Abstract
In this work, a novel DOPO-based spiroorthocarbonate (DSOC), 6,6ʹ-((3,9-diethyl-1,5,7,11-tetraoxaspiro[5.5]undecane-3,9-diyl)bis(hydroxymethylene))bis(6H-dibenzo[c,e][1,2]oxaphosphinine 6-oxide) was synthesized and confirmed with 1H, 31P, 13C NMR, and FT-IR spectra. It was used as anti-shrinkage additive and flame retardant in the cationic polymerization of epoxy resin. The shrinkage of the cured epoxy resin containing DSOC was investigated by measuring the density of the system before and after curing. It was found that the shrinkage of epoxy resin changed from −1.49 to 1.43% with the content of DSOC increased to 20 wt%, not only the shrinkage of epoxy resin was eliminated, but also a slight expansion was achieved. The flame retardancy and thermal stability of the cured epoxy resins were tested by limiting oxygen index (LOI), vertical burning test (UL-94), and thermogravimetric analysis (TGA). LOI values increased from 19.9% for pure E-51 to 30.9% for modified epoxy resins, UL-94 V-0 rating can be obtained with 15 wt% DSOC. The mechanical properties of the modified epoxy resin were also investigated. The results showed that DSOC was a promising anti-shrinkage additive and flame retardant for epoxy resins.
1. Introduction
Epoxy resins (EP) are widely used in various industrial fields such as adhesive, coating, semiconductor encapsulation, and insulating material due to their advantageous properties of adhesion, excellent characteristics of solvent, chemical and moisture resistance, and superior electrical and mechanical properties.[Citation1] However, volume shrinkage during their curing process is a common issue. The volume shrinkage causes dimensional changes and a large buildup internal stress in the material, which are responsible for the inaccuracy in shapes and the decrease in mechanical strength. Commonly, the shrinkage was reduced by adding some expansive spiro-monomers into thermosetting prepolymer reaction system to undergo a copolymerization. If the ratio between them was appropriate, zero shrinkage, even a slight expansion in volume was possible.[Citation2–8] Spiroorthocarbonates (SOCs) can be used as anti-shrinkage additives in the double ring-opening polymerization with a cationic initiator.[Citation9] It aimed to offset chemically volume shrinkage and to overcome the adverse effects of volume shrinkage.[Citation10] SOCs are two-ring structure with the central carbon atom connected to four oxygen atoms. The double cyclic structures are stable under an ordinary condition but can easily undergo ring-opening polymerization with cationic catalysts.[Citation11] When SOCs are compounded with other monomers that undergo cationic polymerization, they can either inhibit the inherent shrinkage that occurred during the polymerization or even induce expansion.[Citation12]
Moreover, conventional epoxy resins are generally flammable and cannot satisfy some applications which require high flame retardancy.[Citation13,14] Epoxy resins can be imparted flame retardance either by adding flame retardants or by introducing reactive flame retardants. In recent years, researchers mainly focus on the latter because the flame retardants can be chemically incorporated into the polymer structure, resulting in some advantages such as more stable flame retardance effect and less gas emission during high temperature processing or burning. At the same time, the reactive flame retardants can minimize some negative impacts on the mechanical properties of the polymer.[Citation1] Phosphorous-containing flame retardants are more environmentally benign than halogen-containing ones. 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and its derivatives have been used as novel phosphorus flame retardants in polymeric materials to improve their flame retardancy.[Citation15–17] According to previous reports, DOPO with an active hydrogen can react with electron deficient compound, such as benzoquinone, maleic acid [Citation18], paraformaldehyde [Citation19], and 4-hydroxybenzoaldehyde [Citation20]. DOPO derivatives may carry various functional groups, which can react with epoxy groups, so that they can be used as reactive flame retardant for epoxy resins.
In this work, a DOPO-based spiroorthocarbonate (DSOC) was synthesized and characterized with 1H, 31P, 13C NMR, and FT-IR spectra. It was used as anti-shrinkage additive and flame retardant in the cationic polymerization of epoxy resin. The shrinkage of the cured epoxy resin containing DSOC was investigated. The flame retardancy and thermal stability of the cured epoxy resins were investigated by limiting oxygen index (LOI), vertical burning test (UL-94), and thermogravimetric analysis (TGA). The mechanical properties of the modified epoxy resin were also investigated.
