Abstract
Using aminomethyl benzimidazole (AMB) as a model of benzimidazole-type drugs, a potential biodegradable drug-loaded polymer poly(lactic acid-co-aminomethyl benzimidazole) (PLAAMB) is synthesized as designed via direct melt polycondensation starting from d,l-lactic acid (LA). When the molar feed ratio LA/AMB is 40/1, the optimal synthetic conditions, including catalyst type and dosage, polycondensation temperature, and copolymerization time are discussed. After the prepolymerization at 140 °C for 8 h, using 0.4 wt% stannous oxide (SnO) as the catalyst, the melt copolymerization at 160 °C for 6 h gives the copolymer with the biggest weight-average molecular weight (Mw) 5300 Da. The structure and properties of the copolymer are systematically characterized with Fourier transform infrared, 1H NMR, gel permeation chromatography, differential scanning calorimetry, and X-ray diffraction. And the investigations on the influences of different molar feed ratios on the properties of PLAAMB show that, the copolymer PLAAMB with the biggest Mw of 9400 Da can be obtained. After the drug model AMB as a monomer is introduced into polylactic acid during the condensation, the Tg of the obtained PLAAMB is lower than the Tg of homopolymer poly(d,l-lactic acid) (PDLLA). The Mw and crystallinity of PLAAMBs can meet the requirement of drug-loaded polymers in the drug delivery.
1. Introduction
Benzimidazole compounds are widely used in many fields, such as organic synthesis, medicinal chemistry, biochemistry, supramolecular chemistry, chemical sensors, and optoelectronic materials.[Citation1–4] Especially, more and more importance has been attached to the benzimidazole drugs for their significant biological activities, e.g. anticancer, antifungal, antiviral, anti-inflammatory, etc. [Citation5–13]. Among them, many drug molecules have a basic skeleton of aminomethyl benzimidazole (AMB) (Figure ).[Citation8–13]
Due to good physical properties, biocompatibility and biodegradability, polylactic acid (PLA) as an environment friendly polymer produced from renewable resource [Citation14–17] can be used in general polymer field as packaging materials, plastic profiles, thin films, nonwovens, and clothing fibers.[Citation18–23] Of course, for the cost reason, PLA materials are more widely used in the biomedical field as bone material, surgical sutures, eye filler, gauze dressings, drug delivery, artificial urethra, and artificial esophagus.[Citation24–27] Therefore, the studies on the low-cost synthesis of PLA materials via direct melt polycondensation and their practical applications in the field of drug delivery have received great attention in recent years.[Citation28–37]
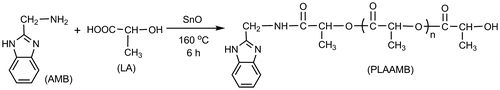
However, there is no report about the drug delivery of benzimidazole drugs loaded by PLA materials. Herein, on the basis of our previous studies on lactic acid (LA) direct melt polymerization [Citation35,38,39] and benzimidazole compounds,[Citation3–6] using AMB [Citation10] as a model of benzimidazole-type drugs, poly(lactic acid-co-aminomethyl benzimidazole) (PLAAMB) as a potential biodegradable drug-loaded polymer is designed, and synthesized (Scheme ). After the discussions on the optimal synthetic conditions, the structures and basic properties of PLAAMBs with different molar feed ratios are systematically investigated by Fourier transform infrared (FT-IR), 1H NMR, gel permeation chromatography (GPC), differential scanning calorimetry (DSC), and X-ray diffraction (XRD). These serial characterizations of the novel copolymer PLAAMB will be advantageous for PLAAMB to be used in drug carrier.
2. Experimental
2.1. Materials
d,l-Lactic acid (LA) was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). O-phenylenediamine was purchased from Aladdin Industrial Corporation (Shanghai, China). Glycine was purchased from J&K Scientific Ltd. (Beijing, China). AMB was self-made from o-phenylenediamine and glycine according to the literature.[Citation5] All other chemicals, including p-toluenesulfonic acid (TSA), stannous chloride (SnCl2), stannous oxide (SnO), zinc chloride (ZnCl2), and zinc oxide (ZnO) were commercially available as analytical grades from Guangzhou Chemical Reagent Factory (Guangzhou, China). All these materials were used without further purification.
