Abstract
We have synthesized novel biobased and environmental friendly mid-chain functional macrophotoinitiators of poly(D,L-lactide) homopolymer (PDLLA-PI-PDLLA) and tetrablock poly(D,L-lactide)-poly(ε-caprolactone) copolymer (PDLLA-PCL-PI-PCL-PDLLA) with controlled molecular weights and narrow molecular weight distributions via ring-opening polymerization (ROP). Mid-chain functional poly(D,L-lactide) (PDLLA-PI-PDLLA) macrophotoinitiator was prepared by ROP of D,L-lactide (DLLA) monomer using a dihydroxy-functional photoinitiator (HO-PI-OH) as the initiator and stannous-2-ethyl hexanoate (Sn(Oct)2) as the catalyst. Tetrablock poly(D,L-lactide)-poly(ε-caprolactone) copolymer (PDLLA-PCL-PI-PCL-PDLLA) with a mid-chain photofunctional group was synthesized via sequential ROP of ε-caprolactone and D,L-lactide using Sn(Oct)2 as the catalyst. The resultant polymers were fully characterized by means of 1H NMR, FTIR, UV–vis, Fluorescence, and GPC techniques. The obtained macrophotoinitiators were used as a prepolymer in photoinitiated free radical polymerization for obtaining AB-type diblock copolymer and ABC-type triblock terpolymer.
1. Introduction
In recent years, there has been a growing interest in the synthesis and characterization of biodegradable and biocompatible polymers. Among them, poly(lactides) (PLA), poly(ε-caprolactone) (PCL), and their copolymers have attracted both academic and industrial attention as a biological and medical material because of their biodegradability, biocompatibility, and non-toxicity.[Citation1–3] The ring-opening polymerization (ROP) of cyclic esters initiated by metal alkoxides/hydroxyl functionalized initiator systems, via coordination–insertion mechanism, is the most efficient approach to produce polymers based on PCL and PLA with controlled molecular weights and lower molecular weight distributions. In the synthesis of PLA- and PCL-based polymers, the most suitable and practical method is ROP initiated technique by metal alkoxides, such as aluminum,[Citation4,5] zinc,[Citation6–8] and tin alkoxides.[Citation9,10] Among them, tin octoate, Sn(Oct)2, is certainly the catalyst most widely used [Citation9,11,12] to synthesize such designed polymers via coordination–insertion mechanism. In particular, when used in conjunction with hydroxyl functional compounds or prepolymers, telecehelics, linear, and star-shaped block copolymers, or networks can be obtained [Citation10,13–15] via corresponding alkyl octoate formation.
Synthesis of well-defined biocompatible and biodegradable amphiphilic block copolymers based on PCL and PLA using Sn(Oct)2/alcohol systems by sequential ROP technique has recently received considerable attention owing to ease of synthesis, outstanding control over polymer molar weight and composition and their extraordinary properties. The sequence in which blocks are synthesized into block copolymers is specific and determined by the choice of monomer and catalyst. When tin octoate used with an alcohol compound as the catalyst/initiator systems well-defined block copolymer of PCL-PLA is formed if the ε-caprolactone is polymerized first, followed by polymerization of L-lactide monomer. Many block copolymers have been synthesized by using this way in the literature. Feijen et al. [Citation16] have reported the preparation of designed PCL and PLA diblock copolymers with tin octoate/alcohol system in satisfactory controlled conditions. Kim et al. [Citation17] reported the synthesis and crystallization behavior of PLLA-b-PCL diblock copolymer obtained by sequential ROP using hydroxyl terminated PCL as the macroinitiator and L-lactide as the monomer. Enantiomeric diblock and triblock copolymers from caprolactone and lactide with various compositions were synthesized using tin octoate/alcohol as initiator system by Pensec and co-workers.[Citation18] Wang et al. [Citation19] and Maglio et al. [Citation20] have reported the synthesis and characterization of PLA-PCL-PLA triblock copolymers by the bulk copolymerization of PCL prepolymers and L-lactide monomer. More recently, novel pentablock copolymer (PLA–PCL–PEG–PCL–PLA)-based nanoparticles for controlled drug delivery and multiblock thermoplastic elastomers based on PCL derivative and PLA have been synthesized and characterized.[Citation14,21] Here, we describe the controlled synthesis of well-defined mid-chain macrophotoinitiators of PDLLA polymer by ROP and PCL-PDLLA tetrablock copolymer by sequential ROP using Sn(Oct)2/alcohol as the initiating systems.
