Abstract
The concept of novel drug delivery systems aims at achieving systematically improved therapeutic success for drugs including those that have limited use because of toxicity, uneven distribution pattern, stability and formulation of difficulties. The paper reveals the potential uses of novel hybrids of oleic acid-g-chitosan and montmorillonite (OA-g-CS/MMT) in controlled drug delivery applications. A calcium channel blocker is a chemical that disrupts the movement of calcium (Ca2+) through calcium channels. The calcium channel blocker used for the present investigation is diltiazem hydrochloride, which forms a class three antianginal drug, and a class IV antiarrhythmic. The drug-loaded novel composites have been prepared by solvent casting and freeze drying of the grafted polymer solution. Drug release was monitored by changing time, percentage of drug loading and pH of the medium. It was observed that the release was much more pronounced in the basic medium than in the acidic medium. The maximum release of drug from OA-g-CS/MMT in both gastric and intestinal fluids was observed within a time period of 12 h.
1. Introduction
One of the most common ways of administering drugs to the body is via injection into the blood stream. The drawbacks of this delivery method are that, the concentration of the injected material is extremely diluted and the material acts on most of the tissues in the body, and may be toxic to some of them. This problem could be solved by the use of controlled drug delivery systems (CDDS). Due to the enhanced efficacy of existing potent drugs at reduced expenses and dosing schedules, CDDS draw significant attraction of drug delivery researchers. Moreover, conventional DDS exhibits fluctuating drug level in the blood, resulting in toxic side effects either by exceeding the maximum or falling below the minimum therapeutic level.[Citation1] Development in biotechnology has led to the development of alternative methods for painful injections, although, the developments have not been rapid enough to compete with the pressing demands.
The oral delivery route remains the most convenient system because it is non-invasive and patient-friendly.[Citation2] Owing to the poor permeability through the intestinal mucosa, the oral administration of biotherapeutic agents is difficult.[Citation3] To explore their therapeutic activity, orally administered biodrugs must be absorbed efficiently from the intestinal lumen into the circulation without being metabolized extensively in the intestine. The two main causes of the poor oral availability of the biodrugs include low permeability across the epithelial mucosa of the gastro intestinal tract and the lack of stability in the luminal environment.[Citation4]
The field of biopolymer–clay mineral composites has attracted attention [Citation5] for oral delivery applications owing to their excellent properties of easy degradation, biocompatibility and tunable mechanical properties.[Citation6] Polymer–clay composites are extending their applicability to the design of new drug release dosage forms with highly specific technological and biopharmaceutical properties, such as swelling, film formation, bioadheshion and cellular uptake.[Citation7]
Clay minerals can be considered as nanocontainers, which are capable of carrying bioactive molecules to the proper site of action at a correct release rate.[Citation8] Montmorillonite (MMT) clay is one of the members of the smectite group, composed of silica tetrahedral sheets layered between alumina octahedral sheets. MMT could absorb dietary toxins, bacterial toxins associated with gastrointestinal disturbance, hydrogen ions in acidosis and metabolic toxins, such as steroidal metabolites associated with pregnancy. Not only does MMT cure minor problems, such as diarrhoea and constipation through local application, it also acts on all organs as well. As a result, MMT is a common ingredient as both the excipient and active substance in pharmaceutical products.[Citation9] In the development of MMT-based controlled release formulations, modulation of their properties may be needed to improve their affinity for bioactive molecules. Both thermal and chemical treatments have been reported to be useful for this purpose.[Citation10] Intercalation with a polymer is one of these treatments: a polymer/clay composite, especially based on MMT is gaining attention because the composite can combine the advantages of a polymer with MMT.[Citation11] In MMT, the imperfection of the crystal lattice and the isomorphous substitution induces a net negative charge that leads to the adsorption of alkaline earth metal ions in the interlayer space. Such imperfection is responsible for the activity and exchange reactions with organic compounds.[Citation12]
Owing to its potential benefits including physiochemical properties, such as formation of gels, films, nanofibres and polycationic nature; and biological properties such as, biodegradability, biocompatibility, mucoadhesiveness, hemocompatibility, non immunogenic and metal binding ability,[Citation13–24] chitosan (CS), obtained by the alkaline deacetylation of chitin derived from the exoskeleton of marine crustaceans has emerged as a useful drug delivery matrix in the field of medicine. The chemical modification of CS by conjugating various functional groups allows the control of hydrophilicity and solubility at neutral and basic pH, thereby opening new opportunities to expand the application of this biopolymer. CS is potentially amenable to undergo chemical modification due to the presence of various functional groups or molecular chains.[Citation25] Oleic acid was chosen as the starting material for designing the intended compounds, because oleic acid has acceptable biocompatibility, which is a monounsaturated omega-9 fatty acid present in various animals and vegetables, and often used as an excipient in pharmaceuticals, and as an emulsifying or solubilizing agent in aerosol products.[Citation26] Through a simple modification of oleic acid by diamines, new amphiphilic molecules were obtained with great transformation in solubility (increase in organic solvents) and configuration (oil to solid). It has been reported that synthetic amphiphiles with cyclic sugars as hydrophilic moieties of oleic acid derivatives are convenient to produce chiral supramolecular fibres and ribbons by self-assembly in aqueous media,[Citation27–34] and the nanostructures can also be generated through a direct self-assembly of oleic acid on a hydroxylated aluminium surface.[Citation35]
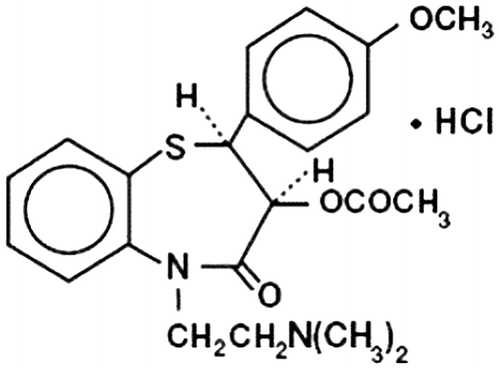
Diltiazem hydrochloride (Figure ), a nondihydropyridine member of the class of drugs known as calcium channel blockers, is used in the treatment of hypertension, angina pectoris and some types of arrhythmia. It is also an effective preventive medication for migraine. It is almost completely absorbed from the gastro intestinal tract (GIT) after oral doses, but undergoes extensive first-pass hepatic metabolism. Its short biological half-life and frequent administration makes it a potential candidate for sustained release preparations, which improve patient compliance.[Citation36]
This paper reveals the potential uses of novel hybrids of oleic acid-g-chitosan and montmorillonite (OA-g-CS/MMT) in controlled drug delivery applications. The drug-loaded novel composites have been prepared by solvent casting and freeze drying of the grafted polymer solution and they are well characterized. The drug release was monitored by changing time, percentage of drug loading and pH of the medium.
