Abstract
A co-polymerizable dendron of first generation synthesized from behera amine was homopolymerized and copolymerized with commercial monomers such as styrene, methyl methacrylate, and N-vinyl pyrrolidone. Characterization of the co-polymers was performed and it was found with the help of FT-IR and proton and carbon NMR that copolymerizable dendron, ACTES (ACrylated TriESter, first-generation dendron of acrylated behera amine) was incorporated in the co-polymer chains. Besides, the reactivity ratio pairs of the ACTES monomer with both styrene and n-vinyl pyrrolidone were determined. The reactivity ratio pair for ACTES and MMA could not be determined by neither gravimetrically nor with NMR. After experiments and calculations, the reactivity ratio pair for styrene and ACTES was found to be rst = 0.27 and rac = 0.44 and for NVP and ACTES rnvp = 0.36 and rac = 1.01. By utilization of the reactivity ratio of ACTES, “Q” and “e” values were determined in order to be able to draw some conclusions about the reactivity behavior during copolymerizations. “Q” = 1.152 and “e” = 0.66 were found with respect to styrene as reference. These values indicates the steric effect of ACTES was more dominant in copolymerizations.
1. Introduction
Copolymerization offers great importance in terms of technological point of view. İnherent properties of homopolymers made from different monomers combined with co-polymer possessing new physical, optical, thermal, and mechanical properties that are not possible with single homopolymer.
Dendrimers are specialty molecules with respect to their molar masses, branching units, monodispersities, and their versatile functionalization abilities. Molecular dimensions can reach up to 10–100 nm depending on their generations, g[n]. Dendronized polymers, on the other hand are a special class of dendrimers that have a polymer chain as a core. These architectures attract much more attentions as well as dendrimers. Thus, detailed reviews were published by different groups.[Citation1–4] Within the studies in the literature, generally comparisons of theoretical and experimental results were made with respect to the topographies of dendritic structures, the amount of dendron on the polymer chain, and their generation on the polymer chain.[Citation5]
Characteristics of co-polymers are reflections of monomer composition. In other words, reactivities of monomers during polymerization play important role to determine the boundaries of co-polymer properties.[Citation6] Reactivity ratios obtained from reaction kinetics of polymers during their propagation phases, “r”, give us important information on the behaviors of monomers during copolymerization and the chance to compare the monomers with each other. Reactivity ratio gives us a clue about the possible composition of the co-polymer chain by relieving the reactivity of monomer in addition addition to the active growing polymer chain.[Citation7]
Although dendronized polymers are in the form of homopolymer and/or co-polymer, copolymerization behaviors of polymerizable dendrons with commercial monomers have not been explored in details yet. In a study made by Krebs et al., first-generation dendronized monomer carrying ethylene glycol-based dendron was copolymerized with styrene, tert-butyl acrylate, tert-butyl methacrylate, and methyl methacrylate. Mainly, reactivity ratios of the g[1]type dendron and co-monomer pairs were determined with an exception of g[2]-type ethylene glycol-based type dendron and styrene pair.[Citation8]
Dendron used in this study, ACTES (di-tert-butyl 4-acryloylamino-4-(2-tert-butoxycarbonylethyl) heptanedioate), is a polymerizable first generation, g[1], dendron derived from Newkome-type dendron bearing easily cleavable tertiary butoxy groups as end groups. These cleavable end groups were used to modify chitosan in order to impart water solubility after the hydrolysis of tertiary butoxide groups.[Citation9] This dendron was also utilized in synthesis of dendritic perylenes that are suitable for the chemical functionalization of inorganic nanoparticles and dendronized polycationic perylenetetracarboxylic acid diimides.[Citation10,11] Newkome-type dendronized norbornene macromonomers were synthesized and polymerized by ring-opening metathesis polymerization to obtain biocompatible polymers.[Citation12] This dendron was also used in synthesis of protein cages to construct synthetic DNA-binding domains,[Citation13] redox active hybrid dendrimers building blocks for nonviral vectors for gene therapies,[Citation14] stabilization of gold particles,[Citation15,16] and catalyst in transfer hydrogenation [Citation17] as well as micelles [Citation18] and ion selective gels after hydrolysis.[Citation19]
A possible application field of hydrolyzed ACTES co-polymers obtained from polymerizable Newkome-type dendron is reduction catalyst of hydrogen. Thus, the details of the compositions of its homopolymer and co-polymers should be explored in detail. In this study, both the reactivity ratios of monomers were explored and co-polymers were characterized. Comonomers were selected as styrene (ST), methyl methacrylate (MMA), and N-vinyl pyrrolidone (NVP), used in large scale as commodity monomers.
