Abstract
Two aromatic diimide-diol monomers N,N′-bis(4-hydroxynaphthyl)oxydiphthalic diimide and N,N′-bis(4-hydroxynaphthyl)biphenyl tetracarboxylic diimide were synthesized from aromatic dianhydrides and 5-amino1-naphthol. Polyimides (PIs) and co-polyimides (co-PIs) were synthesized from these new diols and 4,4′-dichlorodiphenyl sulfone through nucleophilic displacement reaction. The structures of the monomers and PIs and co-PIs were characterized by FT-IR and NMR analysis. The PIs and co-PIs were characterized by X-ray diffraction (XRD), thermogravimetry, differential scanning calorimetry, solution viscosity, solubility test, and dielectric properties test. XRD studies indicated that these PIs and co-PIs were amorphous in nature and were soluble in organic solvents. The PIs and co-PIs showed excellent thermal stability and the 10% weight loss occurred in the range 390–452 °C. The limiting oxygen index values of these PIs and co-PIs were in the range 37.9–42.3 and the value suggests that these PIs and co-PIs can be used as self-extinguishing polymers. These PIs and co-PIs found to have a dielectric constant in the range 3.88–4.56 and have excellent electrical insulation character and can be used as high temperature electrical insulation materials.
1. Introduction
Aromatic polyimides (PIs) are one of the most important classes of high performance polymers (HPP) due to their excellent thermal, mechanical, and electrical properties.[Citation1–6] Polyimides are widely used in electrical, electronics, automotive, and aerospace industries in the form of films, fibers, foams, plastics, adhesives, and coatings.[Citation7,8] In polyimides, the thermal stability is imparted by the high regularity and rigidity of the backbone. The fabrication process for a polymeric material requires that the polymers be either soluble in organic solvents for a casting process or molten below their decomposition temperature for a molding extrusion process. However, aromatic polyimides are insoluble and infusible and very difficult to fabricate into desired articles.[Citation9] Many significant synthetic efforts have been made to improve the processability without decreasing thermal stability. In order to increase the processability, the main approach used for structural modifications includes (i) Incorporation of flexible linkages, it is common to use flexible units containing thermally stable groups such as –O–, –CO, –SO2–, –S–, and –C(CF3)2,[Citation10–13] (ii) Incorporation of kinks in the polyimides chain, the solubility of the polyimides can be increased by the incorporation of a kink in the polyimides backbone through ortho or meta catenation,[Citation14,15] (iii) Incorporation of large pendant groups along the backbone to prevent ordering of molecules,[Citation16–18] and (iv) Incorporation of co-polyimide units, the copolymer moiety disrupt chain symmetry and regularity and improve solubility.[Citation19–21] The commercial polyimides Ultem (General Electric company, USA) based on bisphenol-A(diphthalic anhydride-BPADA) and m-phenylene diamine having flexible –O– & –CH3 group and meta catenation showed good solubility and processability when compared to the corresponding plain polyimides.[Citation22] The objective of this research work was to investigate the effects of introducing flexible –O– & –SO2– linkages, bulky naphthyl group, and co-polyimides moiety on the properties of polyimides. PIs and co-PIs containing flexible units and bulky group were synthesized from two diol monomers. The flexible units, bulky naphthyl group, and co-polyimides moiety in the chain prevent the ordering of molecules, reduce the chain stiffness, and give flexibility, while the aromatic imides units provide rigidity to the polyimides and these two opposite effects on the properties of the PIs and co-PIs were investigated. A comparative study on the properties of the PIs and co-PIs with the reported polyimides for their solubility, thermal stability, and dielectric property was also presented. The PIs and co-PIs reported were studied for possible application as high temperature electrical insulation materials.
2. Experimental
2.1. Materials
The compound biphenyl tetracarboxylic dianhydride (BPDA-97%), oxydiphthalic anhydride (ODPA-97%), 5-amino-1-naphthol (99%), and 4,4′-dichlorodiphenyl sulfone (DCDPS-98%) were purchased from Sigma-Aldrich Chemicals, India. N-methyl-2-pyrrolidone (NMP-99.5%) and toluene (99.8%) were distilled under reduced pressure before use. The reagents pyridine (99.8%), o-cresol (99.0%), N,N-dimethyl formamide (DMF-98%), N,N-dimethyl acetamide (DMAc-99.8%), dimethyl sulfoxide (DMSO-99.9%), and tetrahydrofuran (THF-99.9%) used were analytical grade.
