Abstract
A polymerizable red dye 1-(6-acrylamidohexylamino) anthraquinone (AHAQ) was synthesized and used to prepare colored polymer latexes using styrene and butyl acrylate as main monomers. Influences of the initiator and surfactant amounts, oil/aqueous phase ratio, and AHAQ content on the polymerization process, the particle size, and surface tension of the resulted latex were investigated, and the migration resistance and light fastness of the red latex films were determined. Results showed that the polymerization was carried out smoothly, and the miniemulsion polymerization mechanism was confirmed by monitoring the dimensions of oil phase before and after polymerization. The latex particle size can be adjusted by varying the surfactant amount, and when AHAQ content was 0.2–1 wt% of total monomers, the encapsulation efficiency and dye conversion could be achieved to 98.7 and 96.3%, respectively. The UV–vis spectra of AHAQ and its corresponding covalently colored polymer indicated that the maximum absorption peak was the same, meaning that the chemical structure of chromophore in AHAQ maintains unchanged after undergoing polymerization. The migration resistance and light fastness of the covalently colored latex films were much better than that of the correspondingly non-covalently colored ones.
1. Introduction
People pay increasing attention in environment, safety, and health in the colorants field, and the waterborne polymer system is growingly demanded these years. The dye-containing polymer latexes have been widely applied in many fields, such as coating, paint, and ink industries,[Citation1–6] which can be prepared through emulsion polymerization [Citation7,8] or miniemulsion polymerization [Citation5,9–12] in the presence of oil-soluble dyes. For example, Takasu et al. incorporated copper phthalocyanine and styryl dyes into polystyrene latex particles using miniemulsion polymerization.[Citation13,14] Wu and coworkers encapsulated Sudan Black B by miniemulsion polymerization of styrene,[Citation15] and the colored latexes were also prepared by encapsulation of hydrophilic dyes using a delicate double miniemulsion polymerization.[Citation16] However, because of the poor compatibility and the weak non-covalent interaction between the dye and the polymer matrix, the dye migration from the latex particle or even phase separation is inevitable, leading to unsatisfactory migration fastness and light fastness.[Citation11,17] Incorporating the chromophore of dyes with polymer by covalent bonding is an effective way to enhance the migration resistance.
In our previous work, a series of anthraquinone (AQ) polymerizable dyes were synthesized and covalently colored polymer latexes were prepared through conventional emulsion copolymerization, and the light fastness of the colored latex films were significantly improved.[Citation18–20] However, because the synthesized dyes were barely dissolved in water, it is difficult for these dye molecules to transfer from the monomer droplets to the latex particles through water phase to carry out copolymerization in the conversional emulsion polymerization process, and as a result, the dye conversion was only 82.0% when the dye amount was 1.0% to the total monomers.[Citation18] To improve the incorporation efficiency of the polymerizable dye, a more hydrophilic polymerizable dye was synthesized via six-step organic reaction, and a stable colored polymer latex was prepared with 97.3% conversion of the polymerizable dye through semi-continuous emulsion polymerization.[Citation21]
In this work, in order to simplify the synthesis process, miniemulsion polymerization was explored to prepare covalently colored polymer latex. For this purpose, a red polymerizable dye and its corresponding red nonpolymerizable dye were first synthesized, and the red polymer latexes were then prepared using miniemulsion copolymerization of styrene (St) and butyl acrylate (BA) in the presence of the synthesized dyes. The influences of polymerization recipes on the miniemulsion polymerization were investigated, and the dye migration resistance and light fastness of the covalently colored latex films were studied and compared with the non-covalently colored ones.
2. Experimental
2.1. Materials
Styrene (St) and butyl acrylate (BA) (First Chemical Reagent Factory, Tianjin) were purified by distillation under reduced pressure to remove inhibitor. Ammonium persulfate (APS) (Shanghai Aijian Modern Reagent Co.) was recrystallized in water before use. Sodium dodecyl sulfate (SDS) and hexadecane (HD) were purchased from Aladdin. Ethyl acetate (EA), dichloromethane (CH2Cl2), dimethyl sulfoxide (DMSO), and toluene (Beijing Chemicals) were dried with anhydrous MgSO4 and stored at 4 °C. Acryloyl chloride (Aladdin), propionyl chloride (Aladdin), 1,6-diaminohexane (Aladdin), and 1-nitroanthraquinone (Wuqiao Chemical Co.) were used as received.