2. Experimental
2.1. Materials
Behera amine was synthesized as stated in the literature.[Citation20] Styrene (Aldrich) and methyl methacrylate (MMA, Aldrich) were passed over basic Al2O3 to remove inhibitor just before polymerization. Benzoyl peroxide (Merck) was recrystallized from methanol before use. Acryloyl chloride (Aldrich), triethyl amine (Merck), and dioxane (Aldrich) were used as received.
2.2. Characterization
All 1H NMR and 13C NMR spectra were taken on Varian 400-MHz NMR spectrometer (Varian Associates, Palo Alto, CA) operating at a frequency of 399.986 MHz for proton and 100.587 MHz for carbon in CDCl3 (Aldrich, Milwaukee, WI), and all data are reported as chemical shifts (d) with respect to tetramethyl silane. Fourier-transform infrared (FTIR) spectra of samples were obtained with a Perkin Elmer 1600 FTIR spectrophotometer (Massachusetts, USA). Gel Permeation chromatography analysis was performed using 103, 104, and 105 Å calibrated polystyrene standards. Elution rate is 1 ml/min at 30 °C with THF or chloroform. The thermal behavior of co-polymers was measured with DSC-7 (Perkin Elmer Cetus Instruments, Norwalk, Connecticut, USA) over a temperature range of −50 to 300 °C. The samples were first heated to 300 °C at a heating rate of 10 °C/min, then cooled to −50 °C at a cooling rate of 10 °C/min. Samples were reheated to 300 °C to determine glass transition temperature. Thermo gravimetric analysis of co-polymers was performed (Seiko DT/TGA 6300) over a temperature range of 30–800 °C at a heating rate of 10 °C/min TGA analysis.
2.3. Synthesis of homopolymer
ACTES (1.67 g, 4.77 mmol) was replaced in a schlenk tube and dioxane (4.77 ml) was added as polymerization solvent just enough to obtain the concentration as 1 M. Benzoyl peroxide (11.5 mg) was added as 1 mol% of the ACTES. The solution was purged with nitrogen for 30 min. Tube was sealed, heated to 80 °C, and mixed for 24 h. Polymerization mixture was then precipitated into methanol after 24 h, filtered, and dried (yield 73%).
2.4. Synthesis of co-polymers
General procedure for the synthesis of co-polymers was as follows (MMA co-polymer was given as example) 0.6012 g (1.28 mmol) ACTES and 0.0602 g (0.2528 mmol) benzoyl peroxide (1 mol% of total polymerizable monomers) were placed into schlenk tube and dissolved in 8-ml dioxane. 2.4 g (24 mmol) MMA (removed from inhibitor) was added, purged with nitrogen for 30 min, and sealed. Mixture was heated up to 90 °C in glycerol bath and mixed for overnight. Mixture was precipitated into methanol upon cooling (in which monomers are soluble), then filtered, and dried (yield 84%).
2.5. Reactivity ratio determination
The reactivity ratios “r1” and “r2” for each pair of monomers were determined by three different methods that were used to obtain reactivity ratios monomer pairs, Fineman and Ross [Citation21], Inverted Finemann and Ross [Citation21], and Kelen and Tudos [Citation22]. These methods are the most versatile methods to obtain best correlations within the experimental data and eliminates the use of computerized software.