2. Experimental
2.1. Materials
DOPO was purchased from Shandong Mingshan chemical Co. Ltd, China. Dibutyltin oxide was purchased from Rizhao Lideshi Chemical Co. Ltd, and boron trifluoride ethylamine was purchased from Chengdu Gray West Chemistry Technology Co. Ltd, China. Epoxy resin (E-51, weight per epoxide 185–192 g/eq) was purchased from Wuxi Resin Factory, China.1-methyl-2-pyrrolidone, butyraldehyde, formaldehyde, toluene, hexane, ethyl acetate, and carbon disulfide were purchased from Chinese Medicine Group Chemical Reagent Corp. used as received without further purification. All of the reagents were at analytical grade.
2.2. Synthesis of DMB
2,2-dimethylol-l-butanal (DMB) was synthesized according to the literature.[Citation21] Butyraldehyde (14.42 g, 0.2 mol) and formaldehyde solution (37%, 37.33 g, 0.46 mol) were fed into a 250-mL three-necked round-bottomed flask equipped with a thermometer, magnetic stirrer, an addition funnel, and reflux condenser. The pH value of the solution was adjusted to 9 by alkaline solution (3 wt% Na2CO3 and 2 wt% NaOH), and the pH value of the reaction solution was measured by PHS-3C acidometer. The reaction solution was heated to 30 °C and electromagnetically stirred for 2 h. A small amount of hydrochloric acid was added to neutralize the pH value to 5. The pH value was also determined by the acidometer. The solution was eluted by ethyl acetate, filtrated, and distilled under the vacuum condition to obtain a transparent liquid. The yield of the reaction was 85%.
2.3. Synthesis of DTHA
The synthesis of DOPO-based trihydroxy alcohol (DTHA) is illustrated in Scheme and the details are as follows: DMB (17.18 g, 0.13 mol), DOPO (21.62 g, 0.1 mol), and ethyl alcohol (50 mL) were introduced into a three-necked round-bottomed flask equipped with a magnetic stirrer, condenser, thermo-well, and nitrogen inlet. The mixture was heated to 60 °C and stirred simultaneously under a nitrogen gas atmosphere for 4 h. The pale yellow solution was distilled under vacuum and cooled to obtain a white precipitate. The precipitate was filtered, purified by recrystallizing from ethanol, and dried under vacuum at 100 °C for 8 h. The yield of white solid product was 76% and the melting point of the purified product was 170.5–172.1 °C. FT-IR (KBr, ν, cm−1): 3238 (–OH), 3056 (Ar–H), 2945 (–CH3), 2829 (–CH2–), 1437 (P–C), 1252 (P=O), 1137, 937 (P–O–Ph). 1H NMR (CDCl3, δ, ppm): 7.24–8.19 (8H, Ar–H), 3.56 (1H, –CH–), 3.41 (4H, –CH2O–), 2.09 (3H, –OH), 1.27 (2H, –CH2–), 0.97 (3H, –CH3). 31P NMR (CDCl3, δ, ppm): 37.00.