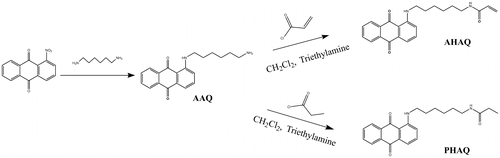
A red polymerizable dye 1-(6-acrylamidohexylamino) anthraquinone (AHAQ) and the corresponding nonpolymerizable dye 1-(6- propionamidohexylamino) anthraquinone (PHAQ) were synthesized according to the literature,[Citation19] and their synthetic reactions were given in Scheme .
2.1.1. Synthesis of 1-(6-aminohexylamino) anthraquinone
In a round-bottom flask equipped with a magnetic stirrer and a condenser, 1-nitroanthraquinone (5.06 g, 20 mmol), potassium carbonate (2.76 g, 20 mmol), and 1,6-diaminohexane (9.28 g, 80 mmol) were suspended in 100 mL DMSO. The reaction mixture was stirred and reacted at 100 °C for 2 h, and the mixture was then cooled to room temperature and poured into 500 mL icy deionized water. The precipitate was collected by filtrating, washing with deionized water three times, and drying at a vacuum oven, and the raw product was then recrystallized from ethyl acetate to obtain 5.94 g red solid (92% yield). 1H NMR (400 MHz, [D6]DMSO, TMS, δ/ppm): 8.20 (d, 1H, AQ 5-H); 8.12 (d, 1H, AQ 8-H); 7.89 (t, 1H, AQ 7-H); 7.83 (t, 1H, AQ 6-H); 7.66 (t, 1H, AQ 3-H); 7.45 (d, 1H, AQ 4-H); 7.26 (d, 1H, AQ 2-H); 3.36 (t, 2H, CH2NH-AQ); 1.67 (t, 2H, CH2NH2); 1.31–1.47 (m, 8H, alkyl H).
2.1.2. Synthesis of AHAQ
To a round-bottom flask equipped with magnetic stirrer and condenser, 100 mL dichloromethane, 1-(6-aminohexylamino) anthraquinone (AAQ) (3.22 g, 10 mmol), and triethylamine (1.5 g, 15 mmol) were added and stirred. Then, the flask was immersed in an ice water bath, and acryloyl chloride (1.27 g, 14 mmol), dissolved in 10 mL CH2Cl2, was dropwise added into the flask in 20 min, and the mixture was stirred for 3 h. After then, the solvent was removed by rotary evaporator, and the raw red product was purified by column chromatography using CH2Cl2/EA (3:1 by volume) as eluent to give 2.94 g of AHAQ (78% yield).
1H NMR (600 MHz, [D6]DMSO, TMS, δ/ppm): 8.17 (d, 1H, AQ 5-H); 8.12 (d, 1H, AQ 8-H); 7.89 (t, 1H, AQ 7-H); 7.84 (t, 1H, AQ 6-H); 7.62 (t, 1H, AQ 3-H); 7.41 (d, 1H, AQ 4-H); 7.20 (d, 1H, AQ 2-H); 6.25 (d × d, 1H, 2-acryloyl H); 6.11 (d, 1H, 3-acryloyl cis H); 5.62 (d, 1H, 3-acryloyl trans H); 3.31 (t, 2H, CH2NH-AQ); 3.14 (t, 2H, CH2NHCO); 1.30–1.71 (m, 8H, alkyl H).
2.1.3. Synthesis of PHAQ
PHAQ was synthesized by a similar procedure as that of AHAQ just using equal mole of propionyl chloride to replace acryloyl chloride.
1H NMR (400 MHz, [D6]DMSO, TMS, δ/ppm): 8.18 (d, 1H, AQ 5-H); 8.11 (d, 1H, AQ 8-H); 7.90 (t, 1H, AQ 7-H); 7.82 (t, 1H, AQ 6-H); 7.63 (t, 1H, AQ 3-H); 7.42 (d, 1H, AQ 4-H); 7.21 (d, 1H, AQ 2-H); 3.39 (t, 2H,CH2NH-AQ); 3.06 (t, 2H, CH2NHCO); 2.07 (m, 2H, CH2CO); 1.30–1.70(m, 8H, alkyl H); 1.01 (t, 3H, CH3).