3. Results and discussion
Behera amine is a first-generation dendron synthesized by Newkome et al. and its synthesis can be found in the literature.[Citation20] Its reaction with acryloyl chloride gave this dendron the capability to be free radically polymerized.[Citation18] Since benzoyl peroxide is as a free radical source, a higher boiling point solvent than the decomposition temperature of benzoyl peroxide, dioxane is the best solvent as polymerization medium due to its capability of to dissolve both reactants and synthesized polymer
3.1. Homopolymerization
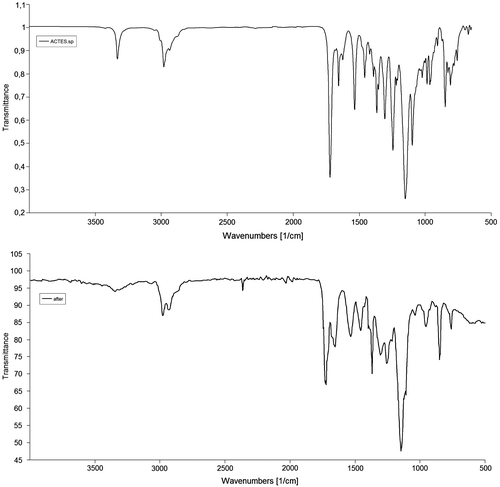
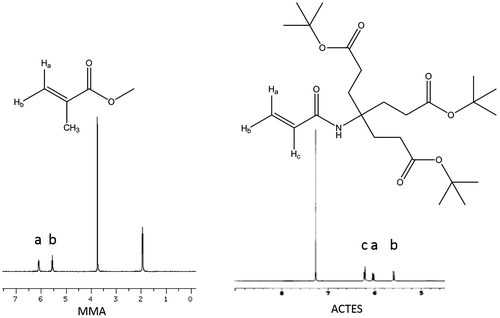
Dendrons of different generations with free radically polymerizable groups such as acrylates does not have the capability to homopolymerize under normal conditions, unless a flexible spacer was attached to the dendron.[Citation23] However, advances occurred to achieve the homopolymerization of these dendrons by several research groups.[Citation24–30] The main requirement to undergo homopolymerization is to sustain high monomer concentration during the polymerization. Molarity of the monomer in solution is around 1–2 M limits. Reasons of this homo polymerization could be a preorganization of dendrons prior to polymerization, so that the polymerizable units were kept at short distance [Citation23] or dense packing of dendrons in bulk leading to anchoring of bulky dendrons to the backbone.[Citation29] The first-generation dendron ACTES, bearing an acrylate group, radically homopolymerized in dioxane with benzoyl peroxide as radical initiator. Characteristic signal of acrylate at 1625, 1421, and 807 cm−1 were all disappeared at the FTIR spectrum (Figure ) of the homopolymer as well as both at 13C NMR and 1H NMR. 1H NMR shifts of acrylate group at 5.59, 6.03, and 6.20 ppm were disappeared together with 13C NMR shifts at 125.5 and 131.7 ppm. The average molecular weight of the homopolymer is about 3429 g/mol and the weight average molecular of the polymer is 4119 g/mol. The PDI is 1.2.
3.2. Copolymerization
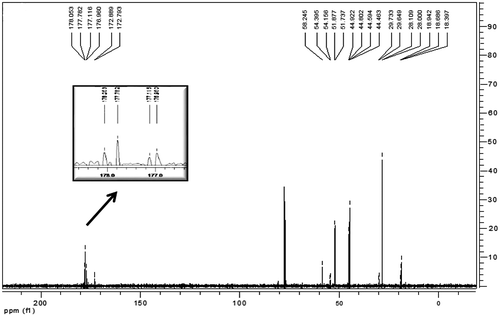
The co-polymer of ACTES with methyl methacrylate (MMA) was synthesized and characterized, ACTES-MMA. Although the characteristic signals of tert-butoxy group at 1726 cm−1 and secondary amine N–H bending signal at 1540 cm−1 did not appear at FTIR spectrum due to the small quantity in the polymerization mixture, C-13 spectrum revealed the formation of co-polymer (Figure ). Signals of Amide and ester carbonyl of the dendron attached to the polymer appeared at 177.1 and 172.7 ppm, respectively. In addition, the signal at 2.20 ppm in proton spectrum is due to the α-protons next to tert-butoxy carbonyls. Moreover, the GPC chromatogram of the co-polymer revealed average molecular weight and weight average molecular weight around 16,500 and 33,000 g/mol, respectively. The polydispersity index of the polymer is 1.8, a normal value for a free radical polymerization performed in solution.