2. Experimental
2.1. Materials
CS (Mw = 450 kDa, 95% deacetylated degree) was obtained from Sigma Aldrich (USA). Sodium oleate was obtained from Nice Chemicals (Kerala, India) and was hydrolyzed with con.HCl, decanted and washed thrice with distilled water prior to use for removing impurities. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) obtained from Alfa Aesar was used as such. Diltiazem hydrochloride was a generous gift from Ultra Tech Labs, Kyrgystan. All other organic solvents were purchased from Merck and used as received.
2.2. Preparation of OA-g-CS/MMT
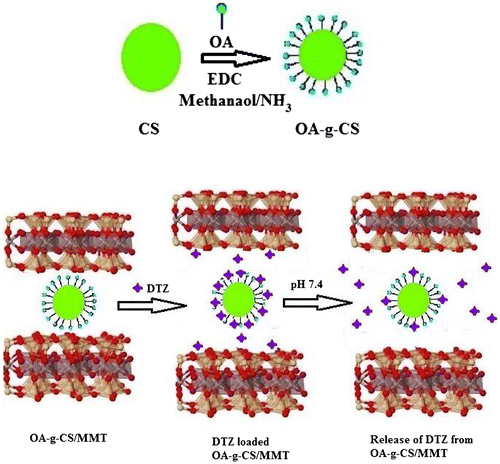
OA-g-CS copolymer was synthesized by a previously reported procedure, via the reaction of the carboxyl group of OA with the primary amino group of CS mediated by EDC. Briefly, CS (5 g) dissolved in 500 mL of 1% (w/v) acetic acid was diluted with 85 mL methanol. After attaining sufficient solubility, OA was added at 0.54 mol L−1. The solution was stirred for 24 h at the room temperature at about 300 rpm. After achieving completeness of the reaction, the reaction mixture was poured onto 250 mL CH3OH/NH3 solution with constant stirring. The precipitate was washed thrice with distilled water and dried at room temperature. To the prepared copolymer, different compositions of MMT (1%, 2%, 3%, 4% and 5%) were added and stirred at 60 °C for 3 h. The composites were allowed to dry at room temperature for three days and were collected for characterization. The schematic representation for the formation of OA-g-CS/MMT composites is represented in (Figure ).
2.3. Characterization of the drug carrier
The FTIR spectrum of different stages of formation of composites based on OA-g-CS/MMT were obtained using a BIORAD-FTS-7PC type FTIR spectrometer. The change in gallery height of the composite material were investigated by WAXD experiments, which was carried out using an X-ray diffractometer (BEDE D-3 system) with Cu Kα radiation at a generator voltage of 40 kv and a generator current of 100 mA. Samples were scanned from 2θ = 1–30° at a scanning rate of 2º/min. The surface morphology of the drug carrier comprising hybrids of OA-g-CS and MMT was analysed using SEM (440, Leica Cambridge Ltd., Cambridge, UK). The powdered specimens were placed on the Cambridge standard aluminium specimen mounts (pin type) with double-sided adhesive, electrically conductive carbon type. The specimen mounts were then coated with 60% gold and 40% palladium for 30 s with 45 mA current in a sputter coater. The coated specimens were then observed on the SEM using an accelerating voltage of 20 kv at a tilt angle of 30° to observe the morphology of the composites, and AFM analysis was performed using Bruker DIMENSION edge with SCAN ASYST instrument in tapping mode.
2.4. Drug loading
The drug diltiazem hydrochloride was loaded into the carrier by continuous shaking using shaking incubator at different pHs ranging from 4.0 to 7.5. Thus, 5 g of OA-g-CS/Na-MMT in powdered form was suspended in a 5% drug solution, water as solvent, under mild stirring and left to swell for 24 h. The absorption of the loaded drug was analysed using UV–visible spectrometer. This experiment was conducted at different concentrations 10, 15, 20 and 25% of the drug.
