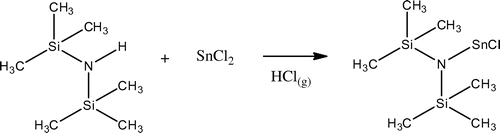Abstract
The polymerization reactions of styrene oxide (SO) at different temperatures for different time intervals were performed by four different tin catalysts, tin(IV) perfluorooctanoate (PFO-Sn), tin(IV) methacrylate (MAc-Sn), tin(II) chloride (SnCl2), and SnCl2/hexamethyldisilazane. Tin(IV) perfluorooctanoate and methacrylate compounds have been prepared from perfluorooctanoic acid, methacrylic acid, and tin tert-butoxide in tert-butanol. The Sn-PFO and Sn-MAc compounds were characterized by 1H, 13C NMR, FTIR, and elemental analysis. These four compounds were tested as catalysts in polymerization of SO and were effective. Polystyrene oxide (PSO) was characterized by 1H, 13C NMR, and gel permeation chromatography. High molecular weight of PSO (~138,000 Da) was recorded for the first time by this study.
1. Introduction
Poly(styrene oxide) homopolymer has been produced from ring-opening polymerization of the styrene oxide by cationic catalysts,[Citation1] anionic catalysts,[Citation2] and mostly coordination catalysts.[Citation3,4] The polymerization of styrene oxide (SO) has been conducted under severe polymerization conditions, such as higher temperature, longer polymerization time, and vacuum conditions.[Citation5,6]
A few reports were published on the applications of poly(styrene oxide) in versatile areas such as hydrophobic nanocarriers for drug delivery,[Citation7] block copolymer micelle formation in water,[Citation7] coating materials, surface modifiers,[Citation8] and in the production of polystyrene carbonate.[Citation9]
There is not enough information and comparison on the ring-opening polymerization of styrene oxide with tin perfluorooctanoate, tin methacrylate catalysts, and tin chloride in the presence of hexamethyldisilazane (HMDS).
In this study, PFO-Sn/MAc-Sn catalysts were synthesized, characterized and used in the polymerization of styrene oxide. In addition to PFO-Sn/MAc-Sn catalysts, tin(II) chloride and tin(II) chloride/HMDS catalysts were also investigated in the polymerization of styrene oxide. The first goal of this study was to prepare and characterize new Sn-catalysts and to investigate their potential application as catalysts in the ring-opening of styrene oxide. The second goal was to prepare high molecular weight polystyrene oxide (PSO) because there is no report about the synthesis of a high molecular weight PSO. The reactivities of the catalysts to ring-opening polymerization of styrene oxide were compared in this study.
2. Experimental
2.1. Materials and instrumentation
Tin(IV) tert-butoxide (99.99%, Aldrich), pentadecafluorooctanoic acid (Merck KGaA, PFOH), (±) styrene oxide (98%, Alfa Aesar), methacrylic acid (98%, Fluka, MAcH), tin(II) chloride (100%, Merck), and hexamethyldisilazane (99%, Fluka, HMDS) were used as received. Tetrahydrofuran (THF) (99.9%, Merck) and tert-butanol (99%, Merck) were dried over activated molecular sieves before use. Polymer syntheses were carried out under nitrogen atmosphere. Tin compounds and polymer were characterized by 1H NMR (Bruker DPX, 400 MHz) and 13C NMR spectroscopy (Bruker DPX, 100 MHz). Infrared spectra of complexes were recorded on a Shimadzu 8201/86601 PC spectrometer. The elemental analysis was carried out with a LECO CHNS-932 elemental analyzer. Gel permeation chromatography (GPC) analysis was performed at 30 °C on a Shimadzu prominence GPC system equipped with a RID-10A refractive index detector, a LC-20AD solvent delivery unit, a CTO-10AS column oven, and a set of two columns, PSS SDV 5 μL
and PSS SDV 5 μL
. THF (HPLC grade) was used as the mobile phase at 1.0 mL/min. The sample concentration was 2 mg/mL, and the injection volume was 50 μL. The calibration curve was made with seven polystyrene standards covering the molecular weight range from 162 to 100,000 Da.