Macrophotoinitiators can be defined as polymers containing end-chain, mid-chain, or pendant photoreactive groups which through a light absorption process can generate active species able to initiate the polymerization and crosslinking of mono- and multi-functional monomers and oligomers.[Citation13,22–24] Recently, the macrophotoinitiators have received great interest, because of their outstanding properties, such as reducing migration, masking unpleasant odors, and improving solubility and compatibility within the formulations.[Citation25–28] There are two ways offered for the preparation of macromolecules possessing photoreactive groups in pendant, end-chain, or mid-chain: (i) synthesis and polymerization of monomers having photoreactive groups [Citation29]; (ii) introduction of photoactive groups into polymer by using photofunctional initiators in a particular polymerization [Citation13] or by reacting functional groups of a preformed polymer with other functional groups of low molecular weight compounds possessing also photoreactive groups.[Citation30,31]
Well-defined macrophotoinitiators can be prepared by ring-opening polymerization (ROP) or atom transfer radical polymerization (ATRP) methods, or by functionalization of the polymers prepared by these techniques (ATRP or ROP) using initiators having suitable functional and photoreactive groups.[Citation32–34] Many well-defined end- and mid-chain functional macrophotoinitiator of polystyrene (PSt),[Citation34–37] Poly(methyl methacrylate) (PMMA), [Citation38] and poly(ε-caprolactone) (PCL) [Citation13,39,40] have been synthesized and characterized by us and the other groups. In this study, we wish to report for the first time the synthesis and characterization of novel well-defined mid-chain macrophotoinitiators of PDLLA homopolymer and tetrablock PDLLA-PCL copolymer, that have a potential to initiate light-induced free radical polymerization, by ring-opening polymerization of D,L-lactide (LA) in the presence of dihydroxy functionalized a photoinitiator and PCL macrophotoinitiator, respectively.
2. Experimental
2.1. Materials
D,L-lactide (DLLA, monomer) (Aldrich) was recrystallized from ethyl acetate. ε-caprolactone (ε-CL, monomer) (Aldrich) and methyl methacrylate (MMA, monomer) were dried over CaH2 followed by distillation under reduced pressure. The photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methylpropan-1-one (HO-PI-OH, Irgacure 2959) was received from Ciba Specialty Chemicals and used without further purification. Stannous 2-ethylhexanoate [Sn(Oct)2] (Aldrich) was used as received. Dichloromethane (CH2Cl2) (Lab-scan), methanol (Lab-scan) and all other chemicals were used as received.
2.2. Characterization
1H NMR spectra were recorded on an Agilent 400 MHz NMR with CDCl3 as the solvent and tetramethylsilane as the internal standard. Fourier transform infrared (FTIR) spectra were measured with a Perkin–Elmer Spectrum Two FTIR spectrophotometer. UV–vis spectra were taken in CH2Cl2 solution by Schimadzu 1601 spectrophotometer. Fluorescence spectra were registered in CH2Cl2 solution by Jasco FP-6300 spectrofluorometer. The molecular weights and molecular weight distributions were determined by gel permeation chromatography (GPC) using a Viscotek GPCmax Autosampler system consisting of a pump, three Visco-GEL GPC columns (G2000HHR, G3000HHR, and G4000HHR), a Viscotek UV detector, and a Viscotek differential refractive index (RI) detector with a THF flow rate of 1.0 mL min−1 at 30 °C. GPC was calibrated with monodisperse polystyrene standards. Average molecular weight values from GPC were calculated from the polystyrene calibration curve (Mn = X. Mn GPC) using the correcting coefficient X. The correction factor, X, was taken as 0.58 for PDLLA containing polymers.[Citation41]
2.3. Synthesis of mid-chain functional macrophotoinitiator of poly(D,L-lactide) (PDLLA- PI-PDLLA)
The ring-opening polymerization (ROP) of D,L-lactide (DLLA) in the presence of Irgacure 2959 (HO-PI-OH) as the initiator and Sn(Oct)2 as the catalyst yielded mid-chain photofunctional poly(D,L-lactide) (PDLLA-PI-PDLLA). The polymerization reactions were carried out in bulk under nitrogen atmosphere at 130 °C. Appropriate amounts of the monomer (DDLA), stannous octoate (Sn(Oct)2), and photoinitiator (HO-PI-OH, Irgacure 2959) (see Table ) were charged into a previously flamed and nitrogen-purged Schlenk tube equipped with a magnetic stirrer. After the polymerization, the mixtures were dissolved in dichloromethane (CH2Cl2) and precipitated twice in excess of methanol. The precipitates were filtered and dried in vacuo at room temperature for 3 days.
Table 1. Synthesis of homo PDLLA-PI-PDLLATable Footnotea and tetrablock PDLLA-PCL-PI-PCL-PDLLATable Footnoteb macrophotoinitiators.