2.5. Drug content and encapsulation efficiency
The amount of drug (%) that is encapsulated in the formulation of composites indicates encapsulation efficiency (EE). The EE of drug from the composites can be calculated in terms of the ratio of drug in the final formulation to the amount of added drug. An accurately weighed amount (100 g) of the formulation of composites was dispersed in 100 mL of tris-HCl buffer. The sample is ultrasonicated for three consecutive periods for 5 min each, with a resulting period of 5 min each. It was left to centrifuge at 3000 rpm for 15 min. The concentration of DTZ in the decanted Tris–HCl buffer and two washing solutions was determined by measuring the absorbance at 237 nm using JASCO UV–visible spectrophotometer. The determinations were made in triplicate.
2.6. Equillibrium swelling studies
Swelling studies of the drug carrier were carried out in a buffer medium. Swelling measurements were performed by immersing dried OA-g-CS/ MMT taken in pre-weighed dialysis bag in the acidic buffer pH 1.2 and basic buffer pH 7.4. The weight of swollen nano-composites was measured at different times after removing the surface water with filter paper. Degree of swelling was calculated as follows:(1)
where Ws = weight of the swollen composite at a given time during swelling.
Wd = weight of the dry composite.
2.7. Dissolution studies
The dissolution studies were performed according to the United States of Pharmacopeia XX111 (USPXX111) dissolution methodology using a dissolution device (Caleve 10ST). A volume of 900-mL dissolution medium was placed in the vessel; temperature (37 °C) of the dissolution medium, paddle rotation speed (50 rpm) and dissolution time (5–6 h) was adjusted by the control unit of the device.
A drug may be expected to remain in stomach for 2–4 h (gastric emptying time) and in the small intestines for 4–10 h. The pH of the stomach varies from 1.0 to 3.0 and in intestines colon and: this value is between pH 7.0 and 8.0. To simulate the GIT, the pH of the dissolution medium was kept at 3.0 during the first 3.5 h of dissolution test, and then it was increased to 7.4, and was kept at that value until the end of the test. In the preparation of the dissolution medium, 8.5 v% phosphoric acid was added to the 900-mL distilled water to reduce pH to the value of 3.0. After 3.5 h, the pH of the medium was increased to 7.4 by adding 5.3 M NaOH to the solution. During the dissolution test, samples were withdrawn from the dissolution medium at certain times, and theconcentration of DTZ in the release medium was determined at 237 nm by using a spectrophotometer (JASCO 650).
2.8. Antimicrobial studies
The antibacterial activities of CS, OA-g-CS and OA-g-CS/MMT were measured according to the method described.[Citation37] The solutions of the samples (1000 mg L−1) were first prepared with 0.1 mol L−1 acetic acid, and then the pH was adjusted to 7.0 with 10% NaOH solution. The minimum inhibitory concentrations (MIC) of the composites were determined by two-fold serial broth dilution and the optical density method. The control contained only nutrient broth and 0.1 mol L−1 acetic acid without samples. After adjusting pH to 7.0 with 10% NaOH solution, all of the samples were inoculated under aseptic conditions with 50 μL of the inoculums of bacteria incubated at 37 ºC for 24 h, and then the turbidity of the cultured medium was mensurated at 610 nm. The lowest concentration of the composites that inhibited the growth of bacteria was considered as the MIC. To examine the effect of pH, the pH of the solution of the composites (1000 mg L−1) was adjusted to 4.0, 7.0 and 10.0 with 10% NaOH solution. The concentrations of both E. coli were adjusted to about 104 CFU mL−1 with sterile distilled water. Four milli litre composite solutions were added to 1 mL of the cell suspensions. The same volume of sterile distilled water was added to the control group. Samples were blended fully and removed after 5 min. Portions (50 μL) were spread on triplicate nutrient agar plates and incubated at 37 ºC for 24 h, and then the numbers of colonies were counted.
3. Results and discussion
3.1. Preparation and characterization of OA-g-CS/MMT
Chemical modification of CS with hydrophobic fatty acid OA has been successfully performed, and the product yield further confirmed carbodiimide as an efficient catalyst for the modification of CS. The prepared co-polymer (OA-g-CS) was then treated with different amounts of MMT to form novel hybrids of (OA-g-CS/MMT), which was used as formulations for the encapsulation and controlled release of DTZ. Drug encapsulation was evaluated at the physiological temperature of 37 °C with pH ranging from 4.0 to 8.0 and the optimization studies are depicted in Table .
Table 1. Optimisation of drug encapsulation.
During the encapsulation of the drug, a neutral pH was maintained at different loadings and the formulations loaded with higher amounts of the drug (25%) exhibited higher encapsulation efficiencies. The results of pH effect on the encapsulation of DTZ OA-g-CS/MMT in the pH range 4–8 were suggesting that the encapsulation of DTZ remains almost constant within experimental error. There was a sharp decrease in the encapsulation of DTZ in OA-g-CS/MMT, when the pH was above eight and below four. This can be explained based on the pKa value of DTZ. The pKa of DTZ is 7.7, which implies that at pH ≤ 7.4, the DTZ exists as mono-charged cations due to protonation of the amine.