2.2. Preparation of monomer miniemulsion
In a typical process, the oil phase was prepared by dissolving AHAQ or PHAQ and HD (4 wt% to the St and BA) in the mixture of St and BA. The aqueous phase was SDS solution. In the miniemulsion preparation, the oil phase and the aqueous phase were mixed together and stirred first by a magnetic stirrer for 20 min at around 500 rpm, and the coarse emulsion was then homogenized for 10 min by an ultrasonicator (100 W, Sonics) in an ice bath.
2.3. Miniemulsion polymerization
A series of covalently red P(St-BA-AHAQ) latexes were prepared according to a typical recipe listed in Table . The monomer miniemulsion was charged into a four-necked flask equipped with a mechanic stirrer, a reflux condenser, a nitrogen inlet, and a thermometer. The system was first stirred at 200 rpm, purged with nitrogen for 5 min, and heated to 75 °C in a water bath, and the polymerization was then initiated by injecting APS solution and the reaction lasted for 2 h.
Table 1. Typical recipes of the colored latex preparation via miniemulsion polymerization.
As a control experiment, non-covalently red P(St-BA)/PHAQ latex was prepared according to the same procedure as P(St-BA-AHAQ) latex using the same amount of PHAQ to substitute for AHAQ.
2.4. Characterization
1H NMR spectra (JNM-ECA600, JEOL) were used to confirm the chemical structure of the synthesized dyes. UV–vis absorption of the dye and the latex polymer dissolved in ethyl acetate were characterized by a UV–vis spectrometer (T6, Pgeneral). The hydrodynamic diameter () and size distribution (poly index) were measured on a Zetasizer instrument (3000HS, Malvern) using dynamic light scattering (DLS) method. The morphology of the latex particles were characterized through a transmission electron microscopy (Hitachi H7650B, TEM). The surface tensions of the monomer miniemulsion and the polymer latex were determined on a tensiometer (DCAT21, Dataphascis).
2.4.1. Monomer conversion
The solid content was determined gravimetrically, and the monomer conversion was calculated by the following equation:
where S is the experimental solid content and Sth is the theoretical solid content assuming all of the monomers were polymerized.
2.4.2. Encapsulation efficiency
Certain amount of the red polymer latex was coated on a glass plate and dried at 60 °C in an oven to form a latex film. Then, the film was dissolved in ethyl acetate to gain a red solution (around 10 g/L), and the visible absorption spectroscopy of the solution was measured on a UV–vis spectrometer (T6, Pgeneral). The dye amount in the red latex film was calculated through the calibration curve of the absorbance (at λmax, 503 nm) vs. dye concentration. The encapsulation efficiency was denoted as the ratio of the dye amount in the latex film and that in the corresponding recipe.
2.4.3. Conversion of polymerizable dye AHAQ
A piece of the latex film (around 1 g) was extracted with methanol in a Soxhlet’ extractor for 6 h, and the unreacted dye in the methanol solution was quantified using the calibration curve of the absorbance (at λmax, 508 nm) vs. dye concentration. The dye monomer conversion was calculated from the following equation:
Here, Wex and W represented the weight of the extracted dye from latex film and the dye used in the recipe, respectively.
2.4.4. Dye migration resistance of the colored polymer latex film
About 1 g of the red latex film with the thickness about 100 μm was put into a conical flask containing 50 mL of methanol, and the flask was sealed and placed in a shaker (HZ-9210 K, HLD Laboratory Equipment Company) which was set at 25 °C and 100 rpm, and UV–vis absorption of the resulted methanol solution was measured every 6 h. Percentage of the dye amount dissolved in methanol to the total amount used in the recipe was calculated and used to characterize the migration resistance of the dye in the latex film to methanol. The higher the percentage is, the weaker the migration resistance will be.
2.4.5. Light fastness of the colored polymer latex film
The red latex films with the thickness about 100 μm were irradiated by a xenon lamp in a xenon artificial weathering system (Chongqing Hanzhan Instrument Co. Ltd) for 7 days. The lamp intensity was 180 W/m2, the temperature in the chamber was 40 °C, and the relative humidity was 50%. The color difference (ΔE) between the aged and original films was measured on a white printing paper every 24 h using a colorimeter (HP-200, Chinaspec).
