized boiled water to remove the remaining NaCl, and excess of epichlorohydrin was evaporated and dried under vacuum to give solid product of BOMP. It was soluble in aromatic hydrocarbons, chloroform and acetone. The synthesis of BOMP is shown in Figure . Yield 88%.
2.4. Synthesis of HVPA
A 500 mL five-necked round bottom flask was taken with 0.5 mol of BOMP, thermometer, funnel, reflux condenser and mechanical stirrer. The contents of the flask were stirred, heated at 70 °C followed by adding 1.0 mol acrylic acid, 7 mL triethylamine and 0.05 wt.% hydroquinone at 6 h, until the acid number was less than 4 mg KOH g−1. The standard alkali solution titration method was used to determine the acid content of the prepolymer. The synthesis of HVPA is shown in Figure . Yield 92%.
2.5. Characterization methods
1H NMR and 13C NMR spectra of HPPP, BOMP and HVPA were taken in CDCl3 on Bruker DPX-300 MHz spectrometer using tetramethylsilane as an internal standard. FT-IR spectra of the synthesized compounds, HPPP, BOMP, HVPA and photocrosslinked polymers were recorded on Perkin-Elmer spectrum one (7800–350 cm−1) spectrometer, using KBr pellets. Medium pressure mercury vapour lamp to the power output of 125 W cm−2 was used for the photocrosslinking study. The hardness of photocrosslinked polymer films was determined by A-Type JIS K6301 hardness tester (Germany).
2.6. Preparation the samples of different viscosity
The viscosity of the photocurable formulation was measured by Brookfield RVDV-II+ viscometer (USA). The viscosity of the formulation such as 5000 cp is prepared by the mixing the HVPA (74.4%) with TEGDA (25.6%) at 10 min. The formulated solution is taken in 600 mL beaker, and then the beaker is inserted at the bottom of Brookfield RVDV-II+ viscometer. The spindle is immersed in the photocurable formulation until the liquid level touches the immersion groove on the spindle shaft. The temperature is set at 23 °C, rpm as 12 and sheer stress as 42% using LV2 spindle. The same instrumental set-up is followed for the preparation of other viscosities such as 4000, 3000, 2000 and 1000 cp. The viscosities and composition of the photocurable formulations are given in Table .
Table 1. The viscosity and composition of photocurable formulation of the mixture.
2.7. Photocrosslinking of acrylated prepolymer
The photocurable formulation containing the prepolymer, HVPA (70%), diluent, TEGDA (30%) and the photoinitiator, DMPA (0.25%) were coated on a thin glass slide for 25 mm thickness, and then exposed to UV-radiation at a distance of 3 cm from the source point. All irradiations were carried out by varying the DMPA concentration at various time intervals. The effect of DMPA concentration on percentage conversion, rate of polymerization (Rp), gel content (%) and hardness were studied.
2.8. Gel content (%)
The gel content (%) of the photocrosslinked polymers was performed in a Soxhlet extractor using acetone as solvent refluxing at 50 °C for 24 h. The swollen sample was isolated and subjected to vacuum drying for sufficiently long time to ensure complete drying. The gel content (%) was determined from the weight difference between the initial weight and final weight of sample.
2.9. Hardness testing
The relative hardness of each of the photocrosslinked polymer films was determined by A-Type JIS K-6301 hardness tester. The photocrosslinked polymer films of more than 25 mm thickness were used for the study. The readings were taken at several places on the same polymer film by pressing the needle of the tester. The average value was taken for hardness of the sample under test.