2.2. Preparation of [(C4H5O2)4Sn2(OC4H9)2O]
Methacrylic acid (2.44 mmol, 0.214 g) was added to a solution of tin tert-butoxide (1.22 mmol, 0.50 g) in 20 mL tert-butanol. The reaction mixture was stirred for 3 h at room temperature. Then, the volatile parts were removed by vacuum evaporator at 50 °C and dried. The resultant product was white solid. Elemental analysis (dimer, C24H38O11Sn2, Mw = 739.97 g/mol), [(C4H5O2)4Sn2(OC4H9)2O] Calcd.: C, 38.96; H, 5.18%. Found: C, 38.10; H, 4.96%. 1H NMR, CDCl3, ppm, δ: 1.29 (s, CH3, OtBu), 1.95 (s, CH3, MAc), 5.67 (s, H, CH2, MAc), 6.24 (s, H, CH2, MAc). 13C NMR, CDCl3, ppm, δ: 17.9 (CH3, MAc), 31.4 (CH3, OtBu), 74.0 (C, OtBu), 127.5 (H2C=, MAc), 135.9 (CMe=, MAc), 172.7 (COO). FTIR (KBr, cm−1): 1646 (C=C), 1556 (strong, νas COO), 1460 (strong, νs COO), 1298, 1264, 1161, 1120, 1082, 940, 850, 752, 652, 590.
2.3. Preparation of [(C8F15O2)4Sn2(OC4H9)2O]
Perfluoroctanoic acid (1.90 mmol, 0.785 g) was added to a solution of tin tert-butoxide (0.95 mmol, 0.39 g) in 20 mL tert-butanol. The reaction mixture was stirred for 3 h at room temperature. Then, the volatile parts were removed by vacuum evaporator at 50 °C and dried. The resultant product was liquid. Elemental analysis (dimer, C40H18O11F60Sn2, Mw = 2051.89 g/mol), [(C8F15O2)4Sn2(OC4H9)2O] Calcd.: C, 23.41; H, 0.88%. Found: C, 22.38; H, 0.61%. 1H NMR, DMSO, ppm, δ: 1.39 (s, CH3, OtBu). 13C NMR, DMSO, ppm, δ: 26.1 (CH3, OtBu), 31.0 (CH3,), 66.9 (C, OtBu), 107.8 (CF2), 110.2 (CF2), 117.8 (CF2), 122.6 (CF3), 159.0 (COO). FTIR (NaCl cell, cm−1): 1674 (υasCOO), 1445 (υs COO), 1360 (CH, OtBu)), 1258 (CF2), 1215, 1157, 1068, 663.
2.4. Polymerization of styrene oxide with tin catalysts
A typical procedure for the polymerization was as follows: each catalyst SnCl2 (15 mg), [(C8F15O2)4Sn2(OC4H9)2O] (20 mg), [(C4H5O2)4Sn2(OC4H9)2O] (15 mg), and SnCl2/HMDS (15 mg/13 mg) were mixed with styrene oxide (1.2 mL) in a vial under nitrogen. The solvent free mixture was stirred at different temperatures and hours as indicated in Table . The monomer conversion was determined by the GPC and NMR measurements. The polymer prepared with SnCl2 catalyst: 1H NMR (CDCl3, ppm), δ: 7.5–7.0 (m, Ph), 4.2–3.39 (t, CH polymer backbone), 3.55–3.50; 3.40–3.37 (m, CH2O polymer backbone).13C NMR (CDCl3, ppm), δ: 139.7, 128.1, 127.5, 126.9 (Ph), 81.8 (s, CH polymer backbone), 74.2 (s, CH2 polymer backbone).
Table 1. Data for PSO obtained from GPC measurements.
The polymer prepared with PFO-Sn catalyst: 1H NMR (CDCl3, ppm), δ: 7.35 (m, Ph), 4.56 (m, CH polymer backbone), 3.68 (m, CH2O polymer backbone). Similar 1H NMR result was obtained for PSO prepared with MAc-Sn catalyst.