2.4. Synthesis of DSOC
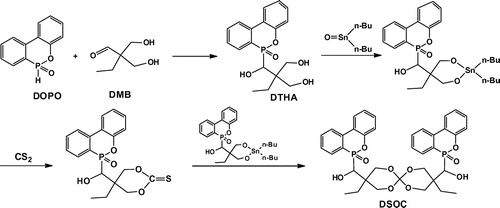
As shown in Scheme , DSOC was synthesized according to the literature.[Citation22–25] DTHA (17.42 g, 0.05 mol), dibutyltin oxide (14.94 g, 0.06 mol), toluene (80 mL), and 1-methyl-2-pyrrolidone (40 mL) were charged in a 250-mL three-necked round-bottomed flask provided with a magnetic stirrer, thermometer, nitrogen inlet, and Dean-Stark trap with a reflux condenser. The reaction mixture was stirred at 120 °C for 10 h, and then cooled to 80 °C, 5 mL of carbon disulfide was added in a drop-wise manner for about 1 h and then the reaction mixture refluxed at 110 °C for 12 h. The solvent and low-boiling residues were removed from the mixture by distillation under reduced pressure. A yellow viscous liquid was obtained; then the liquid was eluted by ethyl acetate and hexane to obtain a white precipitate. The precipitate was filtered and recrystallized from toluene led to white powder with a melting point of 252.7–254.1 °C. The yield of the reaction was 63%. Elem. Anal. Cal.: C, 63.07%; H, 5.44%; O, 22.71%. Found: C, 63.14%; H, 5.42%; O, 22.69%. FT-IR (KBr, ν, cm−1): 1204 (spiro), 3258 (–OH), 3056 (Ar–H), 2955 (–CH3), 2924, 2869 (–CH2–), 1437 (P–C), 1252 (P=O), 1118, 907 (P–O–Ph). 1H NMR (CDCl3, δ, ppm): 7.25–8.19 (16H, Ar–H), 3.83 (8H, –CH2O–), 3.19 (2H, –CH–P), 1.98 (2H, –OH), 1.29 (4H, –CH2–), 0.86 (6H, –CH3). 13C NMR (CDCl3, δ, ppm): 9.7, 20.9, 34.7, 60.7, 77.5, 121.3, 122.3, 125.1, 127.7, 129.2, 132.8, 137.4, 150.1, 154.5. 31P NMR (CDCl3, δ, ppm): 34.00.
2.5. Curing procedure for the modified epoxy resin
Various amounts of DSOC (5, 10, 15, and 20 wt%, respectively) were mixed with E-51 in a 500-mL beaker at room temperature, 3 wt% boron trifluoride ethylamine was added as curing accelerator. The mixed resin was stirred constantly until a homogeneous solution was obtained; then it was put into a vacuum oven to remove air bubble at 60 °C. The resin was poured into a mold, which had been preheated to 120 °C, and cured at 150 °C in an oven for 4 h.
2.6. Determination of epoxy group conversion ratio by FT-IR spectroscopy
The samples before and after curing were studied by FT-IR spectroscopy in a Thermo NEXUS-470 spectrometer. The conversion of epoxy group was followed by observing the absorbance of the epoxy groups at 913 cm−1 (A913) and the benzene groups at 1610 cm−1 (A1610) before and after curing, and the conversion ratio A was calculated by the following formula:
wherein the subscript 0 and t refers to the polymerization time is 0 and t, respectively [Citation26].
2.7. Measuring the shrinkage of the cured epoxy resin with DSOC
Shrinkage was obtained by measuring the density of the system before and after curing. The density of viscous liquid before curing was measured with density bottle. The density of each cured epoxy resins was measured with an electronic hydrometer (MH-20A, Taiwan Matsuhaku Co., Ltd). The specific volume of the system before and after curing was defined as the inverse of the density and the shrinkage was calculated with the following formula:
wherein Δ is the shrinkage ratio of the cured epoxy resin; Vc is the specific volume of cured epoxy resin, and V0 is the specific volume of epoxy resin before curing.
2.8. Characterizations
FT-IR spectra were recorded on a Thermo NEXUS-470 Fourier transform infrared spectrometer using KBr pellet. NMR spectra were measured by Bruker Avance-500 spectrometer with DMSO-d6 as the solvent. The melting point of product was measured with a Buchi B-540 melting point apparatus. The contents of C, H of spiroorthocarbonate were measured with Vario EL Ⅲ elemental analyzers manufactured by Element in Germany. Thermogravimetric analysis (TGA) was performed on STA449C/41G thermal analyzer (NETZSCH company, Germany) at a heating rate of 10 °C/min under N2 atmosphere within the temperature range between ambient to 600 °C. The limiting oxygen index (LOI) values were tested with an HC-2 LOI instrument (Jiangning Company, China) according to ASTM D2863-97. The vertical burning tests (UL-94) were carried out using a CZF-3 flammability meter (Shangyuan Company, China) according to ASTM D 3081. According to ISO 527-2, ISO 4587-2003, the tensile strength, and adhesive strength were tested with TCS-2000 Electric Tensile Tester (Gotech Testing Machines Inc.) at room temperature. Charpy impact tests were performed by GT-7045-MDL Charpy Impact Tester (Gotech Testing Machines Inc.) according to ISO 179-1. The pH value of the reaction solution was measured by PHS-3C acidometer (Shanghai INESA instrument Co., Ltd, China).