3. Results and discussion
The structural conformation of synthesized compounds, HPPP, BOMP and HVPA are carried out by FT-IR, 1H NMR and 13C NMR spectroscopy. The FT-IR spectral data of HPPP, BOMP and HVPA are given as follows: HPPP: (KBr, cm−1): 3416 (–O–H str.); 3137 (Ar–C–H str.); 2946 (–C–H asym. str.); 2890 (–C–H sym. str.); 1620 (Ar–C=C– str.); 1450 (–C–H def str.); 1295 (methyl–C–H str.); 1245 (–C–O str.). BOMP: (KBr, cm−1): 3414 (O–H str.); 3135 (Ar–C–H str.); 2944 (–C–H asym. str.); 2886 (–C–H sym. str.); 1638 (cyclic epoxy –C–H str.); 1617 (Ar–C=C str.); 1458 (methylene –C–H str.); 1294 (methyl –C–H str.); 1239 (epoxy ring half ring str.); 1243 (–C–O str.); 1146 (–C–O–C–str.); 827 (epoxy whole ring str.). HVPA: (KBr, cm−1): 3413 (–O–H str.); 3133 (Ar–C–H str.); 3050 (alkenes=C–H str.); 2943 (–C–H asym. str.); 2885 (–C–H sym. str.); 1745 (C=O str.); 1650 (alkenes –C=C– str.); 1616 (Ar–C=C–str.); 1456 (–C–H bending str.), 1358 (–C–H rock str.); 1293 (methyl C–H str.); 1241 (–C–O str.); 1145 (–C–O–C–str.); 810 (vinyl group –C–H bending str.); 723 (vinyl group C–H rock str.).
1H NMR (δ (ppm), CDCl3, 300 MHz) spectral data of HPPP, BOMP and HVPA are given as follows: HPPP: 1.72 (s, 3H, Ar–C–CH3), 5.22 (s, 2H, Ar–OH), 6.70–7.12 (d, 4H, Ar–H), 7.10–7.50 (d, 1H, Ar–H), 7.41–7.72 (d, 4H, Ar–H). BOMP: 1.71 (s, 3H, Ar–C–CH3), 2.23 (dd, 4H, –O–CH2–), 2.62 (dt, 4H, Ar–O–CH2–), 3.71 (m, 2H, –CH–O–), 6.67–7.12 (d, 4H, Ar–H), 7.10–7.45 (d, 1H, Ar–H), 7.40–7.70 (d, 4H, Ar–H). HVPA: 1.69 (s, 3H, Ar–C–CH3), 2.29 (d, 2H, –CH–OH–), 4.03 (m, 4H, Ar–O–CH2–), 4.29 (m, 2H, –CH2–CH–OH), 4.60 (m, 4H, –CH–OH–CH2–), 5.11 (d, 2H, –COO–CH), 5.52 (d, 2H, –CH=CH2 trans), 6.05 (d, 2H, –CH=CH2 cis), 6.31 (t, 2H, –CH=CH2), 6.88–7.10 (d, 4H, Ar–H), 7.07–7.46 (d, 1H, Ar–H), 7.39–7.68 (d, 4H, Ar–H).
The proton decoupled 13C NMR (δ (ppm), CDCl3, 300 MHz) spectral value of HPPP, BOMP and HVPA are given below: HPPP: 168.34, 140.13, 134.21, 126.13, 114.16, (Ar–C–), 155.31 (Ar–C–O–), 41.12 (Ar–C–CH3), 31.12 (Ar–C–CH3), BOMP: 168.43, 141.30, 135.17, 126.31, 114.24, (Ar–C–), 123.32 (Ar–C–O–CH2–), 72.61 (Ar–C–O–CH2–), 50.53 (–O–CH2–), 47.34 (–CH–CH2–O–), 45.27 (–CH–CH2–O–), 41.12 (Ar–C–CH3–), 31.10 (Ar–C–CH3). HVPA: 167.52 (–CO–O–CH2–), 160.34, 140.31, 134.78, 126.21, 112.41 (Ar–C–), 132.30 (–CH=CH2–), 127.22 (–CH=CH2), 122.32 (Ar–C–O–CH2–), 72.41 (Ar–C–O–CH2–), 69.34 (–CH–OH–), 50.34 (–CH–CH2–O–), 44.72 (–CH–CH2–O–), 41.05 (Ar–C–CH3), 31.03 (Ar–C–CH3).