2.4. Synthesis of mid-chain functional macrophotoinitiator of poly(D,L-lactide)- poly(ε-caprolactone) tetrablock copolymer (PDLLA-PCL-PI-PCL-PDLLA)
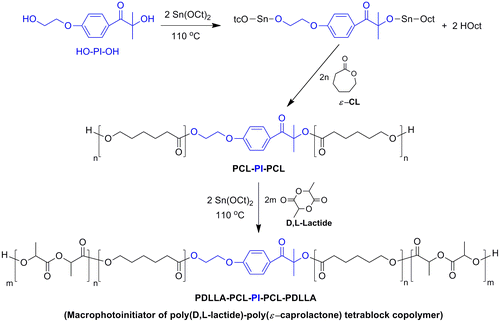
Mid-chain functional macrophotoinitiator of poly(D,L-lactide)-poly(ε-caprolactone) tetrablock copolymer (PDLLA-PCL-PI-PCL-PDLLA) was synthesized via sequential ROP of ε-caprolactone (ε-CL) and D,L-lactide (DLLA) using Sn(Oct)2 as the catalyst. First, dihydroxy-functionalized PCL macrophotoinitiator (PCL-PI-PCL, prepolymer) was prepared by ROP of ε-CL using Irgacure 2959 (HO-PI-OH) as the initiator according to the procedure described in our previous paper [Citation13] ((Yield: 100%, Mn theo = 4800, Mn H NMR = 5000, Mn GPC = 4600 Mw/Mn = 1.36), the detailed polymerization conditions and characterizations were given in Ref. [Citation6]). Then, the obtained dihydroxy-functionalized PCL prepolymer (PCL-PI-PCL) was used as the macroinitiator for subsequent copolymerization with D,L-lactide to yield PDLLA-PCL-PI-PCL-PDLLA tetrablock copolymer. Typically, PCL-PI-PCL (1.00 g) was vacuum-dried at room temperature for 24 h and then placed into a previously flamed and nitrogen-purged Schlenk tube equipped with a magnetic stirrer. DDLA (1.15 g, 8.00 mmol) and Sn(Oct)2 (0.60 mg, 1.49 × 10−3 mmol) were added to the tube, and the polymerization reaction was performed at 130 °C. After 6 h, the polymerization was terminated by cooling the tube to the room temperature, then diluted with methylene chloride and poured into a tenfold excess of cold methanol. The tetrablock copolymer was collected after filtration and dried at room temperature under vacuum to constant weight with a yield of 80%. Mn GPC = 10,200 Mw/Mn = 1.34.
2.5. Photopolymerization
Photopolymerization reactions were carried out in bulk. Appropriate amounts of macrophotoinitiators (PDLLA-PI-PDLLA or PDLLA-PCL-PI-PCL-PDLLA) and monomer (MMA) were added in Pyrex tubes and degassed with nitrogen prior to irradiation by a merry-go-round-type photoreactor equipped with 16 Philips 8 W/08 lamps emitting light nominally at λ > 300 nm and a cooling system. The block copolymers obtained were purified by column chromatography to remove unreacted prepolymers, then precipitated in methanol and dried under vacuum at room temperature for 24 h. Conversions were calculated gravimetrically.
3. Results and discussion
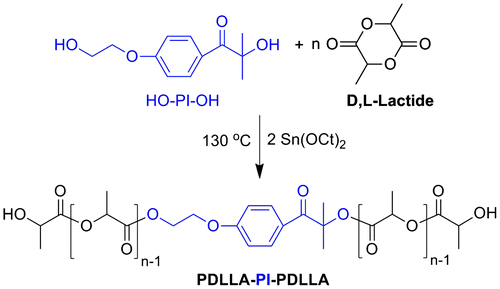
Novel biobased and environmental friendly mid-chain functional macrophotoinitiators of poly(D,L-lactide) homopolymer (PDLLA-PI-PDLLA) and tetrablock poly(D,L-lactide)-poly(ε-caprolactone) copolymer (PDLLA-PCL-PI-PCL-PDLLA) were synthesized by ring-opening polymerization (ROP). PDLLA-PI-PDLLA macrophotoinitiator was synthesized by bulk ROP of DLLA monomer using Irgacure 2959 (HO-PI-OH) as the initiator and Sn(Oct)2 as the catalyst (Scheme ).
In the preparation of tetrablock PDLLA-PCL-PI-PCL-PDLLA macrophotoinitiator, sequential ring-opening polymerization method was applied. The process for the synthesis of tetrablock macrophotoinitiator involved two steps as shown in Scheme . In the first step, ring-opening polymerization of ε-caprolactone started by the dihydroxyl photoinitiator (HO-PI-OH)/Sn(Oct)2 initiating system formed a dihydroxy-functionalized PCL macrophotoinitiator with central PI group (PCL-PI-PCL). In the second step, the obtained this PCL macrophotoinitiator served as macroinitiator for the synthesis of PDLLA-PCL-PI-PCL-PDLLA tetrablock copolymer by ring-opening polymerization of D,L-lactide in the presence of Sn(Oct)2 (Scheme ).