3. Results and discussion
3.1. Synthesis of DTHA and DSOC
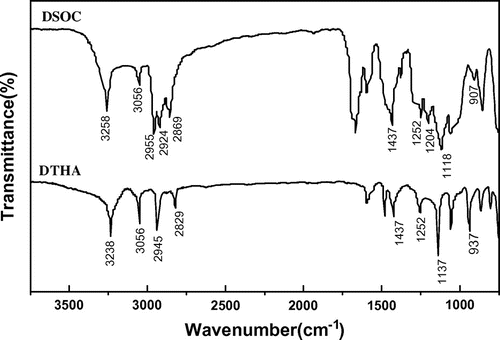
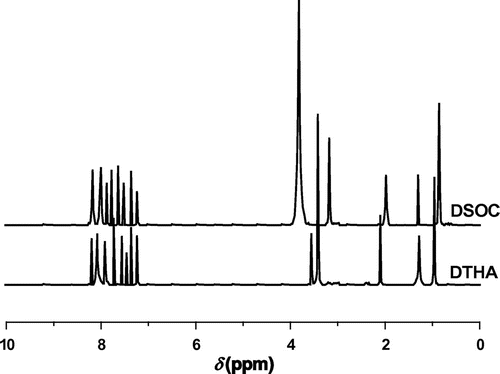
According to Scheme , DTHA was synthesized through the addition reaction of DOPO and DMB. The FT-IR spectrum of DTHA is shown in Figure . It can be seen that the peak at 3238 cm−1 can be assigned to hydroxyl absorption. The peak at about 3056 cm−1 is attributed to aromatic C–H absorption. The absorption corresponds to –CH2 and –CH3 at about 2829–2945 cm−1. The peaks at 1137 and 937 cm−1 can be assigned to P–O–Ph absorption. The P=O absorption is around 1252 cm−1, and the P–C absorption is around 1437 cm−1. The aldehyde absorption near 1719 and 2728 cm−1 does not present. The 1H NMR spectrum of DTHA is shown in Figure . The multiplet between 7.24 and 8.19 ppm are assigned to aryl protons. The peak at 3.56 ppm corresponds to methenyl proton. The peak at 3.41 ppm can be assigned to methylene protons adjacent to hydroxyl. The peak at about 2.09 ppm corresponds to the proton of hydroxyl. The peaks at about 1.27 and 0.97 ppm should be assigned to methylene protons and methyl protons, respectively. 31P NMR spectrum of DTHA exhibits a single peak at 37.00 ppm.
DSOC was synthesized through the reaction of DTHA, dibutyltin oxide, and carbon disulfide (shown in Scheme ). According to the FT-IR spectrum of DSOC (Figure ), the peak at 1204 cm−1 is attributed to spiroorthocarbonate groups. The peak at 3258 cm−1 can be assigned to hydroxyl absorption. The peak at about 3056 cm−1 is associated with aromatic C–H absorption. The absorption of –CH2 and –CH3 is observed at about 2869–2955 cm−1. The peaks at 1252 cm−1 can be assigned to P=O absorption. The peaks at 1118 and 907 cm−1 are attributed to P–O–Ph absorption. The peak at 1437 cm−1 can be assigned to P–C absorption. According to the 1H NMR spectrum of DSOC (Figure ), the peaks at 7.25–8.19 ppm are assigned to aryl protons. The peak at about 3.83 ppm corresponds to the methylene proton connecting with oxygen in the spiro-ring. The peak at 3.19 ppm should be assigned to methenyl proton. The peak at about 1.98 ppm should be assigned to hydroxyl proton. The peak at 1.29 ppm can be assigned to methylene protons and the peak at about 0.86 ppm should be assigned to methyl protons. 31P NMR spectrum of DSOC shows a single peak at about 34.00 ppm.