3. Results and discussion
Two new metal-organic compounds were synthesized by reactions of Sn(OtBu)4 with 1:2 mol ratio of perfluorooctanoic acid and methacrylic acid in alcohol at room temperature to give the products of [(C7F15COO)4Sn2(OtBu)2O] and [(C4H5O2)4Sn2(OC4H9)2O] in accordance with the following reaction:
The reactions were carried out similarly to the preparations of β-diketonate tin(IV) propoxide and tetrafluorophthalate tin(IV) butoxide complexes.[Citation10,11] The formulations of compounds were determined by elemental analysis, FTIR, and NMR measurements. The number of oxo group was one for both compounds. The FTIR spectrum of free perfluorooctanoic acid (PFOH) exhibits intense band at 1775 cm−1 corresponding to asymmetrical stretching vibrations of the carboxyl group.[Citation12] After coordination of PFOH to tin tert-butoxide, the band shifts to low wave numbers ~1674 for νCOOasym and 1445 cm−1 for νCOOsym. In the FTIR spectrum of perfluorooctanoate–Sn(IV) complex the band at ~1674 cm−1 indicates the bonding of carboxylic group. These values are consistent with those detected in a number of carboxylate-metal compounds.[Citation13–15]
The 1H NMR spectra of perfluorooctanoate-Sn and methacrylate-Sn complexes showed singlet at ~1.39 and 1.29 ppm, respectively, because of methyl protons of tert-butoxy groups in Sn-complex.[Citation16] The signal for the COOH proton is absent. Lack of a carboxylic acid proton signal at ~11 ppm indicates that PFOH and MAcH are completely coordinated to tin. The 13C NMR spectra of perfluorooctanoate-Sn and methacrylate-Sn complexes showed characteristic peaks for bonded perfluorooctanoate and methacrylate groups. For example, carboxylate group appeared at 159 ppm for PFO-Sn and 172.7 for Mac-Sn. These values are very characteristic for bonded PFO and Mac groups.[Citation17,18]
SO was polymerized with PFO-tin catalyst at 60 °C for 24 h. The conversion was 96%. When MAc-tin catalyst was used instead of PFO-tin catalyst, a low conversion (70%) was obtained. Polymerization temperature was very effective on the conversion of SO. The conversion increased with the temperature rising from 60 °C to 80 °C and reached a high conversion of 90% at 80 °C for MAc-Sn catalyst after 24 h stirring. These catalysts have covalent and acidic character. Therefore, as expected for acidic catalysts, the molecular weights of polymers (~1500 Da) were very small when compared with basic and ionic catalyst.
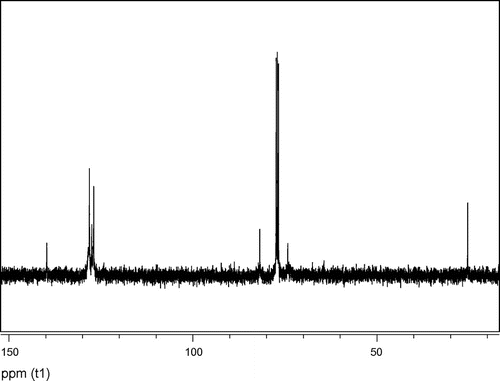
In the 1H NMR spectrum of the PSO prepared with SnCl2, the major signals appear at 4.20–3.39 ppm for methine proton (t, CH polymer backbone), and 3.55–3.50; 3.40–3.37 for methylene protons (m, CH2O polymer backbone), respectively. The other signals at 7.5–7.0 ppm are assigned to the aromatic protons of the PSO side chain. There is a peak around ~4.05 ppm because of OCH proton of isopropanol which was used as a washing solvent. The 13C NMR spectrum of PSO showed the expected peaks due to the methine and methylene carbons of the PSO backbone (Figure ). The main peaks of methine and methylene (due to the head-to-tail linkage) appeared at 81.8 (s, CH polymer backbone) and 74.2 (s, CH2 polymer backbone).
13C NMR result shows that polymer has a regular structure as both region and stereo. These values are consistent with those reported in a number of articles about PSO.[Citation19,20]
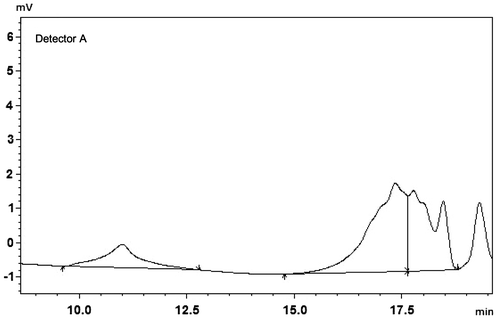
Styrene oxide is notably less reactive than aliphatic and other alicyclic epoxides, but is known to undergo ring-opening with acids and bases.[Citation4] Therefore, it is important to find suitable catalysts for styrene oxide to undergo ring opening polymerization through forming large molecular weights of polymers. The catalyst, SnCl2, is suitable for above purposes and has ionic and basic character. Therefore, as expected for basic catalysts, the molecular weights of polymers (~73,000 Da) were very large when compared with above acidic catalysts, PFO-Sn/MAc-Sn (Table ). However, The SnCl2 catalyst produces polymer having relatively broad molecular distribution and having long tails.