The ring-opening polymerization of DL-lactide (DLLA) was conducted in bulk at 130 °C. The mol ratio of [HO-PI-OH] or [PCL-PI-PCL]/[Sn(Oct)2] was taken as 134/1 for the synthesis of PDLLA-PI-PDLLA and PDLLA-PCL-PI-PCL-PDLLA. The experimental conditions and results for the synthesis of these macrophotoinitiators are summarized in Table . As can be seen from the table, the molecular weight distributions of the resulting macrophotoinitiators are relatively narrow and the measured and theoretical molecular weights generally fit with those determined by 1H NMR analyses, showing the controlled character of the ring-opening polymerization of DLLA with the initiators used. Moreover, the agreements in the molar masses of the macrophotoinitiators (PDLLA-PI-PDLLA or PDLLA-PCL-PI-PCL-PDLLA) indicate that each hydroxyl group derived from the initiators start growth of exactly one polymer chain.
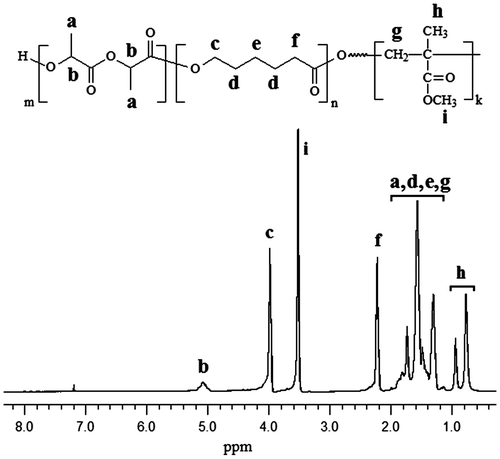
The chemical structure of new macrophotoinitiators could be confirmed by spectroscopic investigations. Figure shows the 1H NMR spectra of the PDLLA-PI-PDLLA and PDLLA-PCL-PI-PCL-PDLLA synthesized. Typical signals of PDLLA and PCL segments were observed. In Figure (a) together with the typical signals of PDLLA units, the signals corresponding to the mid-chain group of PDLLA-PI-PDLLA were also observed. The signals of methyl (–CH3) and methine (–CH–) protons of PDLLA segment were observed at around 1.47–1.55 and 5.07–5.17 ppm, respectively, while the aromatic and methylene (–CH2–) protons of PI group in the mid-point of PDLLA polymer were recorded at around 7.98–8.00, 7.27–7.29, 6.80–6.88 ppm (aromatic) and 4.39–4.43, 4.09–4.18 ppm (OCH2CH2O), respectively. Figure (b) shows the 1H NMR of PDLLA-PCL-PI-PCL-PDLLA macrophotoinitiator. The characteristic peaks of PCL, PDLLA, and PI unit can be clearly seen from this spectrum. The corresponding assignments are given in the Figure (b). The copolymer composition for each segment was determined by 1H NMR spectrum given in Figure (b). The mol percentage compositions of copolymer were found as 42% for PDLLA and 58% for PCL segment. The structure of PDLLA-PI-PDLLA and PDLLA-PCL-PI-PCL-PDLLA were further supported by the observations from FTIR measurements. The IR spectra contain the characteristic CO ester band of PDLLA and PCL segments and the CO keto group of the photoinitiator moiety.
Figure 1. 1H NMR spectra of PDLLA-PI-PDLLA (a) and PDLLA-PCL-PI-PCL-PDLLA (b) macrophotoinitiators in CDCl3.
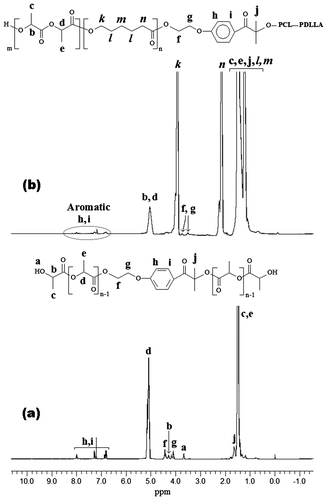
The formations of macrophotoinitiators were also confirmed by GPC measurements (Figure ). The GPC traces are unimodal and narrow indicating that no side reactions occurred during ROP reactions. The peak at lower retention volume (Figure (b)) is ascribed to the PDLLA-PCL-PI-PCL-PDLLA macrophotoinitiator and no signal shoulders attributed to starting polymer (PCL-PI-PCL) can be detected.
Figure 2. GPC traces of PDLLA-PI-PDLLA (Table , Run 1) (a), and PDLLA-PCL-PI-PCL-PDLLA (Table , Run 3) (b), macrophotoinitiators, and PDLLA-PMMA diblock copolymer (c), and PDLLA-PCL-PMMA triblock terpolymer (d).