3.2. Copolymerization of spiroorthocarbonate and epoxy resins
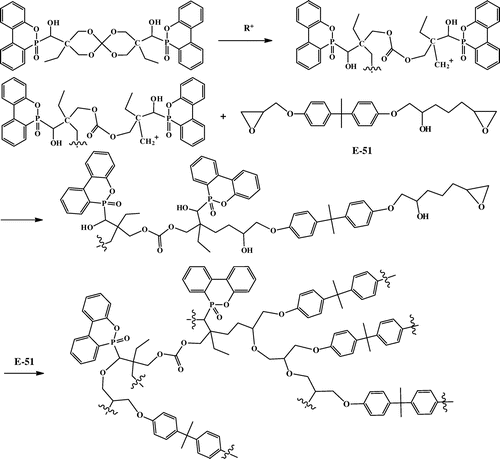
In this paper, boron trifluoride ethylamine, used as latent catalyst, decomposed at 90 °C to release active species, which can initiate self-polymerization and copolymerization of spiroorthocarbonate and epoxy resins by the ring-opening reaction of spiro-rings and epoxy groups. The copolymerization of spiroorthocarbonate and epoxy resins is illustrated in Scheme .
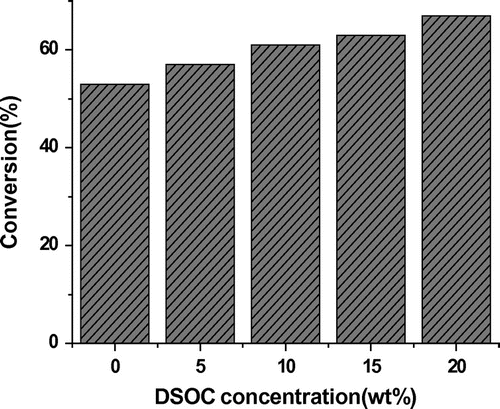
Figure shows the effect of DSOC concentration on the conversion ratio of epoxy group in the polymerization. It can be seen that the epoxy group conversion ratio was 53% in the polymerization of the epoxy resins without addition of DSOC, and the epoxy group conversion ratio increased from 53 to 67% with the increase in DSOC concentration to 20%. This behavior can be explained by the ring-opening copolymerization reaction of the spiroorthocarbonate and epoxy resins. The spiro-ring groups can collide with the epoxy groups to generate branched or crosslinked copolymer. So the DSOC was involved in the curing of epoxy resins and could affect the structure and properties of epoxy resins.
3.3. Shrinkage measurements
The shrinkage of the epoxy resin cured with various amounts of DSOC is summarized in Table . It can be observed that pristine epoxy resin displayed shrinkage of −1.49%. With the increase in DSOC content, the volume shrinkage was reduced. In the case of the polymer with 5 wt% of DSOC, the reduction of the shrinkage was 87%. While at 10 wt% of DSOC, it was observed a positive value of 0.35%, which indicates that not only the shrinkage was compensated but a slight expansion occurred. When the content of DSOC increased to 20 wt%, the material expanded 1.43%. This behavior can be explained by the formation of the poly (ester-carbonate) which contains the highly voluminous DOPO groups which induce large free volume in the polymeric matrix.[Citation10] Therefore, it can be concluded that the synthesized DSOC can effectively reduce the shrinkage of the cured epoxy resin and may induce a slight expansion in volume.
Table 1. Shrinkage ratio of cured epoxy resins with various amounts of DSOC.
3.4. Flammability of cured epoxy resins
The flame retardance of cured epoxy resins was tested by limiting oxygen index (LOI) and vertical burning test (UL-94), as shown in Table . It can be seen that the LOI values significantly increased from 19.9 to 30.9%, when DSOC was incorporated into epoxy network. The DSOC can effectively improve the flame retardancy of epoxy resins. The enhancement of the flame retardance can also be observed from the vertical burning test results. As shown in Table , when the content of DSOC increased to 15 and 20 wt%, the vertical burning test result of the cured epoxy resins was the V-0 ranking in UL-94 test, respectively. The flame extinguished almost instantly as the source of ignition removed, which indicated that DSOC has an excellent flame retardant effect to epoxy resins.
Table 2. LOI measurement and UL-94 test of cured epoxy resins.