2.2. Preparation of PLAAMB
LA and AMB should be prepolymerized before copolymerization according to the previous works on direct melt copolymerization of LA with other monomers.[Citation38–40] After LA and AMB were uniformly mixed as preplanned molar feed ratio, the mixture was directly dehydrated for 8 h at 140 °C under 4000 Pa in a three-necked flask equipped with a mechanical stirring device and a thermometer. After prepolymerization, the selected catalyst was added as predetermined weight percentage of dehydrated reactants (wt%), then the melt copolymerization was carried out at a certain temperature (140–180 °C) and an absolute pressure of 70 Pa for 2–10 h. When the reaction is over, the simple purification via the dissolution in CHCl3 and the subsequent precipitation by CH3OH ordinarily produced a white (or yellowish) powder after drying in vacuo to constant weight. The yield was calculated according to the total weight of the raw materials, it was within the range of 13–44% (in most cases, it was above 31%).
2.3. Characterization
According to the literature,[Citation26,28,31,37,41–43] the relative molecular weight and molecular weight distribution of the polymer were determined by GPC (Waters 1515 pump, Torrance, CA) with tetrahydrofuran (THF) as solvent and with polystyrene (PS) as a reference at 35 °C and a flow velocity of 1 mL min−1. Three Styragel HR columns from Japan covering a molecular weight range of 1 × 103–106 Da were used and calibrated using five PS narrow standards from BF Goodrich (Richfield, Ohio). Molecular weight distributions for the samples were calculated using the Millennium 2010 software from Waters and were reported as PS equivalent values.
Infrared spectra were obtained from a FT-IR spectrometer (Bruker Vector 33, Ettlingen, Germany) by the dichloromethane (CH2Cl2) liquid film method. Proton nuclear magnetic resonance (1H NMR) spectra were recorded with a DRX-400 NMR spectrometer (Bruker instruments, Billerica, MA) with DMSO-d6 as the solvent and tetramethylsilane (TMS) as internal standard.
The crystallinity of PLAAMB was investigated by XRD on a Bruker D8 Advance X-ray diffractometer (Bruker Co., Germany) using Cu Kα radiation with a wavelength of 1.5418 × 10−10 m, and scanning range of 2θ = 5–50° at a scanning speed of 0.03° at 5 s per step. The thermal properties of the polymer were measured by DSC with a PerkinElmer DSC7 thermal analyzer (PerkinElmer, Cetus Instruments, Norwalk, CT). The samples for DSC measurements (an average weight of 4 mg) were scanned at a heating rate of 10 °C min−1 under a nitrogen atmosphere (flow velocity 20 mL min−1), then they were cooled to −30 °C for 5 min and heated again to 150 °C.
3. Results and discussion
Using LA and AMB as raw materials, the copolymer PLAAMBs with different molar feed ratios (LA/AMB = 20/1, 40/1, 80/1, 120/1, and 240/1) are directly synthesized via melt copolycondensation after the conditions for the copolymerization are optimized. The structures and properties of these PLAAMBs are characterized by FT-IR, 1H NMR, GPC, DSC, and XRD.
3.1. Optimal synthetic conditions for PLAAMB
Using GPC to test weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity index (PDI, Mw/Mn) of PLAAMB, the optimal synthetic conditions are discussed. Firstly, different catalysts, such as protonic acid, metal oxides, and metal halides are screened (Table ). It can be observed that, among five available catalysts usually used in the direct melt homo-/co- polymerization of LA,[Citation28,31,37,40,44] the reaction catalyzed by stannous oxide (SnO) gives the biggest molecular weight (run 2). Thus, SnO is selected as the catalyst in the following experiments.
Table 1. The effects of catalyst kinds on the reaction.Table Footnotea
When the molar feed ratio LA/AMB is 40/1, the direct melt copolycondensation is catalyzed by SnO at 160 °C and absolute pressure 70 Pa for 6 h, the influences of catalyst SnO dosage on molecular weight of PLAAMB are shown in Table . Obviously, the molecular weight reaches a maximum value when the weight percent of catalyst SnO quantity is 0.4 wt% of the prepolymer (run 2). Once the quantity is too small, the reaction is so insufficient after a certain time that the molecular weight is not high (run 1). When the quantity of SnO is excessive (runs 3–5), short-chain molecule is apt to be formed through the degradation of polymer, which also is catalyzed by the metal catalyst.[Citation32,36] Therefore, the suitable dosage of catalyst SnO should be 0.4 wt%.
Table 2. The effects of catalyst dosage on the reaction.Table Footnotea
The effects of melt copolymerization time on the reaction are shown in Table . It is obvious that, the molecular weight reaches a maximum value after 6 h (run 3). When the reaction time is too short, the polymerization is insufficient. However, once the reaction time is longer than 6 h, the more serious oxidation and thermal degradation of polymer [Citation32,45] makes the molecular weight to drop, even the color of the purified product becomes deeper (runs 4 and 5). Thus, the appropriate time is 6 h.