The relative number-averaged molecular weights and polydispersities were found from GPC measurements. The 1H NMR was used to measure the absolute molecular weights (Mn, H NMR) of macrophotoinitiators (which agreed with those of the calculated values (Mn theo) based on the monomer conversion and monomer-to-initiator ratio in each case (see Table )) using the following equations:(1)
(2)
(3)
(4)
(5)
where Mn PDLLA-PI-PDLLA, Mn PDLLA-PCL-PI-PCL-PDLLA, and Mn PCL-PI-PCL are the 1H NMR molecular weights of macrophotoinitiators; MLA, MHO-PI-OH, and MDLLA are the molecular weight of the lactic acid unit (72.06 g/mol), photoinitiator (224.26 g/mol), and D,L-lactide (144.13 g/mol); DPPCL, DPPLA, and DPPDLLA are the degree of polymerization for PCL, for PLA units in PDLLA-PI-PDLLA and for PDLLA segments in PDLLA-PCL-PI-PCL-PDLLA; IPLA, IAr, IPDLLA, and IPCL are the integral values of the signals at 5.17–5.07 ppm (CH in PDLLA-PI-PDLLA), at 8.00–6.88 ppm (aromatic protons in photoinitiator group), at 5.22–5.08 ppm (two CH protons of PDLLA segment in PDLLA-PCL-PI-PCL-PDLLA) and at 2.50 ppm (CH2-CO of PCL segment in PDLLA-PCL-PI-PCL-PDLLA), respectively.
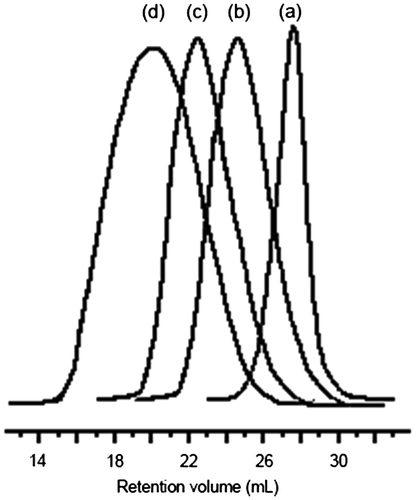
The presence of photoreactive group (PI) into mid-chain of the poly(D,L-lactide) homopolymer and tetrablock poly(D,L-lactide)-poly(ε-caprolactone) copolymer were also evidenced by UV absorption and fluorescence emission measurements. Figure shows UV–vis spectra of PDLLA-PI-PDLLA and PDLLA-PCL-PI-PCL-PDLLA in CH2Cl2 solution. It can be seen that each spectrum exhibits the usual characteristic absorption band of PI derivatives. Fluorescence emission spectrum of the compound PDLLA-PCL-PI-PCL-PDLLA with PDLLA-PI-PDLLA, in CH2Cl2 at room temperature has been shown in Figure . Each spectrum shows the vibrational structure of the aryl ketone chromophore. All these spectroscopic observations confirm that the photochromophoric PI group was protected under the ring-opening polymerization reaction conditions.
Figure 3. UV absorption spectra of PDLLA-PI-PDLLA (0.88 g L−1) and PDLLA-PCL-PI-PCL-PDLLA (1.00 g L−1) macrophotoinitiators in CH2Cl2.
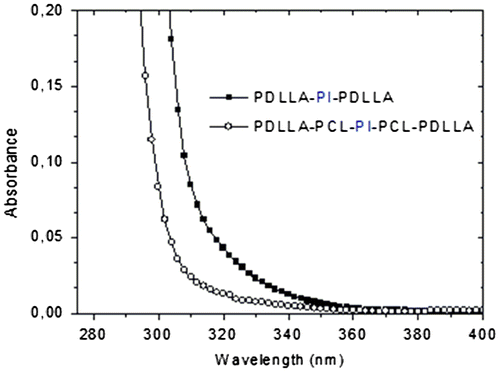
Figure 4. Fluorescence spectra of PDLLA-PI-PDLLA (0.51 g L−1) and PDLLA-PCL-PI-PCL-PDLLA (1.00 g L−1) macrophotoinitiators in CH2Cl2, λexc = 325 nm.