The main reason was that during burning, phosphorus in DSOC was converted to phosphoric acid and further thermal decomposition led to the formation of polyphosphoric acid. The polyphosphoric acid esterified and dehydrated to form phosphorus-rich carbonaceous layer. This protective layer was resistant to higher temperatures and shielded the underlying polymer from attacking by oxygen and radiant heat.[Citation27]
3.5. Thermal stability of cured epoxy resins
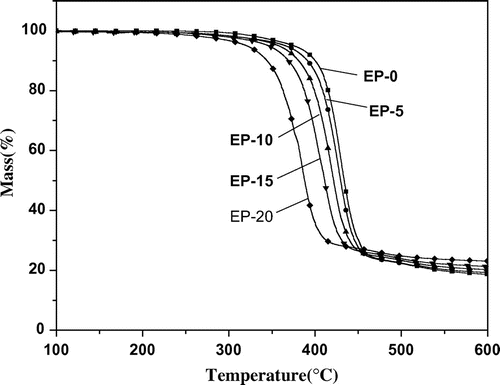
The decomposition behavior of cured epoxy resins was studied with TGATG curves of the cured epoxy resins under nitrogen is presented in Figure . As shown in Figure , the onset degradation temperatures of cured epoxy resins decreased with increasing DSOC content, which is attributed to the poorer stability of O=P–O than –C–C– bond.[Citation1] Although the thermal stabilities decrease with increasing content of DSOC, the cured epoxy resins still exhibit high thermal stability.
Furthermore, it is obvious that the cured epoxy resins show one-stage decomposition processes and the introduction of DSOC can increase the amount of residue. This behavior may be attributed to phosphorous-containing groups that decompose at earlier stages and forms a phosphorous-rich char layer. The layer prevents further decomposition of epoxy resins and leads to a high char yield.[Citation28,29] And the char yields play an important role in improving the flame retardancy of cured epoxy resins.[Citation30,31]
3.6. Mechanical properties
The mechanical properties of the modified epoxy resin were measured and presented in Table . The tensile strength decreased with increased concentration of DSOC, while the adhesive strength and the impact strength increased with increasing content of DSOC. When 20 wt% DSOC was added, the tensile strength decreased from 56.7 to 47.3 MPa, the adhesive strength increased from 15.8 to 20.6 MPa, and the impact strength was up to 14.1 kJ/m2 increased by 107.4%. The toughness of the material had an obvious improvement and it is crucial to improve the application of epoxy resin.
Table 3. Mechanical properties of cured epoxy resins containing different contents of DSOC.
When DSOC cured with epoxy resin, the double spiro-ring structures of DSOC could undergo ring-opening polymerizations with expansions in volume. It could effectively offset the volume shrinkage of the epoxy resin during the curing process, and thus it could reduce the residual shrinkage stress, improve the adhesive properties and impact strength of the material. However, the spiroorthocarbonates are expected to act as flexibilizing agents, it formed flexible poly (ether-carbonate) chains after cationic induced double ring-opening reaction,[Citation11] their low Tg would affect the rigidity of the crosslinked material; but it also improved the conversion ratio of epoxy groups, which can enhance the tensile strength of epoxy resins.
3.7. Fracture surface morphology
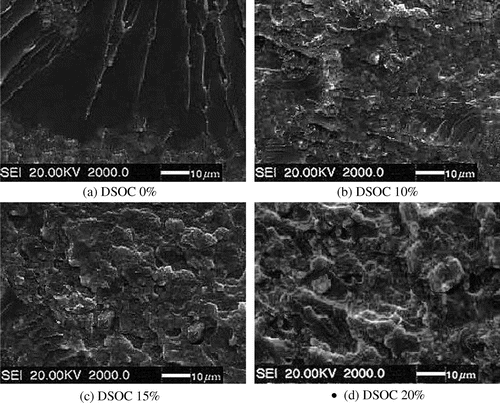
The SEM morphologies of fracture surface after impact testing are presented in Figure . Figure (a) shows the fracture surface morphology of pure EP. It possesses a smooth fracture surface, with the behavior of brittle fracture. Figure (b–d) shows the fracture surface morphology of EP/DSOC composites. The surfaces are very rough, with many ditches and ridges, which can be characterized as ductile fracture. This indicates the copolymerization of DSOC and epoxy resins without phase separation, thus improves the toughness of the material. And it is very important for the properties of cured epoxy resins.