2.2. Measurements
FT-IR spectra were obtained on Spectrum-100 (Perkin Elmer, USA).The 1H-NMR and 13C-NMR spectra were recorded on AVANCE-II 300 MHz (Bruker Biospin, Switzerland). Wide angle X-ray diffractions (XRDs) for polyimides were obtained at room temperature on PANalytical model: X’per PRO using CuKα radiation. Thermogravimetry (TG) analysis was performed on SDT-600 (TA Instruments, USA) at a heating rate of 10 °C/min in nitrogen atmosphere. Differential scanning calorimetric (DSC) analysis was performed on Q20 (TA Instruments, USA) at a heating rate of 10 °C/min under nitrogen atmosphere. The dielectric constant (εr) was determined with HIOKI LCR HiTester 3532-50 at a frequency of 1 MHz. Inherent viscosities were determined at a concentration of 0.5 g/dL in DMSO. The solubility of PIs and co-PIs was determined in various organic solvents at room temperature and on heating.
2.3. Synthesis of monomers
2.3.1. N,N′-Bis(4-hydroxynaphthyl)oxydiphthalic diimide (IVa)
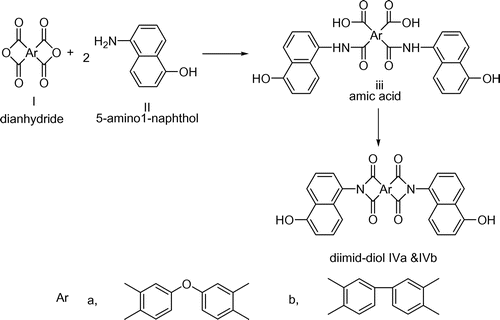
The stable aromatic dimide-diol monomers IVa and IVb were synthesized by the condensation of aromatic dianhydrides and 5-amino1-naphthol through nucleophilic substitution reaction as per the Scheme .[Citation23] The typical procedure adopted for the synthesis of monomer was as follows. To a stirred solution of 5-amino-1-naphthol (0.20 mol) in polar aprotic solvent NMP (60 mL), ODPA (0.10 mol) was added slowly under nitrogen atmosphere and was kept stirred at room temperature for 6 h for amic acid formation. Into the amic acid solutions, toluene (20 mL) was added and the resulting mixture was refluxed at 120 °C for 10 h. The water produced during the conversion of amic acid into imide was removed azeotropically using Dean–Stark trap. After removing the calculated amount of water, the temperature of the reaction mixture was raised to distill off the residual toluene. The reaction mixture was cooled, poured into water, and the precipitated diimide-diol monomer IVa was filtered, washed with excess of dil HCl to remove the unreacted 5-amino1-naphthol and washed with excess of water finally dried in vacuum oven at 80 °C for 24 h. Color: black, Yield: 96%, FT-IR (KBr, cm−1): 3420, 3080, 1775, 1717, 752, and 1375. 1H-NMR (300 MHz, DMSO-d6, ppm): δ 10.44 (s, 2H, Ar, –OH), δ 8.33–8.31 (d, 2H, Ar), δ 8.14–8.11 (d, 2H, Ar), δ 7.72–7.69 (m, 4H, Ar), δ 7.64–7.55 (m, 4H, Ar), δ 7.36–7.33 (t, 2H, Ar), δ 7.20–7.17 (d, 2H, Ar), and δ 6.97– 6.94 (d, 2H, Ar). 13C-NMR (300 MHz, DMSO-d6, ppm): δ 166.78, δ 166.61, δ 160.84, δ 153.59, δ 134.65, δ 131.54, δ 128.12, δ 127.65, δ 127.59, δ 127.34, 126.13, δ 125.31, δ 124.94, δ 123.99, δ 123.64, δ 113.86, δ 113.02, and δ 108.51.
2.3.2. N,N′-Bis(4-hydroxynaphthyl)biphenyl tetracarboxylic diimide (IVb)
The monomer IVb was synthesized from 5-amino-1-naphthol and BPDA using the same procedure as adopted for the synthesis of IVa. Color: brown, Yield: 95%, FT-IR (KBr, cm−1) 3500, 3095, 1770, 1715, 740, and 1385. 1H-NMR (300 MHz, DMSO-d6, ppm): δ 10.44 (s, 2H, Ar–OH), δ 8.52 (s, 2H, Ar), δ 8.47–8.45 (d, 2H, Ar), δ 8.34–8.31 (d, 2H, Ar), δ 8.17–8.15 (d, 2H, Ar), δ 7.67–7.57 (t, 4H, Ar), δ 7.34–7.31(t, 2H, Ar), δ 7.21–7.18 (d, 2H, Ar), and δ 6.97–6.95 (d, 2H, Ar).