3. Results and discussion
3.1. UV–vis absorption spectra of the synthesized dyes
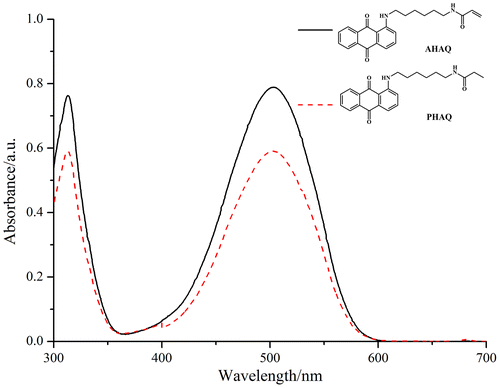
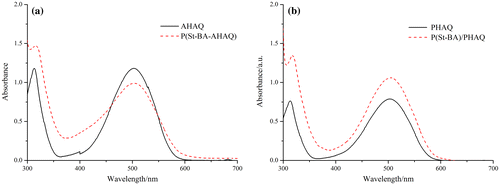
The UV–vis absorption spectra of the red polymerizable dye AHAQ and the nonpolymerizable dye PHAQ were given in Figure . It is clear that both of them possess the same UV–vis absorption characteristics, and the maximum absorption wavelength (λmax) was at 503 nm.
3.2. Fabrication and characterization of the red polymer latex via miniemulsion polymerization
Fabrication of colored polymer latex using miniemulsion polymerization is dependent on many variables such as homogenization device, costabilizer, surfactant, initiator, etc. In this study, the ultrasonification which is a common homogenizing method applied in the laboratory was used to prepare monomer miniemulsion, and HD, a common hydrophobe compound used in the literature,[Citation22] was chosen as costabilizer. The effects of SDS, oil/aqueous phase mass ratio (O/W), APS, and the synthesized dye on the miniemulsion polymerization were investigated.
3.2.1. Characteristics of miniemulsion polymerization
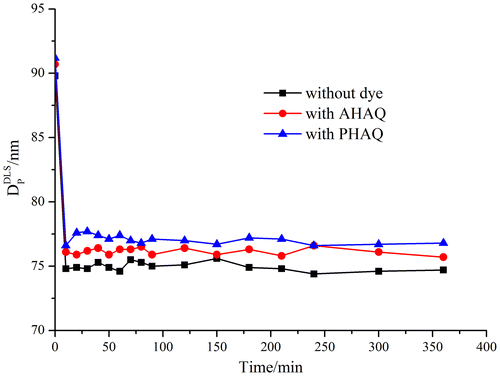
The kinetics investigation of miniemulsion polymerization was mainly focused on single monomer system such as St [Citation22–24] and BA [Citation25] before. Here, miniemulsion copolymerization of St and BA with or without the synthesized dyes was performed under the same conditions with 40 mM SDS to water, and 1.5 wt% of APS and 0.2 wt% of the dye to the St and BA; and the covalently red P(St-BA-AHAQ), non-covalently red P(St-BA)/PHAQ, and colorless P(St-BA) latexes were prepared. In order to confirm the miniemulsion polymerization mechanism, the diameter of oil phase was monitored in the polymerization process. As Figure indicated, the diameter of the monomer droplets decreased immediately right after the addition of the initiator, and about 10 min later, the diameter changed from around 90 to 75 nm. This size decrease was probably due to the addition of APS. Because APS belongs to strong electrolyte, it is ionized into and
in aqueous phase, which certainly compresses the electrical double layer of the monomer droplets to decrease hydrodynamic diameter. After then, the diameter remained approximately constant in the rest of the polymerization time, demonstrating a significant feature of miniemulsion polymerization process.
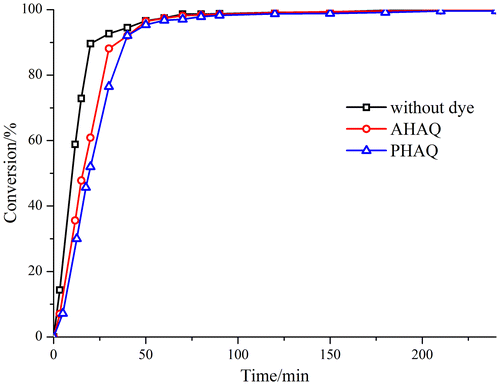
The monomer conversion vs. polymerization time were depicted in Figure . The monomer conversions of all the three polymerization systems rose rapidly after the initiator was introduced. It is notable that for the system without using dye, the monomer conversion was higher than that system containing dye in the first 60 min because of the inhibition effect of the AQ structure of the dyes according to the literature.[Citation18,20] However, all of the final conversions were almost 100%, regardless dye used or not. Thus, the miniemulsion polymerization time was controlled at 2 h in the following study.