Table 3. The effects of melt polymerization time on the reaction.Table Footnotea
When the melt copolymerization is carried out, respectively, at different temperatures for 6 h under the conditions of the molar feed ratio of LA/AMB 40/1, absolute pressure 70 Pa, and catalyst SnO quantity 0.4 wt%, the molecular weights of the resulting polymers are shown in Table . It can be seen that, the appropriate higher temperatures are advantageous to increase molecular chain of the copolymer (runs 1–3). However, when the temperatures are too high (runs 4 and 5), the side reactions, such as thermal degradation and oxidation become apparent.[Citation32,36,45] Even when the temperature is 180 °C, not only the lower molecular weight is obtained, but also the color of the purified product becomes brown with the lowest yield of all (run 5). Therefore, the appropriate temperature should be 160 °C.
Table 4. The effects of melt polymerization temperature on the reaction.Table Footnotea
In a word, PLAAMB with different molecular weight can be obtained by controlling the synthetic conditions. In order to get the higher molecular weight, when the molar feed ratio of LA/AMB is 40/1, the optimal conditions for the synthesis of copolymer PLAAMB via direct melt copolycondensation are as follows: catalyst SnO quantity 0.4 wt%, reaction temperature 160 °C, absolute pressure 70 Pa, and reaction time 6 h. In this case, the maximum Mw can be 5300 Da.
3.2. Structure characterization of PLAAMB
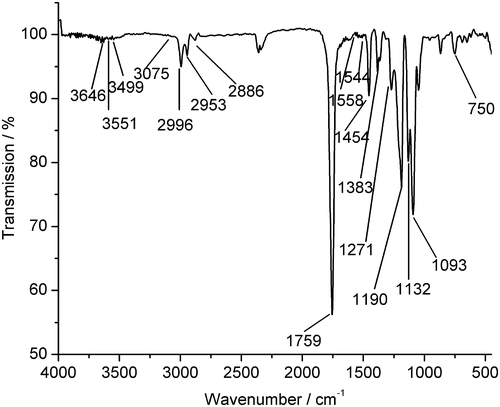
Taking the copolymer synthesized with a molar feed ratio LA/AMB of 40/1 as a representative, the FT-IR curve of PLAAMB is shown as Figure . Compared with the homopolymer poly(d,l-lactic acid) (PDLLA) synthesized via direct melt polycondensation,[Citation30] it is elucidated that these compounds show many similar absorptions in their FT-IR spectra, e.g. the weak absorption of terminal OH group at 3646 cm−1, the absorption of saturated C–H at 2996, 2953, 2886, 1454, and 1383 cm−1, the strongest absorption of ester carbonyl near 1759 cm−1, and the stronger absorption of C–O at 1271, 1190, 1132, and 1093 cm−1. However, the absorption of saturated N–H group at 3551, 3499 cm−1 (Figure ) is not observed in the PDLLA spectrum. Especially, the bands at 1558, 1544, and 1454 cm−1 (Figure ) are the characteristic absorption peaks of the benzimidazole aromatic ring skeleton. And the peak at 750 cm−1 also indicates the structure of ortho-disubstituted benzene ring (benzimidazole ring, Scheme ). These differences show that, the AMB moiety is introduced into the copolymer as designed indeed.
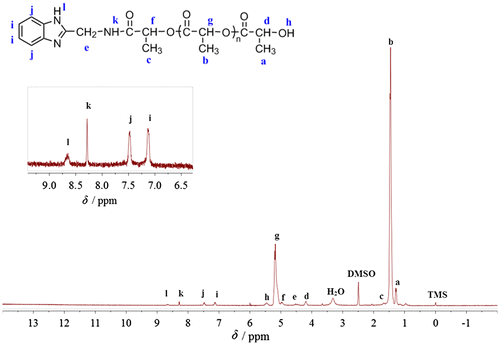
The data of 1H NMR spectrum of PLAAMB synthesized as the molar feed ratio LA/AMB 40/1 (Figure ) are obtained as follows. 1H NMR (DMSO-d6 as solvent and TMS as internal reference), δ, ppm: 1.26–1.29 (Ha, CH3 in terminal PLA segment), 1.40–1.50 (Hb, CH3 in PLA chain), 1.60–1.74 (Hc, CH3 in LA moiety close to AMB moiety), 4.17–4.20 (Hd, CH in terminal PLA segment), 4.43–4.58 (He, CH2 in AMB moiety), 4.94–5.00 (Hf, CH in LA moiety close to AMB moiety), 5.08–5.25 (Hg, CH in PLA chain), 5.43–5.48 (Hh, OH in terminal PLA segment), 7.07–7.18 (Hi, Ar–H in AMB moiety), 7.42–7.53 (Hj, Ar–H in AMB moiety), 8.28 (Hk, NH in the amide bond), and 8.62–8.71 (Hl, NH in AMB moiety).