In the presence of co-catalyst, SnCl2/HMDS (15/13 mg, in 1:1 mol ratio), polymers were obtained with more regular structure and higher molecular weight (138,000 Da). This can be attributed the single site properties of catalyst and basicity of the hexamethyldisilazane which coordinates to tin atom and enhances the catalytic activity and the degree of control on polymerization process. The reaction of tin(II) chloride with 1 equiv of hexamethyldisilazane led to the formation of (Me3Si)2NSnCl as shown in Scheme .[Citation21,22] The SnCl2/HMDS catalyst produces polymer having very high molecular weight and having no tail (Figure ).
All PSO obtained by different catalysts have similar IR spectra. They all show strong characteristic absorption bands of benzene ring (1605, 1582, 1490 cm−1) and C–O group (1080 cm−1), which are similar to PSO obtained with other catalysts.[Citation23]
The ring-opening polymerization of cyclic esters by metal complexes via coordination-insertion mechanism represents the most effective and versatile method for preparing aliphatic polyethers with a good control in terms of molecular weight and stereoselectivity.
PFO is an ancillary ligand and OtBu a nucleophilic group. The presence of chelate ligands (PFO and MAc) tunes the steric properties of the metal center, adjusts its Lewis acidity, and enhances the catalytic activity. NMR data suggest that SO attacks the metal center first and then the nucleophile OtBu ion attacks CH2 carbon atom in SO. Small molecular weights of PSO were obtained with these two catalysts. Acid catalysts generate low molecular weight polymers. Therefore, it can be attributed to their acidic behavior.
4. Conclusion
High molecular weights of the PSO were prepared for the first time with this study. The catalysts SnCl2 and SnCl2/HMDS were more effective in the high molecular weights PSO preparation as compared to the conventional cationic and anionic catalysts. As expected, polymers prepared with acidic catalysts, PFO-Sn and MAc-Sn have low molecular weights. Polymers prepared with tin catalysts (PFO-Sn, MAc-Sn, and SnCl2/HMDS) were more regular than that of prepared with SnCl2. New compounds are suitable as catalysts for ring-opening polymerization of epoxides and still needs further research.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Colclough RO, Gee G, Higginson WCE, Jackson JB, Litt M. The polymerization of epoxides by metal halide catalysts. J. Polym. Sci. 1959;34:171–179.10.1002/pol.1959.1203412715
- Jedliński Z, Kasperczyk J, Dworak A, Matuszewska B. The anionic polymerization of styrene oxide. Polymer structure and direction of ring opening. Die Makromol. Chem. 1982;183:587–591.10.1002/macp.1982.021830308
- Ge L, Huang Q, Zhang Y, Shen Z. Ring-opening polymerization of styrene oxide with rare earth coordination catalysts. Eur. Polym. J. 2000;36:2699–2705.10.1016/S0014-3057(00)00056-2
- Harrold ND, Li Y, Chisholm MH. Studies of ring-opening reactions of styrene oxide by chromium tetraphenylporphyrin initiators. Macromolecules. 2013;46:692–698.10.1021/ma302492p
- Hirose Y, Adachi K. Type-A dipole moment and segmental dynamics of poly(styrene oxide). Polymer. 2005;46:1913–1920.10.1016/j.polymer.2004.12.003
- Yahiaoui A, Belbachir M. Ring-opening polymerization of styrene oxide with Maghnite-H+ as ecocatalyst. J. Appl. Polym. Sci. 2006;100:1681–1687.10.1002/(ISSN)1097-4628
- Cambón A, Rey-Rico A, Barbosa S. Poly(styrene oxide)-poly(ethylene oxide) block copolymers: from “classical” chemotherapeutic nanocarriers to active cell-response inducers. J. Controlled Release. 2013;167:68–75.10.1016/j.jconrel.2013.01.010