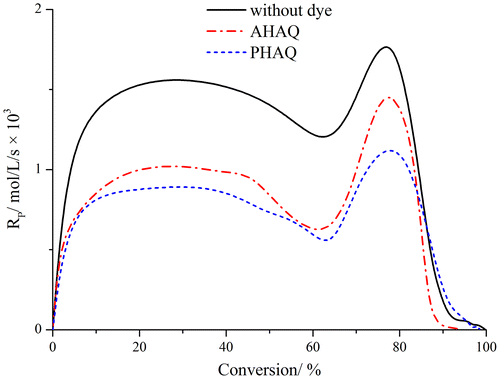
The polymerization rate vs. monomer conversion was plotted in Figure . It is clear that the polymerization kinetics showed a similar trend for all cases. The polymerization went quickly in the first 10% monomer conversion and reached to a maximum plateau, then decreased at around 60% monomer conversion and finally reached another peak at around 80% monomer conversion. The sharp increase of the polymerization rate at high monomer conversion ascribed to a pronounced gel effect. Because of the inhibition of AHAQ and PHAQ,[Citation18,20] the free radical concentration in the dye-containing polymerization systems was lower than that in the system without dye, and as a consequence, the overall polymerization rate was lower as shown in Figure . Along with the polymerization in the presence of AHAQ, AHAQ was gradually incorporated into the polymer; thus, the mobility of the AQ groups decreased compared with the polymerization system with PHAQ. This may be the cause of the difference of polymerization rate with AHAQ and PHAQ.
3.2.2. Influence of APS Amount
Since AQ compound like AHAQ has some inhibition effect on free radical polymerization,[Citation26] sufficient amount of initiator should be used to reach a high monomer conversion in this work. Thus, the miniemulsion polymerizations of St, BA, and AHAQ were carried out with various amounts of APS, and results were listed in Table . The monomer conversion increased with increasing APS amount. When the APS amount was 0.5 wt% of the St and BA, the monomer conversion was only 85.5%. When this amount was equal to or more than 1.5 wt%, the monomer conversion reached nearly 100%. It is also worth to mention that the particle size decreased and particle size distribution narrowed with the increase of APS amount. This may be due to the fact that when the APS amount was insufficient, some of the monomer droplets could not be initiated, and the monomers in these uninitiated droplets were eventually transferred into the initiated latex particles to polymerize by means of conversional emulsion polymerization mechanism, resulting in the large particle size and broaden size distribution. When the APS amount was equal to or more than 1.5 wt%, the droplet nucleation was dominant and as a result, the particle size decreased and its distribution narrowed slowly.
Table 2. Influence of APS amount on the monomer conversion and the particle size and its distribution.Table Footnotea
3.2.3. Effect of SDS amount
Surfactant is the key parameter for the polymerization process and the latex properties. To illustrate the influence of SDS amount, a series of latexes were prepared with various SDS concentrations under the same conditions, and results were given in Table . Experiments showed that the polymerization carried out steadily, and stable colored polymer latexes could be obtained with high monomer conversion. It is obvious that with the increase of the SDS concentration from 10 to 40 mM, the particle diameter decreased from 122 to 75 nm. According to the miniemulsion polymerization principle, the concentration of free surfactants in the polymerization system should be less than its CMC to avoid micelle nucleation, and most of surfactants must be covered on the surface of the monomer droplets to ensure droplet nucleation.[Citation27] Because the occupied surface area per SDS molecule and the O/W were kept unchanged in this work, more amount of SDS could provide larger covered area and as a result, the particle size decreased with the increase of SDS concentration.
Table 3. Properties of the colored latex prepared with different amounts of SDS.Table Footnotea
The surface tension of the polymer latex was also dependent on the SDS concentration. It is believed that in a latex system, surfactant molecules are in an adsorption equilibrium between the particle surface and aqueous phase, and surface tension of the latex is controlled by the free surfactant concentration in aqueous phase. A relatively low SDS total concentration will lead to a low equilibrium free SDS concentration in aqueous phase, bring about a higher surface tension. Thus, the surface tension of the latex lowered from 63.7 to 55.6 mN/m as the SDS concentration increased from 10 to 40 mM according to Table .