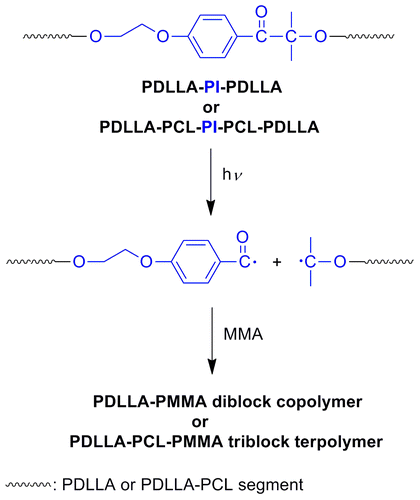
Even more convincing proof for the availability of the PI group in the middle point of the polymer chains was obtained from the photopolymerization studies of the PDLLA-PI-PDLLA and PDLLA-PCL-PI-PCL-PDLLA macrophotoinitiators produced by means of ring-opening polymerization. Photoinduced free radical polymerization reaction of methyl methacrylate (MMA) at room temperature with the macrophotoinitiators (PDLLA-PI-PDLLA or PDLLA-PCL-PI-PCL-PDLLA) is shown in Scheme . UV irradiation of the macrophotoinitiators caused α-scission and yielded two polymer bound radicals, as shown in the following reaction. Both of these polymeric radicals initiate the polymerization of MMA, consequently PDLLA-b-PMMA (AB-type) diblock copolymer or PDLLA-b-PCL-b-PMMA (ABC-type) triblock terpolymer is produced after 30 min irradiation time. The conversion of MMA was found 13% for PDLLA-b-PMMA and 8% PDLLA-b-PCL-b-PMMA copolymer. The unreacted PDLLA-PI-PDLLA or PDLLA-PCL-PI-PCL-PDLLA macrophotoinitiator (~20–25% by weight) was removed by using column chromatography. A control experiment without the macrophotoinitiators gave only negligible amount of polymers after the same irradiation time. Diblock copolymer and triblock terpolymer structures were assigned by GPC and 1H NMR spectral measurements. Figure (c) and (d) shows the GPC traces of PDLLA-b-PMMA diblock copolymer (Mn = 66,000 and Mw/Mn = 1.33) and PDLLA-b-PCL-b-PMMA triblock terpolymer (Mn = 96,000 and Mw/Mn = 1.91) obtained. The characteristic signals of DLLA and/or CL and MMA units were detected in the 1H NMR spectra of diblock copolymers and triblock terpolymers. The copolymers compositions for each segment were determined by 1H NMR spectra of samples. The mol percentage composition of PDLLA-b-PMMA diblock copolymer and PDLLA-b-PCL-b-PMMA triblock terpolymer was found as 30% for PDLLA, 70% for PMMA and 13% for PDLLA, 23% for PCL, 64% for PMMA, respectively. As an example, in the 1H NMR spectrum of PCL-PDLLA-PMMA triblock terpolymer as shown in Figure , the typical signals of CL, DLLA, and MMA units are seen. The peaks in the region of 0.6–1.0 ppm can be attributed to the hydrogens of the methyl group, whose chemical shift is influenced by the tacticity of the polymer.[Citation42] The peak at 0.64 ppm arises from the methyl of the syndiotactic triad, while the peak at 0.84 ppm is due to the heterotactic triad.[Citation43,44]
4. Conclusions
New well-defined mid-chain functional macrophotoinitiator of poly(D,L-lactide) homopolymer (PDLLA-PI-PDLLA) and tetrablock poly(D,L-lactide)-poly(ε-caprolactone) copolymer (PDLLA-PCL-PI-PCL-PDLLA) were successfully prepared through ring-opening polymerization. The structure of polymers and the presence of photochromophoric group, PI, into mid-point of the poly(D,L-lactide) homopolymer and the tetrablock poly(ε-caprolactone)-poly(D,L-lactide) copolymer were evidenced by spectral analyses. The obtained macrophotoinitiator can be used in photopolymerization of vinyl monomers such as MMA. While AB-type block copolymer are formed with the mid-chain functional poly(D,L-lactide) macrophotoinitiator, tetrablock poly(D,L-lactide)-poly(ε-caprolactone) macrophotoinitiator yields ABC-type block copolymer. In addition, these macrophotoinitiators represent a class of potentially useful materials in biomedical applications due to their potential benefits of biocompatibility and nontoxicity of PDLLA and PCL backbones and the polymeric nature of the photoinitiators. Further studies on this line are now in progress.