The structural studies on PLAAMB copolymers with different molar feed ratios by FT-IR and 1H NMR show the similar features as mentioned above. Therefore, these data from FT-IR and 1H NMR indicates that the direct melt copolycondensation of LA and AMB indeed gives the copolymer PLAAMB (Scheme ).
3.3. The effects of molar feed ratios on the molecular weight
According to the previous method,[Citation40,45–47] the molecular weight can be calculated from the 1H NMR data. The resulting molecular weight (MNMR) and the results obtained from GPC are showed in Table . It can be found that the ratio of MNMR/Mn is close to 1.00 in all runs, indicating the results of MNMR can be reliable. Comparing MNMR with the corresponding theoretical value of molecular weight (MT), the differences show the effects of different molar feed ratios on the copolymerization.
Table 5. The effects of different molar feed ratios on yield and Mn of the copolymers.Table Footnotea
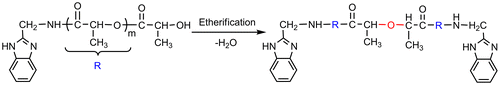
When the molar feed ratio LA/AMB is less than or equal to 20/1, the MNMR is obviously bigger than the theoretical value, and basically twice of the MT (Table , run 1). This may be due to the less proportion of LA in the feed. After PLAAMB as shown in Scheme is generated, the less LA can make the copolymer each other further etherified by the dehydration between the terminal hydroxyl groups.[Citation39,45,47] In the end, the copolymer as shown in Scheme may be formed.
When the molar feed ratio LA/AMB is 40/1, the MNMR is close to the MT, but smaller than the Mn by GPC (Table , run 2). This may be due to the existence of partial etherification reaction. Both this partial etherification by dehydration and the extension of PLA chain by esterification make a peak phenomenon of GPC molecular weight existed within a certain range (Table , runs 1–4). This result further proves the previous conclusions.[Citation39,40,45–47]
Once the molar feed ratio LA/MA is more than or equal to 80/1, the MNMR is obviously smaller than the MT. As reported before,[Citation29,30,36–39,45–47] this may be related to the fact that some LA molecules can escape out of the reaction systems as the form of lactide during the direct melt copolycondensation. Therefore, only when the proportion of LA in the feed is large enough (e.g. LA/AMB = 240/1), the molecular weight of PLAAMB can be obviously increased due to the significant growth of PLA chain (Table , run 5).
In all GPC tests, the GPC flow curves only show a single peak, and the PDI (Mw/Mn) is obviously less than 2.0 (Tables ). These also demonstrate that the direct melt copolycondensation of LA and AMB indeed gives the copolymer PLAAMB. Thus, the drug-loaded polymer PLAAMB with different molecular weight can be obtained by controlling the molar feed ratio LA/AMB in the direct melt copolycondensation of LA and AMB.
Generally, when the PLA biodegradable polymers are used as drug delivery materials, the molecular weights are not more than 30,000 Da.[Citation29,30,34–36,48] As reported in the literature,[Citation49–51] the PLAs material with a molecular weight of 1800 Da can be applied in drug delivery, even PLA copolymers with a molecular weight of only 900 Da can be used as drug delivery device. In this study, the molecular weights of PLAAMBs are between 3100 and 9400 Da (Tables ), each one of them is overwhelmingly higher than 900 Da. Therefore, it can meet the requirement for the drug delivery to the polymer materials.
3.4. Thermal properties of PLAAMB
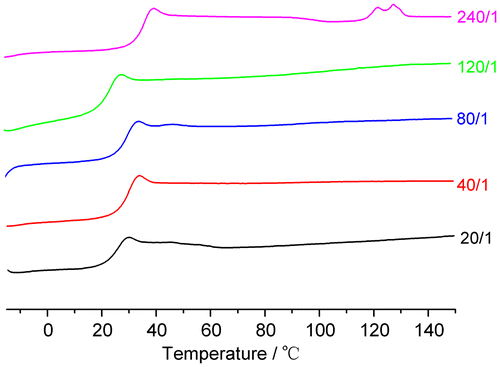
The influences of different molar feed ratios on the thermal properties of PLAAMB are shown in Table . Obviously, due to the introduction of AMB, the glass transition temperature (Tg) of copolymers is significantly lower than that of the homopolymer PDLLA synthesized via the direct melt polycondensation [Citation30] though there is a trend of slight increase for Tg. Meanwhile, the melting temperature (Tm) is not detected in most cases (runs 1–4).