3.2.4. Influences of oil/aqueous mass ratio
O/W is another parameter concerned in the miniemulsion polymerization. A series of P(St-BA-AHAQ) latexes with O/W ranged from 10/90 to 30/70 were prepared, and results were listed in Table . It is well known that the same surfactant concentration will provide the same covered area under the same conditions. With the increase of O/W, more amount of oil (monomers) will be stabilized with the same amount of SDS, and in this case, the size of formed monomer droplets must be increased so as to maintain the total surface area of the droplets unchanged, meanwhile the free SDS molecules in the aqueous was decreased. Thus, with the O/W increased from 10/90 to 30/70, the particle size increased from 55.9 to 91.3 nm, and the surface tension of the colored latex increased from 47.4 to 60.1 mN/m.
Table 4. Properties of the P(St-BA-AHAQ) latexes prepared with different O/W.Table Footnotea
3.2.5. Influences of AHAQ content
The encapsulation efficiency and dye conversion are crucial for the colored latex preparation. As the data shown in Table , the encapsulation efficiency and dye conversion of all runs were higher than 97 and 95%, respectively, and no downtrend was observed when AHAQ content increased from 0.2 to 1.0 wt%. However, when the dye amount was 1.0 wt% in a semi-continuous emulsion polymerization process, the dye conversion was only 82% according to literature.[Citation18] This is an advantage of miniemulsion polymerization against conventional emulsion polymerization in the preparation of covalently colored latexes. Because AHAQ is water insoluble, it is difficult for AHAQ molecules to transfer from monomer droplets to the latex particles in conventional emulsion polymerization process. While in miniemulsion polymerization process, both the particle nucleation and the particle propagation occur in the monomer droplets where all of the monomers including dye exist in, and monomer molecules no longer need to transfer from reservoirs to polymerizing sites, thus both the dye encapsulation efficiency and the dye conversion can be reached high levels.
Table 5. Influence of dye content on the encapsulation efficiency and monomer conversion.Table Footnotea
3.2.6. Morphology of the latex particles
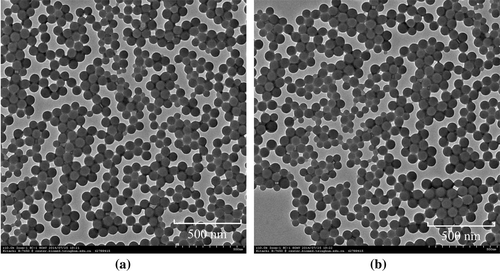
As TEM images in Figure indicated, the morphology of both the covalently colored (sample S7) and non-covalently colored latex (sample S8, the control sample P(St-BA)/PHAQ) was spherical, and the average diameter was about 60 nm, which was smaller than the of sample S7 (75.3 nm) because the hydrodynamic diameter of the latex particles is normally bigger than the diameter of its dried state as reported.[Citation13]
3.3. UV–vis spectra of the dyes and the colored latex polymers
The dyes and the corresponding colored latex polymers were dissolved in ethyl acetate to determine their UV–vis absorption spectra. As shown in Figure (a), the λmax of AHAQ and P(St-BA-AHAQ) appeared at 503 nm, and there was barely noticeable batho- or hypsochromic shift, indicating that the chromophore structure did not change after underwent polymerization, and the chromophores were uniformly distributed in the polymer matrix without stacking or aggregation. The same phenomenon was also observed for PHAQ and P(St-BA)/PHAQ as shown in Figure (b).
3.4. Dye migration resistance
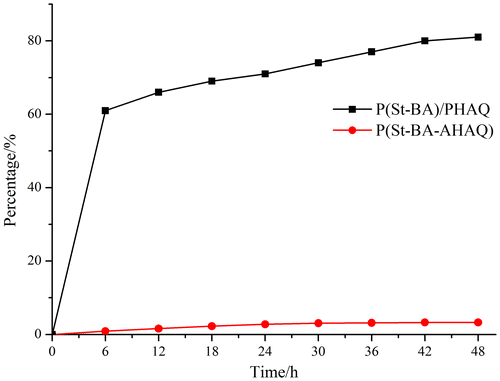
Because methanol is a good solvent of the dyes and a poor solvent of the colored latex polymers, the migration resistances of the colored latex films (sample S7 and corresponding control sample S8) to methanol were determined. As Figure indicated, the dye migration resistance of the P(St-BA)/PHAQ latex film was far worse than that of the P(St-BA-AHAQ) latex film. For example, after 48 h extraction, more than 80% the dye was dissolved into methanol for the P(St-BA)/PHAQ latex film, while this datum was only about 3% for the P(St-BA-AHAQ) latex film. This big difference was due to the interaction of the dye unit and polymer matrix. In the P(St-BA-AHAQ) latex film, since AHAQ units were covalently bonded to the polymer chain, they could not be migrated from the latex film into methanol. To the contrary, in the P(St-BA)/PHAQ latex film, PHAQ molecules were just dispersed in the polymer matrix, so they were easily and quickly dissolved into methanol when the films were immersed into methanol.