Acknowledgments
The authors would like to thank Harran University, Scientific Research Council (HÜBAK), for financial support (Project no: 12160).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lendlein A, Sisson A. Handbook of biodegradable polymers. Weinheim: Wiley-VCH Verlag GmbH & Co.; 2011.10.1002/9783527635818
- Albertsson AC, Varma IK. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules. 2003;4:1466–1486.10.1021/bm034247a
- Pilla S. Handbook of bioplastics and biocomposites engineering applications. Weinheim: Wiley-VCH Verlag GmbH & Co.; 2011.10.1002/9781118203699
- Kowalski A, Duda A, Penczek S. Polymerization of L,L-lactide initiated by aluminum isopropoxide trimer or tetramer. Macromolecules. 1998;31:2114–2122.10.1021/ma971737k
- Bouyahyi M, Duchateau R. Metal-based catalysts for controlled ring-opening polymerization of macrolactones: high molecular weight and well-defined copolymer architectures. Macromolecules. 2014;47:517–524.10.1021/ma402072t
- Contreras J, Pestana J, López-Carrasquero F, Torres C. Synthesis of ε-caprolactone-b-L-lactide block copolymers by mean sequential polymerization, using diphenylzinc as initiator. Polym. Bull. 2014;71:1661–1674.10.1007/s00289-014-1147-9
- Contreras J, Dávila D. Ring-opening copolymerization of L-lactide with ɛ-caprolactone initiated by diphenylzinc. Polym. Int. 2006;55:1049–1056.10.1002/(ISSN)1097-0126
- Chuang HJ, Chen HL, Huang BH, et al. Efficient zinc initiators supported by NNO-tridentate ketiminate ligands for cyclic esters polymerization. J. Polym. Sci. Part A: Polym. Chem. 2013;51:1185–1196.10.1002/pola.26486
- Dechy-Cabaret O, Martin-Vaca B, Bourissou D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004;104:6147–6176.10.1021/cr040002s
- Gardella L, Cavallo D, Colonna S, Fina A, Monticelli O. Novel poly(L-lactide)/poly(D-lactide)/poly(tetrahydrofuran) multiblock copolymers with a controlled architecture: synthesis and characterization. J. Polym. Sci. Part A: Polym. Chem. 2014;52:3269–3282.10.1002/pola.v52.22
- Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 2009;38:3484–3504.10.1039/b820162p
- Kricheldorf HR, Kreiser-Saunders I, Stricker A. Polylactones 48. SnOct2-initiated polymerizations of lactide: a mechanistic study. Macromolecules. 2000;33:702–709.10.1021/ma991181w
- Degirmenci M, Hizal G, Yagci Y. Synthesis and characterization of macrophotoinitiators of poly(ε-caprolactone) and their use in block copolymerization. Macromolecules. 2002;35:8265–8270.10.1021/ma020668t
- Martello MT, Schneiderman DK, Hillmyer MA. Synthesis and melt processing of sustainable poly(ε-decalactone)-block-poly(lactide) multiblock thermoplastic elastomers. ACS Sustainable Chem. Eng. 2014;2:2519–2526.10.1021/sc500412a
- Biela T, Kowalski A, Libiszowski J, Duda A, Penczek S. Progress in polymerization of cyclic esters: mechanisms and synthetic applications. Macromol. Symp. 2006;240:47–55.10.1002/(ISSN)1521-3900
- In’t Veld PJA, Velner EM, Van de Witte P, Hamhuis J, Dijkstra PJ, Feijen J. Melt block copolymerization of ε-caprolactone and L-lactide. J. Polym. Sci. Part A: Polym. Chem. 1997;35:219–226.
- Kim JK, Park DJ, Lee MS, Ihn KJ. Synthesis and crystallization behavior of poly(L-lactide)-block-poly(ε-caprolactone) copolymer. Polymer. 2001;42:7429–7441.10.1016/S0032-3861(01)00217-8
- Pensec S, Leroy M, Akkouche H, Spassky N. Stereocomplex formation in enantiomeric diblock and triblock copolymers of poly (ε-caprolactone) and polylactide. Polym. Bull. 2000;45:373–380.10.1007/s002890070010
- Qian H, Bei J, Wang S. Synthesis, characterization and degradation of ABA block copolymer of L-lactide and ε-caprolactone. Polym. Degrad. Stab. 2000;68:423–429.10.1016/S0141-3910(00)00031-8
- Maglio G, Migliozzi A, Palumbo R. Thermal properties of di- and triblock copolymers of poly(L-lactide) with poly(oxyethylene) or poly(ε-caprolactone). Polymer. 2003;44:369–375.10.1016/S0032-3861(02)00764-4
- Tamboli V, Mishra GP, Mitra AK. Novel pentablock copolymer (PLA–PCL–PEG–PCL–PLA)-based nanoparticles for controlled drug delivery: effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym. Sci. 2013;291:1235–1245.10.1007/s00396-012-2854-0
- Yağci Y Macrophotoinitiators: synthesis and their use in block copolymerization. In: Mishra MK, Nuyken O, Kobayashi S, Yağci Y, Sar B, editors. Macromolecular engineering.New York (NY): Plenium Press; 1995. Chapter 11, p. 151–161.10.1007/978-1-4615-1905-8
- Corrales T, Catalina F, Peinado C, Allen NS. Free radical macrophotoinitiators: an overview on recent advances. J. Photochem. Photobiol. A. 2003;159:103–114.10.1016/S1010-6030(03)00175-8
- Carlini C, Angiolini L, Caretti D, Corelli E. Recent advances on photosensitive polymers: polymeric photoinitiators. Polym. Adv. Technol. 1996;7:379–384.10.1002/(ISSN)1099-1581
- Fouassier J. Photoinitiation, photopolymerisation and photocuring: fundamentals and applications. Munich: Hanser; 1995.
- Allen NS, editor. Photopolymerisation and photoimaging science and technology. Oxford: Elsevier; 1989.