Table 6. The DSC and XRD results of PLAAMBs with different molar feed ratios (LA/AMB).
Only when the molar feed ratio LA/AMB is 240/1 (Table , run 5), there is a Tm detected in the DSC curves (Figure ) with a melting enthalpy (8.72 J g−1), which is smaller than that of PDLLA (17.1 J g−1).[Citation30] Even so, this indicates that the copolymer PLAAMB may be partly crystalline in some cases. Of course, because the structure regularity of the polymer is seriously decreased by the introduction of AMB, the melting enthalpy becomes smaller. This conclusion is further confirmed in the following analyses of XRD characterization.
3.5. Crystallinity of PLAAMB
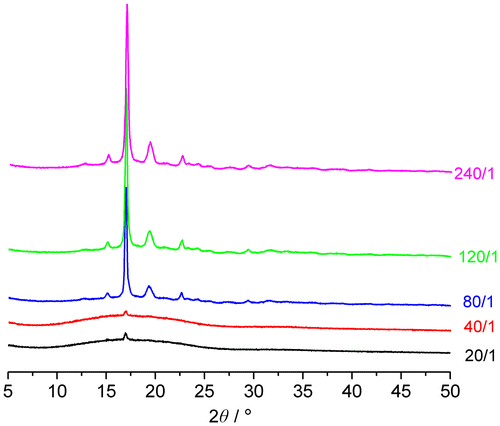
The crystallinity of PLA materials is crucial for their physical and biological properties, especially degradability. The XRD results (Figure ) show that when the molar feed ratio is less than or equal to 40/1, the PLAAMB is basically amorphous. With the increase of the LA proportion, the PLAAMB copolymers gradually become partially crystalline. According to the crystallization peaks of these PLAAMBs to analyze the crystallinity (Xc), the results are shown in Table .
It can be seen that the Xc of these PLAAMB copolymers is lower than that of homopolymer PDLLA.[Citation30] But they significantly have bigger crystallite dimension than PDLLA (Table , runs 3–5). These demonstrate that the introduction of AMB into PLA structure makes the stereoregularity of the molecular structure declined. Even the more AMB feed proportion can make the copolymer amorphous (runs 1–2).
Fortunately, lower or no crystallinity is more beneficial for PLA biodegradable materials to be applied in the biomedical field, especially drug delivery carrier materials, because there will be no residual microcrystalline after degradation in vivo, thereby to avoid unnecessary inflammation.[Citation29,36,48,52] Therefore, we can regulate the molar feed ratio LA/AMB to obtain PLA materials with suitable crystalline property.
4. Conclusions
Using AMB as a drug model, a potential biodegradable drug-loaded polymer PLAAMB is synthesized as designed via direct melt polycondensation starting from LA for the first time. When the molar feed ratio LA/AMB is 40/1, the optimal synthetic conditions, including type and dosage of catalyst, temperature, and time of copolymerization, are discussed in detail. The structures and properties of PLAAMBs at different molar feed ratios are characterized by FT-IR, 1H NMR, GPC, DSC, and XRD. The introduction of AMB into the PLA structure has an important influence on the crystalline property of PLA materials, and it is possible to adjust the crystalline property and molecular weight of the copolymers by changing the molar feed ratios. These investigations provide a basis for the drug delivery study of PLA materials loaded AMB-type drugs, and this simple and practical method via LA direct melt polycondensation can be a reference for the loading of some drugs containing amino group by the biodegradable polymer PLA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu Y, Kumar A, Depauw S, Nhili R, David-Cordonnier MH, Lee MP, Ismail MA, Farahat AA, Say M, Chackal-Catoen S, Batista-Parra A, Neidle S, Boykin DW, Wilson WD. Water-mediated binding of agents that target the DNA minor groove. J. Am. Chem. Soc. 2011;133:10171–10183.10.1021/ja202006u
- Xu TF, Zhang LW, Xu SL, Yang CY, Luo JF, Ding F, Lu XY, Liu YX, Tu ZC, Li SL, Pei DQ, Cai Q, Li HL, Ren XM, Wang SM, Ding K. Pyrimido[4,5-d]pyrimidin- 4(1H)-one derivatives as selective inhibitors of EGFR threonine790 to methionine790 (T790M) mutants. Angew. Chem. 2013;125:8545–8548.10.1002/ange.v125.32