3.5. Light fastness of the colored latex film
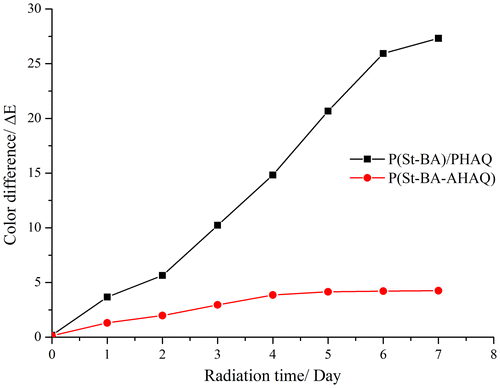
The light fastness of the P(St-BA-AHAQ) and P(St-BA)/PHAQ latex films (from samples S7 and S8, respectively) was determined by xenon arc aging measurement, and the color difference (ΔE) of the aged and original films was utilized to evaluate the light fastness. As depicted in Figure , ΔE of the P(St-BA-AHAQ) film was much smaller than that of the P(St-BA)/PHAQ film, indicating that the covalently bonding chromophores to polymer chains is an effective method to significantly enhance the light fastness of the colored latex film.
4. Conclusion
In this work, covalently red P(St-BA-AHAQ) latexes were fabricated through miniemulsion polymerization. It was found that the miniemulsion polymerization was carried out smoothly, and the AHAQ units were successfully incorporated into polymer chains. When the dye amount was 0.2–1 wt% of St and BA, the encapsulation efficiency and dye conversion could be achieved to 98.7 and 96.3%, respectively. In addition, the dye migration resistance and light fastness of the covalently red P(St-BA-AHAQ) latex film were far better than that of the non-covalently red P(St-BA)/PHAQ one. Accordingly, miniemulsion polymerization is a facile and suitable process to encapsulate water-insoluble dyes, and the resulted covalently colored polymer latex has prominent advantages in migration resistance and light fastness aspects, which can be used as colorful film-forming materials in the fields of waterborne coatings, paints, inks, etc.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Magdassi S, Ben-Moshe M. Patterning of organic nanoparticles by ink-jet printing of microemulsions. Langmuir. 2003;19:939–942.10.1021/la026439h
- Magdassi S, Ben-Moshe M. Aqueous ink composition for ink jets printers comprises oil-soluble dye particles which are dissolved in oil particles of oil-solvent system to form mini-emulsion comprising particles of preset particle size in water. European Patents EP 1,282,670-A2. 2001 May 14.
- Wang X, Chen HD. Colorant mixture useful for forming ink jet inks for ink jet printing, comprises a water insoluble dye and an organic medium comprising ethylenically-unsaturated monomers. European Patents EP 1,319,671-A1. 2003 June 18.