2.4. Synthesis of PIs and co-PIs
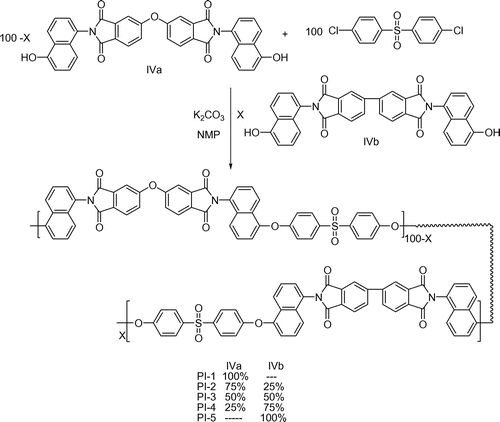
The polyimides (PI-1 and PI-5) and co-polyimides (PI-2 to PI-4) were synthesized by nucleophilic displacement reaction that involved diol monomer and DCDPS as per the Scheme .[Citation24] The procedure adopted for the synthesis of PI-1 was as follows. Into a 100-mL, two-necked, round-bottomed flask equipped with a Dean–Stark trap, diol monomer N,N′-bis(4-hydroxynaphthyl)oxydiphthalic diimide-IVa (0.01 mol), 20 mL of dry NMP, 20 mL of toluene and anhydrous K2CO3 (0.022 mol) were added. The reaction mixture was refluxed with constant stirring at 120 °C under nitrogen atmosphere for 10 h, while removing water azeotropically using Dean–Stark trap. After complete dehydration, the temperature of the system was raised to 160 °C and the remaining toluene was removed. The system was cooled and DCDPS (0.01 mol) was added. The reaction mixture was stirred at 180 °C for further 24 h under nitrogen atmosphere. After complete polymerization, the reaction mixture was cooled and then poured into water and the precipitated polyimide PI-1 was filtered, washed with excess of NaOH to remove unreacted diol monomer, and dried in vacuum oven at 120 °C for 48 h. Using the similar procedure, the polyimide PI-5 was synthesized using the monomer IVb. The co-polyimides PI-2 (75% IVa: 25% IVb), PI-3 (50% IVa: 50% IVb), and PI-4 (25% IVa: 75% IVb) were synthesized using IVa and IVb in various mol% with 4,4′-dichloro diphenyl sulfone.
3. Results and discussion
3.1. Structural characterization of monomers by FT-IR
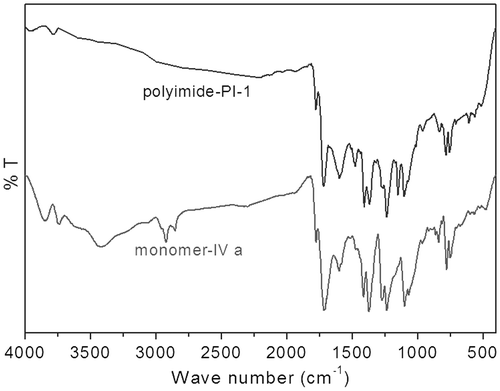
The FT-IR spectrum of monomer N,N′-bis(4-hydroxynaphthyl)oxydiphthalic diimide (Figure ) showed a characteristic absorption at 1775 cm−1 (imides C=O symmetrical stretching), 1717 cm−1 (imides C=O asymmetrical stretching), 752 cm−1 (imide ring deformation), and 1375 cm−1 (C–N stretching) associated with imide ring indicated complete imidization between 5-amino-1-naphthol and ODPA. Phenolic hydroxyl group showed a strong absorption peak at 3420 cm−1 and the aromatic C–H absorption band appeared at 3080 cm−1. Similarly, the FT-IR spectral data of monomer IVb supported the formation of imide ring between BPDA and 5-amino-1-naphthol.