3.1. Percentage double bond conversion
The percentage double bond conversion (PDC) reacted is calculated using the following equation:(1)
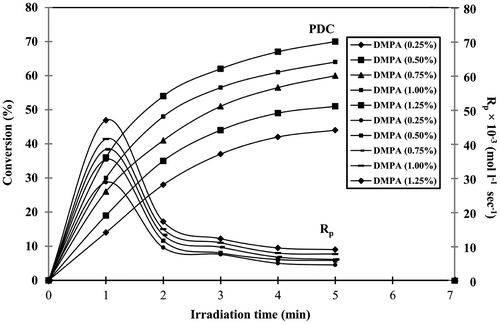
The decrease in the intensity of acrylated carbon–carbon double bond absorbance (Aacrylate) at 810 cm−1 was monitored. The effect of DMPA concentration on PDC is given in Figure . The PDC is increased with increase in the DMPA concentration. At 5 min irradiation time, keeping HVPA and TEGDA concentration as constant by increasing the DMPA concentration from 0.25 to 1.25%, the PDC values increases from 44 to 70%. This may be due to the formation of more free radicals during irradiation and involved in photocrosslinking polymerization reaction. By keeping HVPA, TEGDA and DMPA (0.25%) concentration as constant by increasing the irradiation time from 1 to 5 min, the PDC values are 14 and 44%, respectively. This shows that an increase in the irradiation time results in increase in PDC value which may be due to the availability of more UV energy resulting in the formation of extra free radicals, which participated in photocrosslinking reaction.
3.2. Effect of viscosity
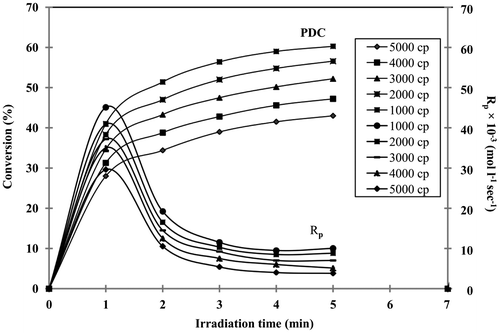
To study the effect of viscosity on PDC, the samples of different viscosities were mixed separately with the photoinitiator, DMPA (1.0%). Then photocurable formulation was placed in thin glass slides to give 25 mm film thickness and cured under UV-radiation at various time intervals. The viscosity of the system affects the kinetics of the photopolymerization, PDC, Rp, flexibility, hardness, gel content (%) and chemical resistance. The effect of viscosity on PDC is shown in Figure . The results show that when the viscosity decreases from 5000 to 1000 cp, the final PDC increases from 42 to 60%, respectively. The increase in the PDC value may be due to decrease in viscosity of the system and the mobility of the free radical reacting with prepolymer is increased.
3.3. Comparison to commercial prepolymer
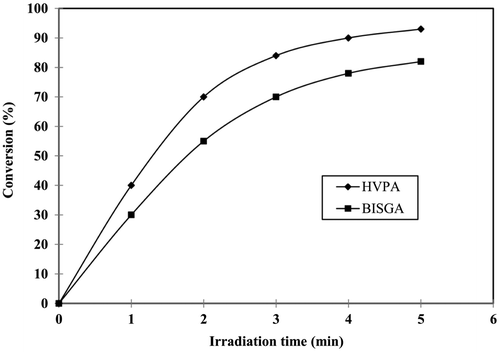
To study the comparison of PDC, two photocurable formulations were prepared using the synthesized prepolymer, HVPA (75%), and commercial prepolymer, BISGA (75%). They were mixed separately with the diluent, TEGDA (25%), in the presence of the photoinitiator, DMPA (2.5%), and cured under UV-radiation at various time intervals. Figure shows the comparison of PDC of the synthesized prepolymer, HVPA, and the commercial prepolymer, BISGA. The final PDC of BISGA and HVPA were found to be 82 and 93%, respectively. This may be due to the presence of phenyl group between two benzene rings which increases the reactivity.
3.4. Rate of polymerization
Rp can be calculated using the following equation:(2)
where [M0] is the monomer concentration before irradiation, (A810)t0, (A810)t1 and (A810)t2 which represent the absorption due to carbon–carbon double bond before and after the exposure during the time periods t1 and t2, respectively. The effect of DMPA concentration on Rp is given in Figure . The Rp depends on the PDC. At 1 min irradiation, keeping HVPA and TEGDA concentration constant, by increasing the DMPA concentration from 0.25 to 1.25%, the Rp values increases from 2.86 × 10−2 to 4.75 × 10−2 mol L−1 s−1. This may be due to the increase in the number of free radicals produced and subsequently participated in photopolymerization reaction. The effect of viscosity on Rp is given in Figure . The results shows that at 1 min irradiation, viscosity decreases from 5000 to 1000 cp, Rp increases from 2.98 × 10−2 to 4.53 × 10−2 mol L−1s−1. The increase in Rp may be due to the reduction in viscosity, which may increase the mobility of free radicals that react with acrylate.