- Lelu S, Novat C, Graillat C, et al. Encapsulation of an organic phthalocyanine blue pigment into polystyrene latex particles using a miniemulsion polymerization process. Polym. Int. 2003;52:542–547.10.1002/(ISSN)1097-0126
- An L, Cai ZJ, Wang WW, et al. A thermo-sensitive imaging coating derived from polymer nanoparticles containing infrared absorbing dye. Eur. Polym. J. 2014;52:166–171.10.1016/j.eurpolymj.2014.01.002
- Hu ZK, Xue MZ, Zhang Q, et al. Nanocolorants: a novel class of colorants, the preparation and performance characterization. Dyes Pigm. 2008;76:173–178.10.1016/j.dyepig.2006.08.026
- McDonald CJ, Bouck KJ, Chaput AB, et al. Emulsion polymerization of voided particles by encapsulation of a nonsolvent. Macromolecules. 2000;33:1593–1605.10.1021/ma991284e
- Yu DG, An JH, Bae JY, et al. Preparation and characterization of acrylic-based electronic inks by in situ emulsifier-free emulsion polymerization for electrophoretic displays. Chem. Mater. 2004;16:4693–4698.10.1021/cm049704o
- Tiarks FK, Landfester K, Antonietti M. Encapsulation of carbon black by miniemulsion polymerization. Macromol. Chem. Phys. 2001;202:51–60.10.1002/(ISSN)1521-3935
- Takasu M, Kawaguchi H. Preparation of colored latex with polyurea shell by miniemulsion polymerization. Colloid Polym. Sci. 2005;283:805–811.10.1007/s00396-004-1248-3
- Zhao X, Meng Q, Liu JW, et al. Hydrophobic dye/polymer composite colorants synthesized by miniemulsion solvent evaporation technique. Dyes Pigm. 2014;100:41–49.10.1016/j.dyepig.2013.07.028
- Landfester K. Miniemulsion polymerization and the structure of polymer and hybrid nanoparticles. Angew. Chem.-Int. Edit. 2009;48:4488–4507.10.1002/anie.v48:25
- Takasu M, Shiroya T, Takeshita K, et al. Preparation of colored latex containing oil-soluble dyes with high dye content by mini-emulsion polymerization. Colloid Polym. Sci. 2003;282:119–126.10.1007/s00396-003-0902-5
- Takasu M, Shiroya T, Takeshita K, et al. Improvement of the storage stability and photostability of colored latex prepared by miniemulsion polymerization. Colloid Polym. Sci. 2004;282:740–746.10.1007/s00396-003-1008-9
- Zhao X, Zhou SX, Chen M, et al. Effective encapsulation of sudan black B with polystyrene using miniemulsion polymerization. Colloid Polym. Sci. 2009;287:969–977.10.1007/s00396-009-2053-9
- Zhao X, Zhou SX, Chen M, et al. Encapsulation of hydrophilic dyes with polystyrene using double miniemulsion technique. J. Appl. Polym. Sci. 2011;119:3615–3622.10.1002/app.v119.6
- Zhao X, Liu JW, Dai HJ, et al. Preparation of composite nano-colorants using mini-emulsion/solvent evaporation technique. CIESC J. 2014;65:1118–1125.
- Li BT, Shen J, Ji WJ, et al. Preparation of covalently-colored polymer latex via batch emulsion polymerization. Chin. J. Chem. 2012;30:2338–2342.10.1002/cjoc.201200541
- Li BT, Shen J, Jiang YM, et al. Preparation and properties of covalently colored polymer latex based on a new anthraquinone monomer. J. Appl. Polym. Sci. 2013;129:1484–1490.10.1002/app.38848
- Li BT, Shen J, Liang RB, et al. Synthesis and characterization of covalently colored polymer latex based on new polymerizable anthraquinone dyes. Colloid Polym. Sci. 2012;290:1893–1900.10.1007/s00396-012-2718-7
- Li BT, Jiang YM, Wang JS, et al. Preparation and properties of a new polymerizable anthraquinone dye and its covalently colored polymer latex. Acta Polym. Sin. 2013;11:1445–1492.
- Chern CS, Chen TJ, Liou YC. Miniemulsion polymerization of styrene in the presence of a water-insoluble blue dye. Polymer. 1998;39:3767–3777.10.1016/S0032-3861(97)10347-0
- Landfester K, Bechthold N, Förster S, et al. Evidence for the preservation of the particle identity in miniemulsion polymerization. Macromol. Rapid Commun. 1999;20:81–84.10.1002/(ISSN)1521-3927
- Ugelstad J, Hansen FK, Lange S. Emulsion polymerization of styrene with sodium hexadecyl sulfate/hexadecanol mixtures as emulsifiers-initiation in monomer droplets. Makromolekulare Chemie-Macromolecular Chemistry and Physics. 1974;175:507–521.10.1002/macp.1974.021750214
- Yildiz U, Capek I, Sarov Y, et al. Kinetics and colloidal parameters of miniemulsion polymerization of butyl acrylate. Polym. Int. 2009;58:1411–1421.10.1002/pi.v58:12
- Odian G. Principles of polymerization. Hoboken: Wiley; 2004.10.1002/047147875X
- Asua JM. Miniemulsion polymerization. Prog. Polym. Sci. 2002;27:1283–1346.10.1016/S0079-6700(02)00010-2