4. Conclusion
A novel DOPO-based spiroorthocarbonate was synthesized successfully and confirmed with 1H, 31P, 13C NMR, and FT-IR spectra. It was used as anti-shrinkage additive and flame retardant in epoxy resin curing. The shrinkage of the cured epoxy resin containing DSOC was investigated by measuring the density of the system before and after curing. It was found that shrinkage of epoxy resin changed from −1.49 to 1.43% with the content of DSOC increased to 20 wt%, not only the shrinkage of epoxy resin was eliminated, but also a slight expansion was achieved. The flame retardancy and thermal stability of the cured epoxy resins were investigated by LOI, UL-94 test, and TGA. LOI values increased from 19.9% for pure E-51 to 30.9% for modified epoxy resins, and UL-94 V-0 rating can be obtained with 15 wt% DSOC. TGA indicates that the incorporation of DSOC significantly enhanced the char yield. The tensile strength of cured epoxy resins decreased, the adhesive strength increased, and the toughness was notably improved. The results showed that DSOC was a promising anti-shrinkage additive and flame retardant for epoxy resins.
Disclosure statement
No potential conflict of interest were reported by the authors.
Additional information
Funding
References
- Gao LP, Wang DE, Wang YZ, Wang JS, Yang BA. A flame-retardant epoxy resin based on a reactive phosphorus-containing monomer of DODPP and its thermal and flame-retardant properties. Polym. Degrad. Stab. 2008;93:1308–1315.10.1016/j.polymdegradstab.2008.04.004
- He PS, Zhou ZQ, Pan CY. An epoxy resin copolymer with zero shrinkage. J. Mater. Sci. 1989;24:1528–1532.
- Chappelow CC, Pinzino CS, Chen SS, Kotha SP, Glaros AG, Eick JD. Tetraoxaspiroalkanes for polymerization stress reduction of silorane resins. J. Appl. Polym. Sci. 2008;108:3738–3747.10.1002/(ISSN)1097-4628
- Ge J, Trujillo-Lemon M, Stansbury JW. A mechanistic and kinetic study of the photoinitiated cationic double ring-opening polymerization of 2-methylene-7-phenyl-1,4,6,9-tetraoxa-spiro[4.4]nonane. Macromolecules. 2006;39:8968–8976.10.1021/ma061284w
- Wang CS, Zhou BL. Study on epoxy resin modified with prepolymers containing spiro orthocarnonate. J. Mater. Sci. Lett. 1999;18:1259–1262.10.1023/A:1006668117964
- Chen WS, Chang YL, Hsiang HI, Hsu FC, Shen YH, Yen FS. Mechanical and dielectric properties of NiZn ferrite powders-CTBN modified epoxy resin coatings. Polym. Plast. Technol. Eng. 2011;50:568–572.10.1080/03602559.2010.543737
- Fotsing ER, Miron F, Eury Y, Ross A, Ruiz E. Bonding analysis of carbon/epoxy composites with viscoelastic acrylic adhesive. Composites Part B: Eng. 2012;43:2087–2093.10.1016/j.compositesb.2012.03.009
- Liu Y, Wu W, Chen Y, Shi PP, Liu MC, Wu X. The effects of polyamic acid on curing behavior, thermal stability, and mechanical properties of epoxy/DDS system. J. Appl. Polym. Sci. 2013;127:3213–3220.
- Thompson VP, Williams EF, Bailey WJ. Dental resins with reduced shrinkage during hardening. J. Dent. Res. 1979;58:1522–1532.10.1177/00220345790580051701
- Acosta Ortiz R, Berlanga Duarte ML, Robles Olivare JL, Sangermano M. Synthesis of the fluorine spiroorthocarbonate and the evaluation of its antishrinking activity in the cationic photopolymerization of an epoxy resin. Des. Monomers Polym. 2013;16:323–329.
- Acosta Ortiz R, Berlanga Duarte ML, Savage Gomez AG, Sangermano M, Valdez AEG. Novel diol spiro orthocarbonates derived from glycerol as anti-shrinkage additives for the cationic photopolymerization of epoxy monomers. Polym. Int. 2010;59:680–685.