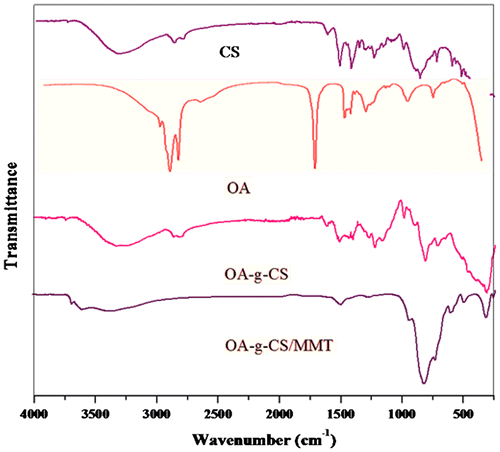
The characteristic vibration peak of CS and its derivatives were clearly observed (Figure ), and the broad peak at 3440 cm−1 was assigned to –OH and –NH stretching vibration and the weak peak at 2920 cm−1 was attributed to –CH stretch. The peak at 1030 cm−1 was due to the C–O–C stretching and the saccharine structure. The characteristic amide group vibration at 1739 and 1590 cm−1 became pronounced for the grafting of OA with CS. Peaks at 2924, 2854, 1464 and 1182 cm−1 characteristic of –CH2 vibrations confirmed the incorporation of the bulky methyl group onto CS. By virtue of amide formation, peak of the primary amino group in the range of 3000– 3600 cm−1 showed a significant decrease of the modified products. MMT shows characteristic absorption bands at 3400 cm−1 due to –OH stretching band for adsorbed water. The characteristic peak at 1115 and 1035 cm−1 is due to Si–O stretching (out-of-plane) and Si–O stretching (in-plane) vibration for layered silicates, respectively. Peaks at 915, 875 and 836 cm−1 are attributed to Al AlOH, AlFeOH and AlMgOH bending vibrations, respectively. IR spectral assignments for diltiazem hydrochloride revealed characteristic peaks at 3056, 2837, 2393, 1740, 1679, 839, 781 and 3035 cm−1 frequencies in the region of 400–4000 cm−1. Similar peaks were observed for the formulations, which indicate that there was no drug polymer interaction. Shifts of several absorption bands at 3614, 1634 and 1418 cm−1 were attributed to the formation of (OA-g-CS)/MMT hybrids.
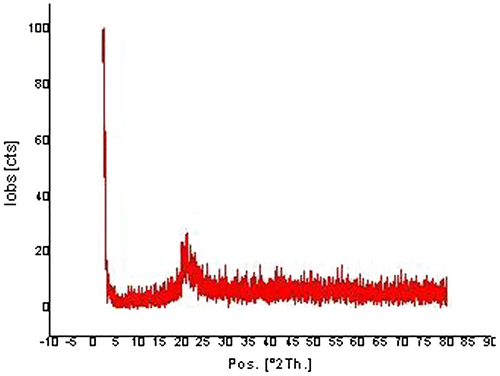
The XRD pattern of the composite matrix is shown in (Figure ). Results indicate that the crystalline structure of DTZ [Citation38] was absent in the diffraction pattern of the formulation indicating the conversion of crystalline to amorphous nature. Moreover, the XRD profile of MMT shows a diffraction peak at 2θ = 7.6º corresponding to the distance of clay sheets with d-spacing 1.16 nm.[Citation39] Incorporation of the polymer onto MMT leads to the formation of a composite (OA-g-CS)/MMT. No diffraction peak was observed in this final formulation and it can be concluded that the clay layers are completely exfoliated.[Citation40]
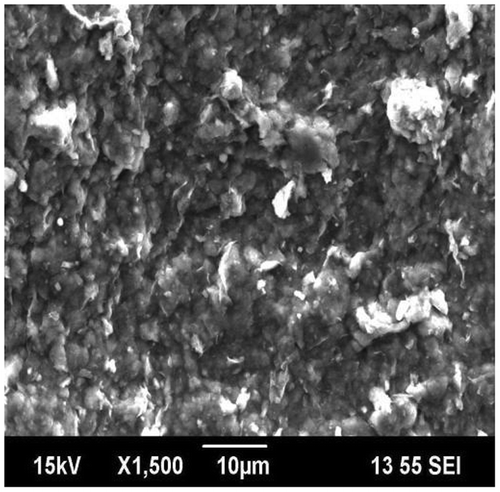
SEM is used to investigate the surface morphology of the drug carrier vehicle, which formed hybrids comprising OA-g-CS and MMT, which is represented in Figure . The composites of OA-g-CS/MMT contains coarse and indulant surface, and this observation can be attributed to the insertion of polymer onto clay leading to exfoliation. Moreover, OA-g-CS and MMT shows good compatibility in the hybrid, which is desirable for excellent drug encapsulation properties.
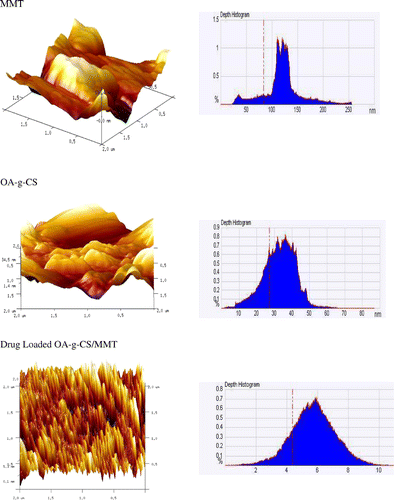
The AFM (3-D) images of MMT, OA-g- CS and 25% drug-loaded OA-g-CS /MMT shown in Figure . The images show difference in the surface properties of MMT and drug-loaded MMT. MMT posseses a rough surface and drug-loaded composites show the formation of high degrees of entanglement (agglomerations), emphasizing the encapsulation of drug inside the matrix.