3.2. Structural characterization of monomers by 1H-NMR and 13C-NMR
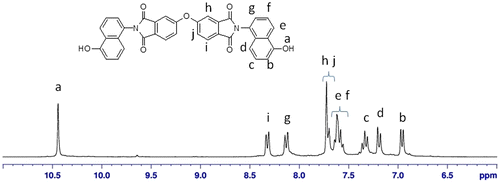
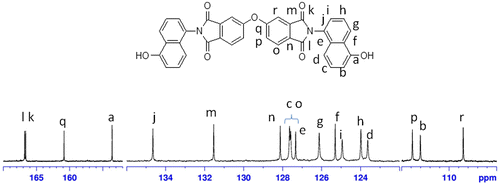
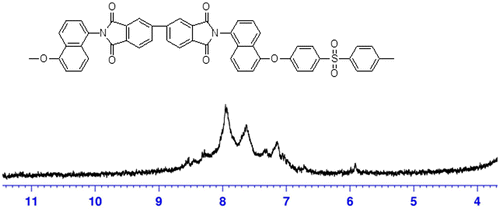
The 1H-NMR spectrum of monomer N,N′-bis(4-hydroxynaphthyl)oxydiphthalic diimide (IVa) is given in Figure . The phenolic proton appeared as a singlet at 10.44 δ ppm. The aromatic protons ortho and para to the hydroxyl group appeared as a doublet at δ 6.97–6.94 ppm and δ 7.20–7.17 ppm, respectively. The aromatic protons meta to the hydroxyl group appeared as a triplet at δ 7.36–7.33 ppm. The aromatic protons designated as ‘e’ and ‘f’ in Figure merged together and appeared as a multiplet at δ 7.64–7.55 ppm. The aromatic proton ortho to the imide nitrogen atom and designated as ‘g’ appeared as a doublet at δ 8.14–8.11 δ ppm. The singlet protons designated as ‘h’ and the doublet protons designated as ‘j’ merged together and appeared at δ 7.72–7.69 ppm. The protons designated as ‘i’ appeared as doublet at δ 8.33–8.31 ppm. The protons designated as ‘h’, ‘i’, and ‘j’ appeared in the farthest downfield region in the range of δ 7.69–8.36 ppm because of the presence of electron withdrawing imide groups. The 1H-NMR spectrum supported the formation of monomer IVa between ODPA and 5-amino 1-naphthol. The 13C-NMR of the monomer N,N′-bis (4-hydroxynaphthyl)oxydiphthalic diimide along with the assignments of the carbon atoms is shown in Figure , and DEPT-135 spectrum was also recorded in order to confirm the structure by way of assigning protonated and non-protonated carbon atoms of the monomer. The NMR analysis of diol monomer IVb was also in accordance with the proposed structure.
3.3. Synthesis and characterization of PIs and co-PIs
The results of polymerization are given in Table . The inherent viscosities that are measures of molecular weight of the polymers, measured at room temperature were in the range 0.30–0.46 dL/g indicating the formation of polymer of moderate molecular weight. The absence of peak for the phenolic protons in 1H-NMR of all PIs and co-PIs supported the formation of polymer but not an oligomer.
Table 1. Properties of PIs and co-PIs.
3.4. Structural characterization of PIs and co-PIs by FT-IR
The FT-IR spectrum of the polyimides PI-1 is given in Figure , which showed a characteristic absorption band at 1777 cm−1 (imide C=O symmetrical stretching), 1715 cm−1 (imide C=O asymmetrical stretching), 755 cm−1 (imide ring deformation), and 1367 cm−1 (C–N stretching) associated with imide ring indicated that the imide ring of the diol monomer was stable during the high temperature nucleophilic displacement polymerization reaction. The disappearance of strong absorption due to –OH group at 3420 cm−1 that was present in the FT-IR of the monomer and the appearance of the new stretching at 1140 cm−1 (S=O, symmetrical stretching) corresponds to the –SO2– group indicated complete nucleophilic displacement polymerization of –Cl atom of DCDPS by –OH group of diimide-diol monomer and successive formation of aromatic ether bonds occurred. Similarly, the FT-IR spectral data of the polyimides PI-5 and co-polyimides (PI-2, PI-3, and PI-4) also supported the proposed structures.
3.5. Structural characterization of PIs and co-PIs by 1H-NMR
The 1H-NMR spectrum of PI-1 (100% mol IVa) is given in Figure showed signal in the range δ 7.20–7.80 ppm. The disappearance of the singlet peak that was present in the 1H-NMR of the monomer IVa at δ 10.44 ppm for the phenoloc protons indicated the complete polymerization of diol monomer. The observed 1H-NMR spectra were fully consistent with the chemical structure of the polyimide PI-1. Similarly, the 1H-NMR spectrum of PI-5 (100% mol IVb) is given in Figure , which showed signals in the range 7.14–7.96 δ ppm and supported the proposed structure. The FT-IR and 1H-NMR spectral data are consistent with the proposed structures for PIs and co-PIs. In all the 1H-NMR of the polyimides, the absence of detectable peak for phenolic protons indicated complete conversion of monomer into polymer.