- Xiong JF, Luo SH, Huo JP, Liu JY, Chen SX, Wang ZY. Design, synthesis, and characterization of 1,3,5-tri(1H-benzo[d]imidazol-2-yl)benzene-based fluorescent supra- molecular columnar liquid crystals with a broad mesomophic range. J. Org. Chem. 2014;79:8366–8373.10.1021/jo5016954
- Xiong JF, Li JX, Mo GZ, Huo JP, Liu JY, Chen XY, Wang ZY. Benzimidazole derivatives: selective fluorescent chemosensors for the picogram detection of picric acid. J. Org. Chem. 2014;79:11619–11630.10.1021/jo502281b
- Peng P, Xiong JF, Mo GZ, Zheng JL, Chen RH, Chen XY, Wang ZY. A concise synthesis of benzimidazoles via the microwave one-pot batch reaction of amino acids up to a 10-g scale. Amino Acids. 2014;46:2427–2433.10.1007/s00726-014-1794-z
- Mao ZZ, Wang ZY, Mei WJ, Yang K. Synthesis of novel unsymmetric bisbenzimidazoles. Chin. J. Chem. 2010;28:818–824.10.1002/cjoc.v28:5
- Narasimhan B, Sharma D, Kumar P. Benzimidazole: a medicinally important heterocyclic moiety. Med. Chem. Res. 2012;21:269–283.10.1007/s00044-010-9533-9
- Balboni G, Fiorini S, Baldisserotto A, Trapella C, Sasaki Y, Ambo A, Marczak ED, Lazarus LH, Salvadori S. Further studies on lead compounds containing the opioid pharmacophore Dmt-Tic. J. Med. Chem. 2008;51:5109–5117.10.1021/jm800587e
- Gudmundsson KS, Sebahar PR, Richardson LD, Miller JF, Turner EM, Catalano JG, Spaltenstein A, Lawrence W, Thomson M, Jenkinson S. Amine substituted N-(1H- benzimidazol-2ylmethyl)-5,6,7,8-tetrahydro-8-quinolinamines as CXCR4 antagonists with potent activity against HIV-1. Bioorg. Med. Chem. Lett. 2009;19:5048–5052.10.1016/j.bmcl.2009.07.037
- Milletti F, Storchi L, Goracci L, Bendels S, Wagner B, Kansy M, Cruciani G. Extending pKa prediction accuracy: high-throughput pKa measurements to understand pKa modulation of new chemical series. Eur. J. Med. Chem. 2010;45:4270–4279.10.1016/j.ejmech.2010.06.026
- Bauer J, Kinast S, Burger-Kentischer A, Finkelmeier D, Kleymann G, Rayyan W, Schropple K, Singh A, Jung G, Wiesmuller KH, Rupp S, Eickhoff H. High-throughout-screening-based identification and structure-activity relationship characterization defined (S)- 2-(1-aminoisobutyl)-1-(3-chlorobenzyl)benzimidazole as a highly antimycotic agent nontoxic to cell lines. J. Med. Chem. 2011;54:6993–6997.10.1021/jm200571e
- Skerlj R, Bridger G, McEachern E, Harwig C, Smith C, Wilson T, Veale D, Yee H, Crawford J, Skupinska K, Wauthy R, Yang W, Zhu YB, Bogucki D, Di Fluri M, Langille J, Huskens D, De Clercq E, Schols D. Synthesis and SAR of novel CXCR4 antagonists that are potent inhibitors of T tropic (X4) HIV-1 replication. Bioorg. Med. Chem. 2011;21:262–266.10.1016/j.bmcl.2010.11.023
- Kaushik D, Khan SA, Chawla G. Synthesis of (substituted benzamidostyryl) lH-benzimidazoles and their screening for anti-inflammatory activity. Med. Chem. Res. 2012;21:459–467.10.1007/s00044-011-9552-1
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013;31:877–902.10.1016/j.biotechadv.2013.04.002
- Raquez JM, Habibi Y, Murariu M, Dubois P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013;38:1504–1542.10.1016/j.progpolymsci.2013.05.014
- Bishai M, De S, Adhikari B, Banerjee R. Copolymerization of lactic acid for cost-effective PLA synthesis and studies on its improved characteristics. Food Sci. Biotechnol. 2013;22:73–77.10.1007/s10068-013-0051-7
- Jiménez-Bonilla P, Salas-Arias J, Esquivel M, Vega-Baudrit JR. Optimization of microwave-assisted and conventional heating comparative synthesis of poly(lactic acid) by direct melt polycondensation from agroindustrial banana (musa AAA cavendish) and pineapple (ananas comosus) fermented wastes. J. Polym. Environ. 2014;22:393–397.10.1007/s10924-014-0667-6
- Luo YF, Wang ZY, Song XM, Mao ZZ. Star-shaped polylactic acid. Prog. Chem. 2008;20:1578–1587.