3.2. Effect of MMT content on the swelling ratios of (OA-g-CS/MMT) composites
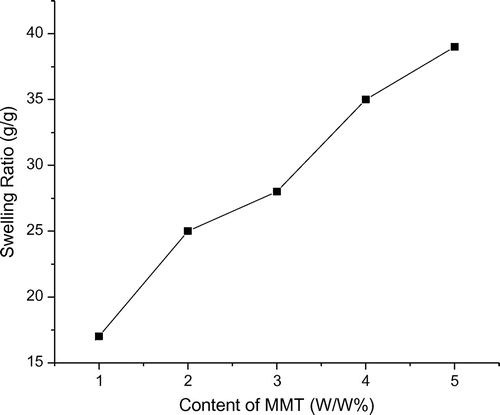
The weight ratios of MMT varied from 1.0 to 5.0% to study the effect of MMT content on the swelling ratios of (OA-g-CS/MMT) composites. With increasing the amount of MMT content, the swelling ratios of the composites are found to increase. The swelling ratios were found to be high for 5% MMT at pH 7.4, and MMT having high swelling capacity enhances the swelling degree of the composites (Figure ).
3.3. Effect of swelling medium pH
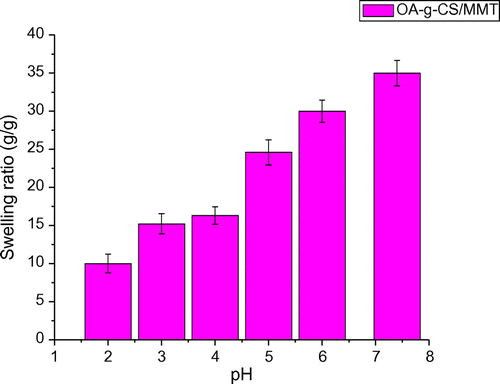
To investigate the equilibrium swelling behaviour of (OA-g-CS/MMT) composites at various pH levels, the samples are swollen in different buffer solutions of pH 2.0, 4.0, 6.0 and 8.0 with an ionic strength of 0.1 M at 37 °C. As shown in (Figure ), the swelling ratios of the composites increase with the increase in pH values. This is mainly attributed to the graft co-polymer formed between CS and OA. The ionization of the amino groups occurs as the solution becomes less acidic, resulting in the electrostatic repulsion between the ionized groups, which cause swelling degree of the hydrogel to reach to a relatively large value. This characteristic behaviour of a pH-sensitive controlled release system is desirable and effective as the pH of stomach is acidic, whereas that of intestine is basic.
3.4. Swelling studies
It is important to investigate the swelling behaviour of the composites under varied pH conditions because of the different pH in the gastric (pH 1–3) and the intestine (pH 7–8).The swelling behaviour of the polymer network depends upon the nature of the polymer, polymer solvent compatibility and degree of crosslinking. The swelling behaviour depends upon mass transfer limitations, ion exchange and ionic interaction in the case of ionic networks.[Citation41] It can be seen that swelling increases with time and levels off around 3 h at pH 1.2, and after 7 h at pH 7.4 .In addition, the swelling ratio of all the samples at pH 7.4 are higher compared with those of the same composites at pH 1.2, implying their swelling property is highly dependent on pH values. Owing to the high swelling capacity, MMT accounts for the enhanced swelling degree, which may act as a physical barrier for the moisture to exude from the composites. This water retention behaviour is favourable for cell adhesion. The hydrophilicity can be varied by tuning the extent of grafting and incorporation of clay. Structural ability and swelling behaviour of the carrier matrix strongly depends on the pH of the delivery site for practical use in biomedical applications. In nanohybrids, the biopolymers are intercalated in between the silicate layers and absorption, and desorption of fluids will be slower in a system which contains immobilized phases. Owing to their mucoadhesive properties, MMT incorporated nanohybrids can retain the stability of the material.