3.6. Solubility characteristics of PIs and co-PIs
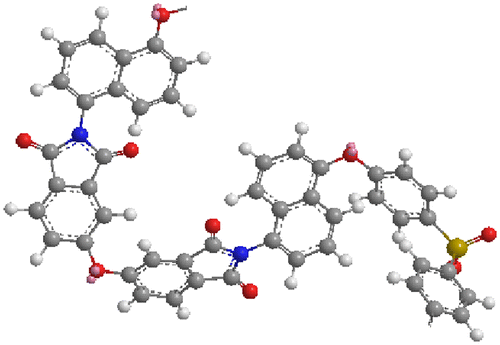
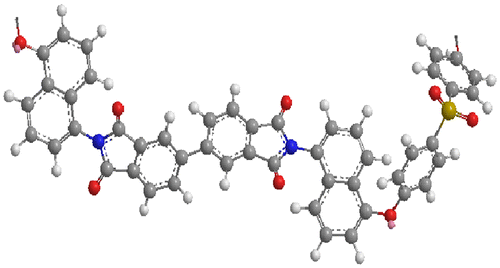
The solubility of the PIs and co-PIs was checked at 5.0 wt% concentration in different organic solvents at room temperature or on heating. The solubility characteristics of the PIs and co-PIs are given in Table . All the PIs and co-PIs were soluble in polar aprotic solvents NMP and DMSO at room temperature. However, they were insoluble in less polar solvents including toluene and THF. The PI-1 based on ODPA showed higher solubility than the polyimides PI-5 based on rigid BPDA. This is because PI-1 has flexible –O– group and was supported by the molecular modeling of the polyimides. The molecular modeling of the polyimide PI-1 (Figure ) showed more twisted structures deviating from the linearity than the polyimides PI-5 (Figure ). Due to such a twisted shape, the dense packing of the chains was disturbed which led to increase in free volume, the diffusion of solvents molecules between the polymer chains was facilitated and that led to better solubility. The co-polyimides PI-3 (50% IVa and 50% IVb) which has twisted structure undergo swelling in DMF, DMAc, THF, and o-cresol. An increase in particle size was observed visually within a minute and such polymers can be used to remove the organic solvent impurities. The co-polyimides containing more percentage of ODPA moieties showed higher solubility. The solubility increased with increasing flexibility of polyimides chain. The solubility of the polyimides was found to decrease in the following order PI-1 > PI-2 > PI-3 > PI-4 > PI-5. The solubility behavior was comparable with the PIs and co-PIs reported by Yang et al. [Citation25] derived from 1,1-Bis[4-(4-aminophenoxy)phenyl]-1-phenyl ethane (BAPPE) and aromatic dianhydrides containing flexible –O– & –CH3 and phenyl units as side group.
Table 2. Solubility characteristics of PIs and co-PIs.
3.7. Thermal properties of PIs and co-PIs
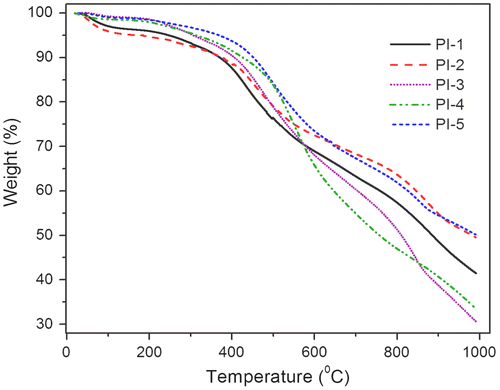
The thermal stability of the PIs and co-PIs was evaluated by TG analysis. The TG curves for the polyimides are given in Figure , and the thermal analysis data are summarized in Table . All the PIs and co-PIs showed similar decomposition behavior. The temperature for 10% gravimetric loss (T10) is an important criterion for the evaluation of thermal stability in heat resistant PIs and was found to be in the range 390–452 °C and is comparable with the PIs and co-PIs reported by Koohmareh et al. which has 10% weight loss in the range 395–560 °C.[Citation20] The char yield at 800 °C was in the range 48–62% and is comparable with the PIs and co-PIs (42–68%) reported by Koohmareh et al. The high char yields of these PIs and co-PIs may be due to the presence of rigid imide group and naphthalene moiety in the chain backbone. The maximum degradation (Tmax) of the PIs and co-PIs was occurred at a temperature in the range 475–550 °C. The PI-5 (100% IVb/DCDPS) showed higher thermal stability and high char yield when compared to the other polyimides, which can be attributed to rigid imides ring based on BPDA unit in the polyimides chain and close chain–chain packing of the PI-5 molecules. Similarly, the PI-1 (100% IVa/DCDPS) showed less thermal stability which might be due to the presence of flexible ODPA unit in the chain which led to less ordering of chain. TG analysis indicated that these PIs and co-PIs were thermally stable and were comparable with the commercially available and literature polyimides.
Table 3. Thermal properties of PIs and co-PIs.