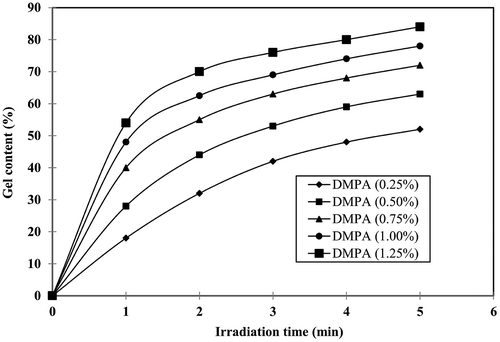
3.5. Gel content (%)
The gel content (%) of the photocrosslinked polymers can be calculated using the following equation:(3)
where W0 is the initial weight of polymer and W1 is the weight of the extracted polymer. Figure represents the effect of DMPA concentration on gel content (%). By keeping HVPA, TEGDA and DMPA (1.25%) concentration constant by increasing the irradiation time from 1 to 5 min, the gel content (%) values increases from 55 to 85%, respectively. The results show that gel content (%) increases with increase in irradiation time. At 5 min irradiation time, keeping HVPA and TEGDA concentration constant by increasing the DMPA concentration from 0.25 to 1.25%, the gel content (%) values increases from 50 to 85%, respectively. This may be due to increase in number of free radicals resulting in formation of highly crosslinked polymer.
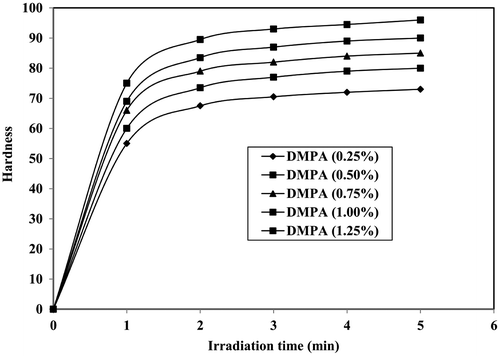
3.6. Hardness of photocrosslinked polymers
The hardness of photocrosslinked polymers were measured and presented in Figure . At 5 min irradiation time, keeping HVPA and TEGDA concentration constant by increasing the DMPA concentration from 0.25 to 1.25%, the hardness values increases from 71 to 96, respectively. This showed that the hardness of the crosslinked polymers increased with increasing DMPA concentrations, which was due to increase in the crosslink density of photocrosslinked polymers. By keeping HVPA, TEGDA and DMPA (1.0%) concentration constant by increasing the irradiation time from 1 to 5 min, the hardness value increases from 69 to 90, respectively. This is due to increasing number of free radicals resulting in the increase in crosslink density of photocrosslinked polymer.[Citation22]
4. Conclusions
The photocurable prepolymer, HVPA was synthesized from HPPP and BOMP, and confirmed by FT-IR, 1H NMR and 13C NMR spectroscopy. The photocrosslinking studies were performed by irradiating the photocrosslinkable mixture containing the HVPA with TEGDA in the presence of various concentrations of DMPA using medium pressure mercury vapour lamp. It was found that PDC, gel content (%) and hardness of the photocrosslinked polymers increased with increasing irradiation time and DMPA concentration, whereas Rp increased rapidly, then decreased and became constant. To compare PDC of the synthesized polymer, HVPA, and commercially available polymer, BISGA, the PDC of HVPA was higher than BISGA, which indicated that reactivity of HVPA was higher than BISGA, which showed that synthesized prepolymer, HVPA, is suitable for industrial applications such as coating, UV-curable inks, etc. The unique properties of the synthesized prepolymer – the PDC, reactivity and hardness – are higher and curing is also very fast. The production cost of the synthesized prepolymer, HVPA, is one-third of the actual cost of production of other commercial prepolymer.
Acknowledgements
The authors would like to thank Anna University, Chemistry Department, A. C Tech, Chennai, CLRI, Chennai and IIT, Chennai for helping us to conduct experiments for determination of the characterization of the polymers.