3.5. In vitro release studies
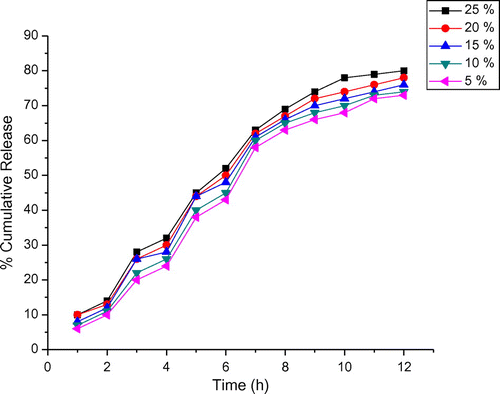
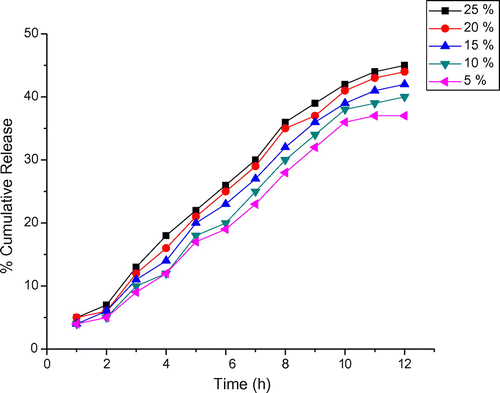
Drug release behaviour of the formulations was evaluated by performing the in vitro release experiments in simulated gastric (1.2) and intestinal pH (7.4) conditions. (Figures and ) show the percentage cumulative release of DTZ from the composite, and it is evident that increasing the pH from 1.2 to 7.4, a considerable increase in the cumulative release is observed. For both pH values, an initial burst release was observed, which may be due to the steric strain presented by the bulky units of OA at pH different from that of encapsulation. Moreover, 25% DTZ- polymer composites have shown longer drug release rates than the other composites containing different amounts of the drug. This suggests that the drug release behaviour of the composites is pH sensitive. Hence, the drug release depends on the pH of the medium and nature of the polymer matrix, and the results suggest that the drug in the matrix is suitable for the basic environment of large intestine, colon and rectal mucosa. More than 50% of the drug is released at pH 7.4 within 5 h. Owing to the large surface area and organized structures, MMT can accommodate substances in its gallery spaces to form composites. The interaction between silicate layers and guest molecules can be coulumbic interactions, van der Waals forces and hydrogen bonding. The release profile shows an initial burst release, which indicates that a significant amount of DTZ initially associated with the composites remained on their surfaces by weak interaction between OA-g-CS/MMT and DTZ. Due to the availability of more free void spaces through which a lesser number of drug molecules could transport, the release rate becomes quite slower at the lower amount of the drug. Drug release and molecular transport parameter were correlated using an empirical equation The percentage of DTZ released from the composite is 13% within 3 h in simulated gastric fluid (pH 1.2), and the equilibrium release in simulated intestinal fluid (pH 7.4) reaches nearly 80% of the initial drug content within 12 h.
3.6. Release mechanism studies
The release data of drug can be calculated by the equation(2)
where Mt = amount of drug release at time t
= total amount of drug released at infinite time
K = release constant and is related to the structural and geometric properties of the dosage form.
n = release exponent indicating the type of drug release mechanism.
This equation has been used frequently in the literature, due to its utility in describing the relative importance of Fickian (n = 0.5) and zero order (n = 1) mechanism in drug diffusion. In zero-order kinetics the release rate remains constant until the entire active ingredient has been delivered. The term zero-order means time-independent rate and so as being independent of quantity of drug remaining. According to Peppa’s equation, there are two distinct physical realistic meanings in the two special cases, where n values were calculated using the method of least squares. The release exponents of n, rate constant of log k and the correlation coefficient R2 from the matrix were obtained and they are listed in Table , indicating a release from erosion type to a swelling-controlled, non-Fickian-type mechanism.
Table 2. Values of n for different drug loadings at pH 1.2 and pH 7.4.
3.7. Antimicrobial studies
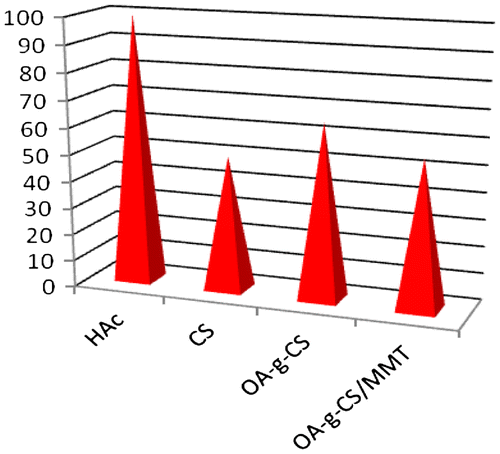
Solutions of CS, OA-g-CS, OA-g-CS/MMT and drug-loaded OA-g-CS/MMT were spotted in a colony of E. coli by the agar diffusion method. With increase in the concentration of OA-g-CS, the antimicrobial activity was found to increase.[Citation42] OA-g-CS/MMT developed a small zone compared to OA-g-CS. No zones were developed for drug-loaded OA-g-CS/MMT (Figure ). The zones developed by OA-g-CS/MMT assured the release of drug at pH 7.4, which was earlier determined by UV-vis spectrometer. The difference in the zonal development of OA-g-CS and OA-g-CS/MMT was evidence for the structural changes between the copolymer and the composite. The inactivity of OA-g-CS/MMT compared to OA-g-CS served as a proof for the effective encapsulation of drug. The material showed anti-bacterial activity as it involved CS, which has inherent anti-bacterial activity. Since, owing to the structure, the CS tail is exposed to the environment, it is free to exhibit its properties. However, in drug-loaded OA-g-CS/MMT, the shrinkage in size has forced the system to show no toxicity against bacteria. Moreover, after the controlled and complete release of the drug, the carrier vehicle comprising the polymeric material and clay system pose no threat to the body system as it is biodegradable and CS possessing antimicrobial activity can kill the micro organism, thereby making the system bio-compatible. In addition to biodegradability, it lowers toxicity as well as dosage frequency. The effect of pH on the anti-bacterial activity of composites was studied from pH 4.0 to 8.0 and upon increasing the pH, anti-bacterial activities of the composites also increased. As OA-g-CS shows strong antibacterial activity at pH 7.0,[Citation43] the prepared composites also exhibited more activity at pH 7.0, which could eliminate the effect of acetic acid on the bacterial strength.