3.8. Limited oxygen index – self-extinguishing polymer
The limiting oxygen index (LOI) test is commonly used to assess the relative flammability of a material by studying its tendency to sustain a flame by varying the oxygen concentration in a small enclosure. The LOI corresponds to the minimum volume concentration of oxygen in an oxygen/nitrogen mixture required to just support the burning of a test specimen. LOI values for different materials were determined experimentally. Since air comprises about 21% oxygen by volume, any material with a LOI less than this will burn easily in air, while those with a higher LOI will not burn. The higher the value of LOI, the safer the material. Theoretically, char yield can also be used as criteria for evaluating LOI of the polymer in accordance with Van Krevelen and Hoftyzer equation.[Citation26]
The LOI values of PIs and co-PIs calculated were in the range 37.9–42.3 (Table ) confirming the usefulness of these polymers as flame retardants. Such PIs can be classified as self-extinguishing polymers. The LOI values are close to the commercial Kapton® film (LOI = 37) derived from pyromellitic anhydride and oxydianiline. All the samples of the present study can effectively be used as flame retardant materials. The LOI values of the polymers were increased by incorporation of diimide, and it was found to be highest in the case of plain polyimides PI-1 and PI-5 and the LOI value decreased for random co-polyimides.
3.9. Phase transitions of PIs and co-PIs
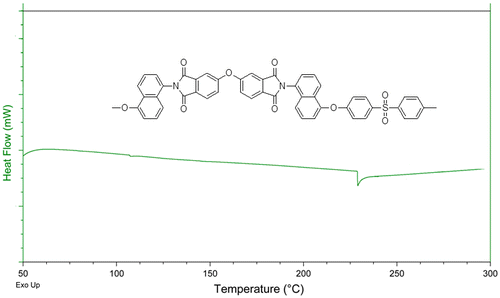
Tg is an important parameter to decide on the characteristic and applications of a polymer. The glass transition temperature exhibited by a polymer is dependent upon the chain flexibility, interaction, and structure (chain chemical makeup of the material). In general, factors increasing the stiffness of the polymeric molecular segments will tend to increase Tg, while the flexible chain will have low Tg. The Tg values of the PIs and co-PIs were evaluated by DSC during second scan in nitrogen atmosphere at a heating rate of 10 °C/min. Representative DSC curve is given for the PI-1 in Figure . The Tg values were observed clearly for the polyimides PI-1(228 °C) and PI-5 (280 °C), co-polyimides PI-4 (270 °C). The Tg values were close to the commercially available polyimides (laRC-TPI, Tg = 264 °C, Ultem, Tg = 220 °C). The Tg value was high for PI-5 because of the rigid backbone based on BPDA. The Tg value was low for PI-1 because of the ODPA backbone which has flexible ether linkages and prevents ordering of the polymer chain. In the random co-polyimides PI-4, there are no longer two distinct phases and the random copolymers exhibit a single Tg at 270 °C which lies between the two Tg’s of the individual polymer comprising the co-polyimides. For the co-polyimides, PI-2 and PI-3 the change in the sample’s Cp at Tg may be smaller and might not be detected by the DSC instrument.
3.10. XRD and morphology of PIs and co-PIs
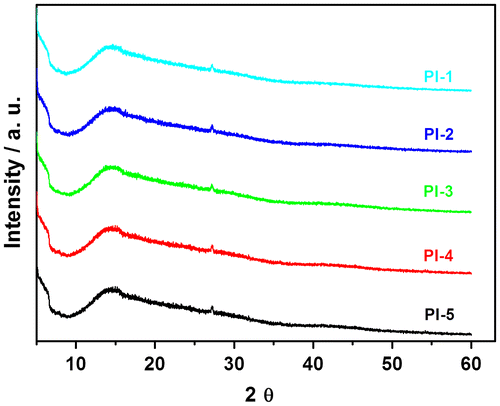
In order to study the crystalline or amorphous nature of the PIs and co-PIs and investigate the effect of flexible –O– & –SO2– group and bulky naphthalene moiety on the crystallinity extent, WXRD measurements at room temperature in the region of 2θ = 5–60° were done for all PIs and co-PIs. XRD patterns of the PIs and co-PIs are given in Figure . The diffractograms indicated that in the wide angle region, the PIs and co-PIs have very broad peaks at about 2θ = 15°. These peaks are so broad that they may be considered as amorphous halos. Weak reflections were observed for all the PIs and co-PIs at 2θ = 27° and the interplanar distance the ‘d’ were 3.30 Å. The amorphous nature of these PIs and co-PIs was reflected in their solubility, which is in agreement with the general rule that the solubility increases with increasing amorphous character. This amorphous character was due to the presence of flexible –O– & –SO2– group and bulky naphthalene moiety, which prevent the chain–chain interaction leading to amorphous morphology. The XRD studies were supported by solubility behavior and molecular modeling.