- Jiang XS, Luo XW, Yin J. Polymeric photoinitiators containing in-chain benzophenone and coinitiators amine: effect of the structure of coinitiator amine on photopolymerization. J. Photochem. Photobiol. A. 2005;174:165–170.10.1016/j.jphotochem.2005.02.008
- Wang Y, Jiang X, Yin J. Novel polymeric photoinitiators comprising of side-chain benzophenone and coinitiator amine: photochemical and photopolymerization behaviors. Eur. Polym. J. 2009;45:437–447.10.1016/j.eurpolymj.2008.10.039
- Davidson RS. Polymeric and polymerisable free radical photoinitiators. J. Photochem. Photobiol. A. 1993;69:263–275.10.1016/1010-6030(93)85092-M
- Degirmenci M, Sarac MA, Genli N. Synthesis and characterization of mid-chain macrophotoinitiator of poly(ε-caprolactone) by combination of ROP and click chemistry. Polym. Bull. 2014;71:1743–1755.10.1007/s00289-014-1152-z
- Ohar H, Dolynska L, Tokarev V. Synthesis and application of macrophotoinitiators obtained via benzoin tethering with copolymers of maleic anhydride. Chem. Chem. Technol. 2013;7:125–130.
- Degirmenci M, Cianga I, Hizal G, Yagci Y. Synthesis, characterization and application of polymeric photoinitiators prepared by atom transfer radical polymerization and ring-opening polymerization. Polym. Prepr. 2002;43:22–23.
- Degirmenci M, Genli N. Synthesis of well-defined telechelic macrophotoinitiator of polystyrene by combination of ATRP and click chemistry. Macromol. Chem. Phys. 2009;210:1617–1623.10.1002/macp.v210:19
- Temel G, Arsu N. One-pot synthesis of water-soluble polymeric photoinitiator via thioxanthonation and sulfonation process. J. Photochem. Photobiol. A. 2009;202:63–66.10.1016/j.jphotochem.2008.11.012
- Durmaz YY, Yilmaz G, Yağci Y N-alkoxy pyridinium ion terminated polystyrenes: a facile route to photoinduced block copolymerization. J. Polym. Sci. Part A: Polym. Chem. 2007;45:423–428.10.1002/(ISSN)1099-0518
- Degirmenci M, Cianga I, Yagci Y. Synthesis of well-defined polystyrene macrophotoinitiators by atom-transfer radical polymerization. Macromol. Chem. Phys. 2002;203:1279–1284.10.1002/1521-3935(200207)203:10/11<1279::AID-MACP1279>3.0.CO;2-H
- Degirmenci M, Alter S, Genli N. Synthesis and characterization of well-defined mid-chain functional macrophotoinitiators of polystyrene by combination of ATRP and “click” chemistry. Macromol. Chem. Phys. 2011;212:1575–1581.10.1002/macp.v212.15
- Degirmenci M Synthesis and characterization of novel well‐defined end‐functional macrophotoinitiator of poly(MMA) by ATRP J. Macromol. Sci. Part A-Pure Appl. Chem. 2005;42:21–30.10.1081/MA-200040949
- Degirmenci M. Synthesis of novel well-defined end-functional macrophotoinitiator of poly(ε-caprolactone) by ring-opening polymerization. Polym. J. 2004;36:542–548.10.1295/polymj.36.542
- Degirmenci M, Besli PA, Genli N. Synthesis of a well-defined end-chain macrophotoinitiator of poly(ε-caprolactone) by combination of ring-opening polymerization and click chemistry. J. Polym. Res. 2014;21:540–549.10.1007/s10965-014-0540-2
- Save M, Schappacher M, Soum A. Controlled ring-opening polymerization of lactones and lactides initiated by lanthanum isopropoxide, 1. General aspects and kinetics. Macromol. Chem. Phys. 2002;203:889–899.10.1002/1521-3935(20020401)203:5/6<889::AID-MACP889>3.0.CO;2-O
- César-Oliveira MAF, Zaioncz S, Oliveira ARS, et al. Síntese de copolímeros metacrílicos através da modificação química do poli(metacrilato de metila) de massa molar controlada [Synthesis of methacrylic copolymers by chemical modification of poly(methyl methacrylate) with controlled molecular weight]. Polímeros. 1999;9:156–162.10.1590/S0104-14281999000400026
- Vangani V, Rakshit AK. Synthesis and characterization of homopolymer of 2-ethylhexyl acrylate and its copolymers with acrylamide, acrylonitrile, and methyl methacrylate. J. Appl. Polym. Sci. 1996;60:1005–1013.10.1002/(ISSN)1097-4628
- Gong S, Ma H, Wan X. Atom transfer radical polymerization of methyl methacrylate induced by an initiator derived from an ionic liquid. Polym. Int. 2006;55:1420–1425.10.1002/(ISSN)1097-0126