- Acosta Ortiz R, Savage Gomez AG, Berlanga Duarte ML, Sangermano M. The effect of hydroxyspiro-orthocarbonates on the cationic photopolymerization of an epoxy resin and on the mechanical properties of the final polymer. Polym. Int. 2012;61:587–595.10.1002/pi.v61.4
- Lu SY, Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002;27:1661–1712.10.1016/S0079-6700(02)00018-7
- Shieh JY, Ho TH, Wang CS. Aminosiloxane-modified epoxy resins as microelectronic encapsulants. Angew. Makromol. Chem. 1995;224:21–32.10.1002/apmc.1995.052240103
- Zhang C, Huang JY, Liu SM, Zhao JQ. The synthesis and properties of a reactive flame-retardant unsaturated polyester resin from a phosphorus-containing diacid. Polym. Adv. Technol. 2011;22:1768–1777.10.1002/pat.v22.12
- Levchik S, Piotrowski A, Weil E, Yao Q. New developments in flame retardancy of epoxy resins. Polym. Degrad. Stab. 2005;88:57–62.10.1016/j.polymdegradstab.2004.02.019
- Perret B, Schartel B, Stöß K, et al. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Euro. Polym. J. 2011;47:1081–1089.10.1016/j.eurpolymj.2011.02.008
- Lin CH, Wang CS. Novel phosphorous-containing epoxy resins Part I. Synthesis and properties. Polymer. 2001;42:1869–1878.
- Shieh JY, Wang CS. Synthesis of novel flame retardant epoxy hardeners and properties of cured products. Polymer. 2001;42:7617–7625.10.1016/S0032-3861(01)00257-9
- Liu YL. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer. 2001;42:3445–3454.10.1016/S0032-3861(00)00717-5
- Shi HY, Zhang JX, Tian ZJ, Xue CX. Preparation of 2,2-dimethylolbutyric acid. Fine Chem. 2003;20:101–104.
- Shaika S, Kuroda Y, Ishii Y. Preparation of orthocarbonates from thallous alkoxides and carbon disulfide. J. Org. Chem. 1972;37:4198–4200.
- Park YJ, No KH, Kim JH, Suh IH. Synthesis and X-ray crysyallographic characterization of spiro orthocarbonates. Bull. Korean Chem. Soc. 1992;13:375–381.
- Yuan JY, Pan CY. Synthesis of spiro orthocarbonate, a kind of expansion monomer. Chin. Adhes. 2000;9:32–35.
- Xu XQ, Zhou L, Liang B, Wu YM, Wang CS. Synthesis of copolymers containing double spiro orthocarbonate and used as anti-shrinkage additives in epoxy resin composite. Polym.-Plast. Technol. Eng. 2014;53:753–759.10.1080/03602559.2013.869697
- Wang CS, Zhou BL. Synthesis of prepolymer containing spiro orthocarbonate and its copolymerization with epoxy resin. 2000;16:28–31.
- Liu P, Liu MM, Gao C, Wang F. Preparation, characterization and properties of a halogen-free phosphorous flame-retarded poly(butylene terephthalate) composite based on a DOPO derivative. J. Appl. Polym. Sci. 2013;130:1301–1307.10.1002/app.39318
- Wang CS, Lin CH. Synthesis and properties of phosphorus-containing epoxy resins by novel method. J. Polym. Sci. Part A: Polym. Chem. 1999;37:3903–3909.10.1002/(ISSN)1099-0518
- Wang CS, Shieh JY. Phosphorus-containing epoxy resin for an electronic application. J. Appl. Polym. Sci. 1999;73:353–361.10.1002/(ISSN)1097-4628
- Lin CH. Synthesis of novel phosphorus-containing cyanate esters and their curing reaction with epoxy resin. Polymer. 2004;45:7911–7926.10.1016/j.polymer.2004.09.023
- Liu YL, Hsiue GH, Lee RH, Chiu YS. Phosphorus-containing epoxy for flame retardant. III: using phosphorylated diamines as curing agents. J. Appl. Polym. Sci. 1997;63:895–901.10.1002/(ISSN)1097-4628