Additional information
Funding
References
- Fouassier JP. Photoinitiation, photopolymerization and photocuring: fundamentals and applications. New York (NY): Hanser; 1995.
- Decker C, Moussa K. Real-time kinetic study of laser-induced polymerization. Macromolecules. 1989;22:4455–4462.
- Fouassier JP, Ruhlmann D, Erddalane A. Photoinduced polymerization reactions in the presence of light stabilizers: reactivity of the photoinitiator in solution and in bulk. Macromolecules. 1993;26:721–728.10.1021/ma00056a025
- Holman R, Oldring P. UV and EB curing formulation for printing inks, coatings and paints. London: SITA; 1988.
- Pappas SP. UV curing science and technology. Stamford (CT): Marketing Corporation; 1985.
- Randell DR. Radiation curing of polymers. London: Royal Society of Chemistry; 1987.
- Roffey CG. Photopolymerization of surface coatings. New York (NY): Wiley; 1982.
- Thompson LF, Wilson CG, Bowden MJ. Introduction to microlithography. Washington (DC): American Chemical Society; 1994.
- Fouassier JF, Rabek JF. Radiation curing in polymer science and technology – volume II. London: Chapman and Hall; 1993.10.1007/978-94-011-1876-7
- Allen NS. Photopolymerisation and photoimaging science and technology. New York (NY): Elsevier; 1989.10.1007/978-94-009-1127-7
- Hoyle CE, Hensel RD, Grubb MB. Temperature dependence of the laser-initiated polymerization of a thiol-ene system. J. Polym. Sci., Part A: Polym. Chem. 1984;22:1865–1873.
- Andrzejewska E, Zych-Tomkowiak D, Andrzejewski M, Hug GL, Marciniak B. Heteroaromatic thiols as co-initiators for type II photoinitiating systems based on camphorquinone and isopropylthioxanthone. Macromolecules. 2006;39:3777–3785.10.1021/ma060240k
- Wang CS, Shieh J-Y. Synthesis and properties of epoxy resins containing bis(3-hydroxyphenyl) phenyl phosphate. Eur. Polym. J. 2000;36:443–452.10.1016/S0014-3057(99)00091-9
- Hourston DJ, Zia Y. Semi- and fully interpenetrating polymer networks based on polyurethane–polyacrylate systems. IV. Grafted semi-1-interpenetrating polymer networks. J. Appl. Polym. Sci. 1984;29:629–635.
- Li Z, Xiao M, Nie J. Synthesis and photopolymerization of 2-(acryloyloxy)ethyl pyrrolidine-1-carboxylate. Des. Monomers Polym. 2008;11:235–242.10.1163/156855508X316845
- Acosta Ortiz R, Savage Gomez AG, Berlanga Duarte ML, Garcia Valdez AE. The effect of a dithiol spiroorthocarbonate on mechanical properties and shrinkage of a dental resin. Des. Monomers Polym. 2015;18:73–78.10.1080/15685551.2014.947555
- Wang K, Wu W, Zhu X, Yu Q. Synthesis and photopolymerization of 2,2 di((acryloyloxy)methyl)butyl bis(2-(acryloyloxy)ethyl)carbamate. Des. Monomers Polym. 2012;15:31–39.10.1163/156855511X606128
- Xiao M, He Y, Nie J. Novel bisphenol a epoxide–acrylate hybrid oligomer and its photopolymerization. Des. Monomers Polym. 2008;11:383–394.10.1163/156855508X332522
- Li C, Cheng J, Chang W, Nie J. Photopolymerization kinetics and properties of a trifunctional epoxy acrylate. Des. Monomers Polym. 2013;16:274–282.10.1080/15685551.2012.747143
- Mohammed IA, Hamidi RM. Synthesis of new liquid crystalline diglycidyl ethers. Molecules. 2012;17:645–656.10.3390/molecules17010645
- Pan G, Du Z, Zhang C, Li C, Yanng X, Li H. Synthesis, characterization, and properties of novel epoxy resin containing naphthalene moiety. Polymer. 2007;48:3586–3693.
- Kim BK, Lee KH, Kim HD. Preparation and properties of UV-curable polyurethane acrylates. J. Appl. Polym. Sci. 1996;60:799–805.10.1002/(ISSN)1097-4628