3.11. Application of PIs and co-PIs – high temperature electrical insulation materials
Polyimides are used as electrical insulation materials and more desirable than any other materials because they are thermally stable at temperatures exceeding 250 °C, twice the operating temperature of most common dielectric materials. Polyimides would not lose their dielectric stability even at high temperature but most common polymers used as dielectric materials exhibit severe decrease in dielectric strength around 70 °C due to phase transition of the polymer.[Citation27] The dielectric constant is an important parameter for selecting electrical insulation material.[Citation28] The dielectric constants of the PIs and co-PIs determined at 1 MHz are presented in the Table and are in the range 3.88–4.56. The plain polyimides PI-5 (100% IVb) has less free volume among all and has high dielectric constant 4.56, which can be attributed to rigid imides ring based on BPDA unit in the polyimides chain and close chain–chain packing of the PI-5 molecules. The co-polyimides PI-3 (made up of 50% IVa and 50% IVb) have high free volume showed lowest dielectric constant 3.88. The plain PI-1(100% IVa) having flexible ether and pendant group has the dielectric constant 3.98, which might be due to the presence of flexible ODPA unit in the chain which led to less ordering of chain. The co-polyimides PI-2 (75% IVa and 25% IVb) and PI-4 (25% IVa and 75% IVb) have high free volume than the PI-5 has dielectric constant 4.30 and 4.32, respectively. The dielectric property indicated that PIs and co-PIs have electrical insulation character and are comparable with the commercial polyimide Kapton-Du Pont Co.,(є = 3.40) and UPILEX-ICI America.(є = 3.30–3.50). PIs and co-PIs can be used as insulation materials for electrical items such as cables, generators, electric motors, and other units operating at elevated temperature.
4. Conclusion
We have successfully synthesized diimide-diols monomers and PIs and co-PIs with high yield. All the PIs and co-PIs exhibit excellent solubility, and the PI-3 undergoes swelling and can be used to remove the organic solvents impurity. All the PIs and co-PIs exhibit good thermal stability and the LOI values of PIs and co-PIs were in the range of 37.9–42.3, such PIs can be used as self-extinguishing polymers. The dielectric constants were in the range 3.88–4.56. PIs and co-PIs can be used as insulation materials for electrical items such as cables, generators, electric motors, and other units operating at elevated temperature. Thus, these aromatic PIs and co-PIs are considered as processable HPP.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yafeng G, Yuming Z, Man H, Haiyun W, Yiping C, Tong Z. Synthesis and characterization of fluorinated polyimides derived from 1,4-bis-[4-amino-2-(trifluoromethyl)-phenoxy] benzene/tetrafluoride benzene. Des. Monomers Polym. 2014;17:590–600.
- Mallakpour S, Zadehnazari A. Tailored synthesis of nanostructured polymer thin films from optically active and thermally stable poly (amide-co-imide)s containing hydroxyl pendant groups in a green ionic solvent. Polym. Plast. Technol. Eng. 2012;51:1097–1105.10.1080/03602559.2012.689060
- Thiruvasagam P. Synthesis and characterization of AB-type monomers and polyimides – a review. Des. Monomers Polym. 2013;16:197–221.10.1080/15685551.2013.771307
- Thiruvasagam P, Venkatesan D. Synthesis and characterization of processable aromatic polyimides. High Perform. Polym. 2010;22:682–693.10.1177/0954008309360932
- Parhami A, Shojai Nasab S, Abbasi M, Jafarazad R, Bahmani R. Synthesis and characterization of novel nanostructured, organosoluble, thermally stable and wholly aromatic poly(amide-imide)s from a new diimide-diacid monomer by direct polycondensation. Des. Monomers Polym. 2014;17:736–745.10.1080/15685551.2014.918012
- Paşahan A, Koytepe S, Ekinci E. Synthesis characterization of a new organosoluble polyimide and its application in development of glucose biosensor. Polym. Plast. Technol. Eng. 2011;50:1239–1246.
- Huo H, Sun H, Mo S, Yang S, Fan L. Preparation of meltable aromatic polyimides and their adhesive properties. J. Macromol. Sci. Pure Appl. Chem. 2011;48:880–889.10.1080/10601325.2011.614853
- Gholam AK. Synthesis and characterization of new disperse-red functionalized polyimide for use as nonlinear optical. Des. Monomers Polym. 2012;15 275–288
- Yang HH. Aromatic high strength fibers. New York, NY: Wiley; 1989.