- Rasal RM, Janorkar AV, Hirt DE. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010;35:338–356.10.1016/j.progpolymsci.2009.12.003
- Rhim JW, Park HM, Ha CS. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013;38:1629–1652.10.1016/j.progpolymsci.2013.05.008
- Lantano C, Alfieri I, Cavazza A, Corradini C, Lorenzi A, Zucchetto N, Montenero A. Natamycin based sol-gel antimicrobial coatings on polylactic acid films for food packaging. Food Chem. 2014;165:342–347.10.1016/j.foodchem.2014.05.066
- Okamoto H, Katagiri Y, Nakano M, Usuki A. A slow shape-recovery polymer based on polylactic acid. J. Appl. Polym. Sci. 2014;131:41004.
- Hammonds RL, Gazzola WH, Benson RS. Physical and thermal characterization of polylactic acid meltblown nonwovens. J. Appl. Polym. Sci. 2014;131:40593.
- Ko NR, Yao KJ, Tang CB, Oh JK. Synthesis and thiol-responsive degradation of polylactide-based block copolymers having disulfide junctions using ATRP and ROP. J. Polym. Sci. Part A: Polym. Chem. 2013;51:3071–3080.10.1002/pola.26335
- Dorresteijn R, Ragg R, Rago G, Billecke N, Bonn M, Parekh SH, Battagliarin G, Peneva K, Wagner M, Klapper M, Müllen K. Biocompatible polylactide-block-polypeptide-block-polylactide nanocarrier. Biomacromolecules. 2013;14:1572–1577.10.1021/bm400216r
- Bakkour Y, Darcos V, Coumes F, Li SM, Coudane J. Brush-like amphiphilic copolymers based on polylactide and poly(ethyleneglycol): synthesis, self-assembly and evaluation as drug carrier. Polymer. 2013;54:1746–1754.10.1016/j.polymer.2013.01.042
- Qiu JS, Xing CY, Cao XJ, Wang HT, Wang L, Zhao LP, Li YJ. Miscibility and double glass transition temperature depression of poly(L-lactic acid) (PLLA)/Poly(oxymethylene) (POM) blends. Macromolecules. 2013;46:5806–5814.10.1021/ma401084y
- Moon SI, Kimura Y. Melt polycondensation of L-lactic acid to poly(L-lactic acid) with Sn(II) catalysts combined with various metal alkoxides. Polym. Int. 2003;52:299–303.10.1002/(ISSN)1097-0126
- Zhao YM, Wang ZY, Wang J, Mai HZ, Yan B, Yang F. Direct synthesis of poly(D,L-lactic acid) via melt polycondensation and its application in drug delivery. J. Appl. Polym. Sci. 2004;91:2143–2150.10.1002/(ISSN)1097-4628
- Zhao YM, Wang ZY, Yang F. Characterization of poly(D,L-lactic acid) synthesized via direct melt polymerization and its application in Chinese traditional medicine compound prescription microsphere. J. Appl. Polym. Sci. 2005;97:195–200.10.1002/(ISSN)1097-4628
- Takasu A, Narukawa Y, Hirabayashi T. Direct dehydration polycondensation of lactic acid catalyzed by water-stable Lewis acids. J. Polym. Sci. Polym. Chem. 2006;44:5247–5253.10.1002/(ISSN)1099-0518
- Duan JF, Du J, Zheng YB. Synthesis and characterization of a novel biodegradable polymer poly(lactic acid-glycolic acid-4-hydroxyproline). J. Appl. Polym. Sci. 2007;103:3585–3590.10.1002/(ISSN)1097-4628
- Maharana T, Mohanty B, Negi YS. Melt-solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009;34:99–124.10.1016/j.progpolymsci.2008.10.001
- Yang F, Song FL, Pan YF, Wang ZY, Yang YQ, Zhao YM, Liang SZ, Zhang YM. Preparation and characteristics of interferon-alpha poly(lactic-co-glycolic acid) microspheres. J. Microencapsul. 2010;27:133–141.10.3109/02652040903052010
- Mao CX, Luo SH, Wang QF, Xiong JF, Wang ZY. Synthesis and characterization of a novel functional biodegradable material, poly(lactic acid-co-borneol). Des. Monomers Polym. 2012;15:575–586.10.1080/1385772X.2012.688340
- Mao CX, Luo SH, Wang QF, Xiong JF, Wang ZY. Synthesis of poly(lactic acid-co-menthol) via direct melt polycondensation and its characterization. J. Appl. Polym. Sci. 2012;125:E339–E346.10.1002/app.36968
- Marques DS, Gil MH, Baptista CMSG. Improving lactic acid melt polycondensation: the role of co-catalyst. J. Appl. Polym. Sci. 2013;128:2145–2151.