4. Conclusions
Novel hybrids of chitosan-g-oleic acid and MMT as a delivery vehicle was formulated and loaded with different amounts of drug DTX. The swelling studies of the composites have been reported. The drug release was monitored by changing time, percentage of drug loading and pH of the medium and it was observed that the release was much more pronounced in the basic medium than the acidic medium. The percentage of DTZ released from the composite is 13% within 3 h in simulated gastric fluid (pH 1.2), and the equilibrium release in simulated intestinal fluid (pH 7.4) reaches nearly 80% of the initial drug content within 12 h. Moreover, after the controlled and complete release of the drug, the carrier vehicle comprising the polymeric material and clay system pose no threat to the body system, thereby making the material a potential candidate for the controlled release of DTX.
Acknowledgements
The authors thank the Head, Department of Chemistry, University of Kerala, Trivandrum, for providing laboratory facilities.
References
- Jogani V, Jinturkar K, Vyas T, Misra A. Recent patents review on intranasal administration for CNS drug delivery. Recent Pat. Drug. Deliv. Formul. 2008;2:25–32.
- Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery. Drug Discovery Today. 2006;11:905–912.10.1016/j.drudis.2006.08.005
- Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discovery. 2003;2:289–294.10.1038/nrd1067
- Colombo P, Sonvico F, Colombo G, Bettini R. Novel platforms for oral drug delivery. Pharm. Res. 2009;26:601–609.10.1007/s11095-008-9803-0
- Park JK, Choy YB, Kim JM, Hwang SJ. Clay-enriched silk biomaterials for bone formation. Int. J. Pharm. 2008;359:198–205.10.1016/j.ijpharm.2008.04.012
- Nagahara LA, Ferrari M, Grodzinski P. Nanofunctional materials in cancer research: challenges, novel methods, and emerging applications. MRS Bull. 2009;34:406–413.10.1557/mrs2009.116
- Nanda R, Sasmal A, Nayak PL. Preparation and characterization of chitosan-polylactide composites blended with cloisite 30B for control release of the anticancer drug paclitaxel. Carbohydr. Polym. 2011;83:988–998.10.1016/j.carbpol.2010.09.009
- Zheng H, Rao Y, Yin Y, Xiong X, Xu P, Lu B. Preparation, characterization, and in vitro drug release behaviour of 6-mercaptopurine carboxymethyl chitosan. Carbohydr. Polym. 2011;83:1952–1960.10.1016/j.carbpol.2010.10.069
- Aguzzi PC, Viseras C, Caramella C. Use of clays as drug delivery systems: possibilities and limitations. Appl. Clay Sci. 2007;36:22–34.10.1016/j.clay.2006.06.015
- Joshi GV, Kevadiya BD, Patel HA, Bajaj HC, Jasra RV. Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int. J. Pharm. 2009;374:53–62.10.1016/j.ijpharm.2009.03.004
- Viseras C, Cerezo P, Sanchez R, Salcedo I, Aguzzi C. Current challenges in clay minerals for drug delivery. Appl. Clay Sci. 2010;48:291–303.10.1016/j.clay.2010.01.007
- Fejer I, Kata M, Eros I, Berkesi O, Dekany I. Release of cationic drugs from loaded clay minerals. Colloid. Polym. Sci. 2001;279:1177–1186.
- Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998;24:979–989.10.3109/03639049809089942
- Kumar S, Dutta J, Dutta PK. Preparation, characterization and optical property of chitosan-phenothiazine derivative by microwave assisted synthesis. J. Macromol. Sci. Part A Pure Appl. Chem. 2009;46: 1095–1110.10.1080/10601320903256539
- Kumar S, Koch J. Physiochemical, circular dichroism-induced helical conformation and optical property of chitosan azo-based amino methanesulfonate complex. J. Appl. Polym. Sci. 2012;124:4897–4904.
- Schiffman JD, Schauer CL. Cross-linking chitosan nanofibers. Biomacromolecules. 2007;8:594–605.10.1021/bm060804s
- Kumar S, Dutta J, Dutta PK. Chitin and chitosan for versatile applications. Asian Chitin J. 2007;3:117–127.