- Huang Y, Gao L, Ding M. Polymerization of styrene oxide catalyzed by a diethylzinc/α-pinene oxide system. J. Polym. Sci.: Part A: Polym. Chem. 1999;37:4640–4645.10.1002/(ISSN)1099-0518
- Harrold ND, Li Y, Chisholm MH. Studies of ring-opening reactions of styrene oxide by chromium tetraphenylporphyrin ınitiators. Mechanistic and stereochemical considerations. Macromolecules. 2013;46:692–698.10.1021/ma302492p
- Chandler CD, Fallon GD, Koplick AJ, West BO. The structures of mono and bis β-diketonate tin(IV) alkoxide complexes. Austr. J. Chem. 1987;40:1427–1439.10.1071/CH9871427
- Yalçın G, Kayan A. Ring-opening polymerization of isopropylglycidyl ether (IPGE) with new catalysts of Ti, Sn, Al-alkoxides and comparison of its reactivity. Des. Monomers Polym. 2012;15:405–416.10.1080/1385772X.2012.686693
- Wang L, Han J, Sheng J, Tian H, Fan Z. Rare earth perfluorooctanoate [RE(PFO)3] catalyzed one-pot Mannich reaction: three component synthesis of β-amino carbonyl compounds. Catal. Commun. 2005;6:201–204.10.1016/j.catcom.2004.12.009
- Su CK, Chuang HJ, Li CY, Yu CY, Ko BT, Chen JD, Chen MJ. Oxo-bridged bimetallic group 4 complexes bearing amine-bis(benzotriazole phenolate) derivatives as bifunctional catalysts for ring-opening polymerization of lactide and copolymerization of carbon dioxide with cyclohexene oxide. Organometallics. 2014;33:7091–7100.10.1021/om500784a
- Yalcin G, Yildiz U, Kayan A. Preparation of Al, Ti, Zr-perfluoroheptanoate compounds and their use in ring opening polymerization. Appl. Catal., A. 2012;423:205–210.10.1016/j.apcata.2012.02.042
- Mert O, Kayan A. Synthesis and characterization of substituted salicylate zirconium compounds and their catalytic activity over ε-caprolactone. J. Inclusion Phenom. Macrocyclic Chem. 2014;80:409–416.10.1007/s10847-014-0429-z
- Zoller T, Jurkschat K. Novel trialkanolamine derivatives of tin of the type [N (CH2CMe2O)2(CH2)nOSnOR] m (m = 1, 2; n = 2, 3; R = t-Bu, 2,6-Me2C6H3) and related tri-and pentanuclear tin (IV) oxoclusters. Syntheses and molecular structures. Inorg. Chem. 2013;52:1872–1882.
- Sayılkan H, Arpaç E, Şener E. The modification of aluminium tri-sec-butoxide with different alcohols and chelating ligands: hydrolysis and condensation of the products. Syn. React. Inorg. Met.-Org. Chem. 1997;27:1437–1452.10.1080/00945719708003149
- Martinez-Ferrero E, Boubekeur K, Ribot F. Carboxylate-containing tin(IV) ısopropoxides: synthesis and characterization of [Sn(OiPr)(O2CR)2]2 [R = (CH3)CCH2, C6H5, CH3]. Eur. J. Inorg. Chem. 2006;4:802–807.10.1002/(ISSN)1099-0682
- Misaka H, Sakai R, Satoh T, Kakuchi T. Synthesis of high molecular weight and end-functionalized poly(styrene oxide) by living ring-opening polymerization of styrene oxide using the alcohol/phosphazene base ınitiating system. Macromolecules. 2011;44:9099–9107.10.1021/ma202115w
- Brocas AL, Mantzaridis C, Tunc D, Carlotti S. Polyether synthesis: from activated or metal-free anionic ring-opening polymerization of epoxides to functionalization. Prog. Polym. Sci. 2013;38:845–873.10.1016/j.progpolymsci.2012.09.007
- Cabeza JA, Fernández-Colinas JM, García-Álvarez P, Polo D. Diaminogermylene and diaminostannylene derivatives of gold(I): novel AuM and AuM 2 (M = Ge, Sn) complexes. Inorg. Chem. 2012;51:3896–3903.10.1021/ic3001575
- Álvarez-Rodríguez L, Cabeza JA, García-Álvarez P, Polo D. Organic amides as suitable precursors to stabilize stannylenes. Organometallics. 2013;32:3557–3561.10.1021/om400476c
- Seung SLN, Young RN. Polymerization of styrene oxide initiated by iodine and preparation of styrene oxide-2-methyl-2-oxazoline block copolymer. J. Polym. Sci.: Polym. Lett. Ed. 1980;18:89–96.