- Xiong JF, Luo SH, Wang QF, Wang ZY, Qi J. Synthesis and characterization of a novel flame retardant, poly(lactic acid-co-3,3'-diaminobenzidine). Des. Monomers Polym. 2013;16:389–397.10.1080/15685551.2012.747156
- Xiong JF, Wang QF, Peng P, Shi J, Wang ZY, Yang CL. Design, synthesis and characterization of a potential flame retardant poly(lactic acid-co-pyrimidine-2,4,5,6-tetramine) via direct melt polycondensation. J. Appl. Polym. Sci. 2014;131:40275.
- Wang ZY, Zhao HJ, Wang QF, Ye RR, Finlow DE. Synthesis of poly(D,L-lactic acid) modified by cholic acid via direct melt copolycondensation and its characterization. J. Appl. Polym. Sci. 2010;117:1405–1415.
- Kowalski A, Duda A, Penczek S. Polymerization of L,L-Lactide initiated by aluminum isopropoxide trimer or tetramer. Macromolecules. 1998;31:2114–2122.10.1021/ma971737k
- Shaver MP, Cameron DJA. Tacticity control in the synthesis of poly(lactic acid) polymer stars with dipentaerythritol cores. Biomacromolecules. 2010;11:3673–3679.10.1021/bm101140d
- Jiang W, Huang W, Cheng N, Qi YB, Zong XP, Li H, Zhang QX. Isotactic polycondensation of L-lactic acid with biogenic creatinine. Polymer. 2012;53:5476–5479.10.1016/j.polymer.2012.09.044
- Ye RR, Wang ZY, Luo SH, Yang LT, Xiao X. Synthesis and characterization of the bio- material poly(lactic acid-co-Nε-carbobenzoyloxy-L-lysine) via direct melt copolymerization. J. Appl. Polym. Sci. 2010;121:420–426.
- Luo SH, Wang ZY, Mao CX, Huo JP. Synthesis of biodegradable material poly(lactic acid-co-glycerol) via direct melt polycondensation and its reaction mechanism. J. Polym. Res. 2011;18:2093–2102.10.1007/s10965-011-9619-1
- Wang ZY, Luo YF, Ye RR, Song XM. Synthesis of novel biodegradable material poly(lactic acid-trimesic acid) via direct melt copolycondensation and its characterization. J. Polym. Res. 2011;18:499–508.10.1007/s10965-010-9442-0
- Luo SH, Wang QF, Xiong JF, Wang ZY. Synthesis of biodegradable material poly(lactic acid-co-sorbitol) via direct melt polycondensation and its reaction mechanism. J. Polym. Res. 2012;19:9962–9971.10.1007/s10965-012-9962-x
- Zhou SB, Deng XM, Li XH. Synthesis and characterization of biodegradable low molecular weight aliphatic polyesters and their use in protein delivery systems. J. Appl. Polym. Sci. 2004;91:1848–1856.10.1002/(ISSN)1097-4628
- Wang N, Wu XS, Lujan-Upton H, Donahue E, Siddiqui A. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic glycolic acid oligomers. 1. Synthesis and characterization. J. Biomater. Sci. Polym. Ed. 1997;8:905–917.10.1163/156856297X00083
- Wang N, Wu XS. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid oligomers: Part II. Biodegradation and drug delivery application. J. Biomater. Sci. Polym. Ed. 1998;9:75–87.10.1163/156856297X00272
- Wang ZY, Hou XN, Mao ZZ, Ye RR, Mo YQ, Finlow DE. Synthesis and characterization of biodegradable ply(lactic acid-co-glycine) via direct melt copolymerization. Iran. Polym. J. 2008;17:791–798.
- Wu XM, Branford-White CJ, Zhu LM, Chatterton NP, Yu DG. Ester prodrug-loaded electrospun cellulose acetate fiber mats as transdermal drug delivery systems. J. Mater. Sci. Mater. Med. 2010;21:2403–2411.10.1007/s10856-010-4100-y