- Nishat N, Rasool R, Parveen S, Manisha, Khan SA. Antimicrobial polychelates: synthesis and characterization of ransition metal chelated barbituric acid–formaldehyde resin. Int. J. Polym. Mater. Polym. Biomater. 2012;61:41–51.10.1080/00914037.2011.584226
- Mirzaei EB, Ramazani SA, Shefiee M, Danaei M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2013;62:605–615.10.1080/00914037.2013.769165
- Prasanna K, Deepthi MV, Ashamol A, Sailaja RN. Effect of nanoclay content and pH of the medium on the swelling characteristics of grafted and cross-linked mixed chitosan derivatives. Polym. Plast. Technol. Eng. 2013;52:352–360.10.1080/03602559.2012.721442
- Peng Z, Li Z, Shen Y. Influence of chemical cross-linking on prop-erties of gelatin/chitosan microspheres. Polym. Plast. Technol. Eng. 2012;51:381–391.10.1080/03602559.2011.639830
- Peng Z, Li Z, Shen Y, Zhang F, Peng X. Fabrication and properties of gelatin/chitosan composite hydrogel. Polym. Plast. Technol. Eng. 2012;51:739–748.10.1080/03602559.2012.663037
- Sadrolhosseini AR, Noor ASM, Moksin M, Abdi MM, Mohammadi A. Application of polypyrrole multi-walled carbon nanotube composite layer for detection of mercury, lead and iron ions using surface plasmon resonance technique. Int. J. Polym. Mater. Polym. Biomater. 2013;62:284–293.10.1080/00914037.2012.664209
- Yu Y, Jia F, Li S, Yan S, Ling C, Yuan K. Prerequisite characteristics of nanocarriers favoring oral insulin delivery: nanogels as an opportunity. Int. J. Polym. Mater. Polym. Biomater. 2013;62:450–460.10.1080/00914037.2012.719139
- Vishwanath B, Shivakumar HR, Sheshappa RK, Ganesh S, Prasad P, Guru GS, Bhavya B. In-vitro release study of metoprolol succinate from the bioadhesive films of pullulan-polyacrylamide blends. Int. J. Polym. Mater. Polym. Biomater. 2012;61:300–309.10.1080/00914037.2011.584227
- Sivakumar S, Bansal V, Cortez C, Chong SF, Zelikin AN, Caruso F. Degradable, surfactant‐free, monodisperse polymer encapsulated emulsions as anticancer drug carriers. Adv. Mater. 2009:1820–1832.
- Fuhrhop JH, Helfrich W. Fluid and solid fibers made of lipid molecular bilayers. Chem. Rev. 2003;93:1565–1575.
- Estroff LA, Hamilton AD. Water gelation by small organic molecules. Chem. Rev. 2004;104:1201–1212.10.1021/cr0302049
- Fuhrhop JH, Wang T. Bolaamphiphiles. Chem. Rev. 2004;104:2901–2911.10.1021/cr030602b
- Shimizu T, Masuda M. Stereochemical effect of even−odd connecting links on supramolecular assemblies made of 1-glucosamide bolaamphiphiles. J. Am. Chem. Soc. 1997;119:2812–2823.10.1021/ja961226y
- Nakazawa I, Masuda M, Okada Y, Hanada T, Yase K, Asai M, Shimizu T. Spontaneous formation of helically twisted fibers from 2-glucosamide bolaamphiphiles: energy-filtering transmission electron microscopic observation and even−odd effect of connecting bridge. Langmuir. 1999;15:4757–4766.10.1021/la981714e
- Masuda M, Shimizu T. Conformational and thermal phase behavior of oligomethylene chains constrained by carbohydrate hydrogen-bond networks. Carbohydr. Res. 2000;326:56–65.10.1016/S0008-6215(00)00020-3
- Shimizu T. Bottom up synthesis and structural properties of selfassembled high axial ratio nanostructures macromol. Rapid Commun. 2002;23:311–319.10.1002/1521-3927(20020401)23:5/6<311::AID-MARC311>3.0.CO;2-U
- Smitz E, Engmerts JBFN, Kellogg RM, Doren HAV. Fabrication of microspheres via solvent volatization induced aggregation of self-assembled nanomicellar structures and their use as a ph-dependent drug release system. Liq. Cryst. 1997;23:488–498.
- Liascukiene I, Aissaoui N, Asadauskas SJ, Landoulsi J, Lambert J. Lambert. Ordered nanostructures on a hydroxylated aluminum surface through the self-assembly of fatty acids. Langmuir. 2012;28:5116–5126.10.1021/la2051542
- Collares-Buzato CB, Jepson MA, Simmons NL, Hirst BH. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur. J. Cell Biol. 1998;76:85–96.10.1016/S0171-9335(98)80020-4
- Xing K, Chen XG, Li YY, Liu CS, Cha DS, Park HJ. Antibacterial activity of oleoyl-chitosan nanoparticles: a novel antibacterial dispersion system. Carbohydr. Polym. 2008;74:114–120.10.1016/j.carbpol.2008.01.024
- Moinuddin F, Subodh Mondal, Satish CS. Diltiazem hydrochloride trapping in poly (methacrylic acid-co-acrylamide) microparticles: in-vitro drug delivery studies. Int. J. Polym. Mater. Polym. Biomater. 2013; 62: 469–476.
- Van Olphen H. Wiley Intersciences. Published by Krieger Pub Co.; 1991.
- Mahdavinia GR, Marandi GB, Pourjavadi AG. Semi-IPN carrageenanbased nanocomposite hydrogels: synthesis and swelling behavior. J. Appl. Polym. Sci. 2010;118:2989–3000.10.1002/app.32700
- Holton RA. Synthesis of new beta-lactams. Eur. Patent Appl. EP 400971. 1990.
- Liu XF, Guan YL, Yang DZ, Li Z, Yao KD. Synthesis and property studies of N-carboxymethyl chitosan. J. Appl. Polym. Sci. 2001;79:1324–1333.
- Huang Lu, Cheng Xiaojie, Liu Chensheng. Preparation, characterization, and antibacterial activity of oleic acid-grafted chitosan oligosaccharide nanoparticles. Front. Biol. China. 2009;4:321–329.10.1007/s11515-009-0027-4