- Harris FW, Beltz MW, Hergenrother PM. New readily processable polyimides. SAMPLE Journals. 1987;23:6–9.
- Thiruvasagam P, Vijayan M. Synthesis of poly(amide-imide)s from new diacid monomers: study of structure-property relationship and applications. High Perform. Polym. 2012;24:210–217.10.1177/0954008311435800
- Thiruvasagam P, Vijayan M. Synthesis of new diacid monomers and poly(amide-imide)s: study of structure–property relationship and applications. J. Polym. Res. 2012;19:9845.10.1007/s10965-012-9845-1
- Thiruvasagam P, Venkatesan D. Synthesis of diimide-diacid monomers and poly(amideimide)s: effects of flexible linkages and pendant hexafluoro isopropylidine unit on processability, thermal stability and electrical properties. Polym. Plast. Technol. Eng. 2012;51:1133–1140.10.1080/03602559.2012.680564
- Maiti S, Das S. Synthesis and properties of polyesterimides and their isomers. J. Appl. Polym. Sci. 1981;26:957–978.10.1002/app.1981.070260319
- Fang X, Wang Z, Yang Z, Gao L, Li Q, Ding M. Novel polyimides derived from 2,3,3′,4′-benzophenonetetracarboxylic dianhydride. Polymer. 2003;44:2641–2646.10.1016/S0032-3861(03)00181-2
- Thiruvasagam P. Synthesis and characterization of new unsymmetrical diamine monomer and polyimides. Des. Monomers Polym. 2014;17:166–175.10.1080/15685551.2013.840497
- Zeng K, Zhou S, Fan H, Yang G. Synthesis and characterization of highly organosoluble polyimides based on a new asymmetric dianhydride. Des. Monomers Polym. 2014;15:53–62.
- Tan LS, Simko SR. Aromatic polyimides based on an asymmetrically benzamide-pendanted benzidine. Polymer Preprint (Am. Chem. Soc., Div. Polym. Chem). 1995;36:441–442.
- Vinogradova SV, Churochikina NA, Vygodakii Ya A, Korshak VV. Synthesis and properties of mixed cardic polyimides. Polym. Sci. U.S.S.R. 1973;15:1922–1926.
- Gholam AK, Najmeh M. Synthesis and characterization of some new thermally stable polyimides and copolyimides bearing bipyridine side-chain groups. J. Polym. Res. 2011;18:983–991.
- Chao M, Kou K, Wu G, Zhang J, Li N, Zhang D. Synthesis and properties of semicrystalline copolyimides based on 4,4′-diamino diphenylether and 1,3-bis(4-aminophenoxy) benzene. J. Macromol. Sci. Phys. 2012;51:2003–2014.
- de Abajo J, de la Campa JG. Processable aromatic polyimides. Adv. Polym. Sci. 1999;140:23–59.10.1007/3-540-49815-X
- Mehdipour-Ataei S, Keshavarz S. Use of new diimide-dinaphthols in preparation of novel thermally stable poly(ester-imide)s. J. Appl. Polym. Sci. 2003;89:2567–2572.10.1002/(ISSN)1097-4628
- Sayyed MM, Maldar NN. Novel poly (arylene-ether-ether-ketone)s containing preformed imide unit and pendant long chain alkyl group. Mater. Sci. Eng., B. 2010;168:164–170.10.1016/j.mseb.2009.11.015
- Yang CP, Chen RS, Hsu MF. Synthesis and properties of soluble 3,3,4,4-benzo phenone tetracarboxylic dianhydride copolyimides based on 1,1-bis[4-(4-amino phenoxy)phenyl]-1-phenylethane and commercial dianhydrides. J. Polym. Res. 2002;9:245–250.10.1023/A:1021355819315
- Mallakpour S, Zeraatpisheh F. Novel heat resistant nanostructure poly(amide–imide)s containing new TMA-based diacid via conventional polycondensation reaction in an ionic green medium: synthesis, morphology, and thermal properties. Des. Monomers Polym. 2013;16:313–322.10.1080/15685551.2012.747147
- Aaron FB, Rui M, Chenchen W, Rampi R, Gregory AS. Structure–property relationship of polyimides based on pyromellitic dianhydride and short-chain aliphatic diamines for dielectric material applications. J. Appl. Polym. Sci. 2013;130:1276–1280.
- Ghaemy M, Berenjestanaki FR, Bazzar M. Organosoluble, thermally stable and low dielectric constant fluorinated polyimides containing 2,4,5-triphenylimidazole moiety in the main chains. Des. Monomers Polym. 2014;17:101–110.10.1080/15685551.2013.840473