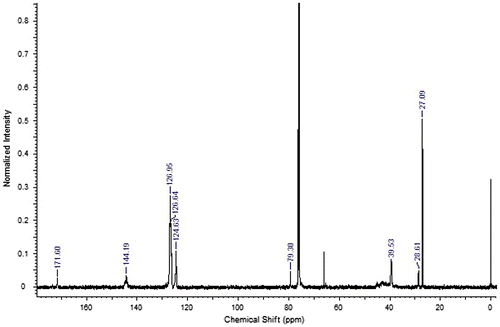
Co-polymer of ACTES with styrene was also synthesized and characterized ACTES-ST. The absence of double bond signals of dendron at 3012 cm−1 (C–H stretching) and 807 cm−1 (bending) and appearance of characteristic signals of ACTES monomer at 1720 cm−1 (ester) and 1682 cm−1 (amide) together with the characteristic signals of aromatic ring of styrene can be regarded as the formation of the co-polymer. Ester peak of the attached dendron can be seen in C13 spectrum together with the signals between 120 and 145 ppm coming from the aromatic ring of polystyrene (Figure ). Signals of tert-butoxy methyl protons of ACTES monomer and beta methyl protons to the ester carbonyl coincide with polystyrene chain signals. However, α-methyl protons to ester carbonyl appeared at 2.55 ppm.
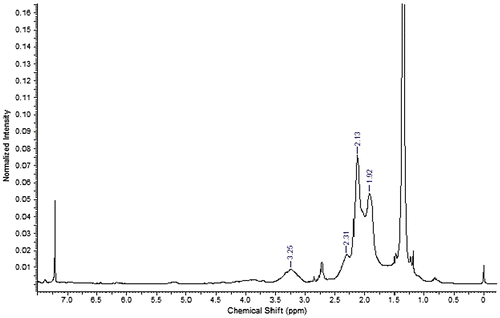
FT-IR spectrum of ACTES-NVP co-polymer revealed the ester signal of ACTES monomer 1720 cm−1. However, amide signal of ACTES, normally at 1682 cm−1 was blocked by lactam carbonyl signal of NVP monomer. Proton NMR spectrum has α-methyl protons to ester carbonyl signal at 2.31 ppm together with characteristic signal of pyrrolidone ring at 3.25, 2.13, and 1.92 ppm (Figure ).
Glass transition temperatures of ACTES-MMA and ACTES-ST appeared at 100 and 80 °C, respectively. These co-polymers exhibited three different decomposition stage. In TGA of ACTES-ST, first and second decompositions with an 8% loss at 254 °C and 10.1% loss at 307 °C were attributed to the loss due to the thermal degradation of dendrons attached to the main polymer chain. The largest degradation pattern of 73.4% at 395 °C is linked with main polystyrene chain degradation. Also the char yield of the ACTES-ST co-polymer is about 4% under inert conditions. ACTES-NVP co-polymer revealed a broad glass transition between 56 and 60 °C. Thermo gravimetric analysis contains 30% weight loss probably coming from decomposition of dendrons at 230 °C, 38% weight loss from the decomposition of pyrrolidone ring at 313 °C and main chain degradation at 416 °C. Graphs are shown in supplementary information.
3.3. Reactivity ratio determination
It has crucial importance to determine (stop) the conversions below 10% because only under low conversions, monomers exhibited their real reactivities toward their selves or other monomers. By this way, main compositions on the co-polymer can be determined and subsequently reactivity ratios can be obtained by applying different feeding ratios.
Time required for conversion up to 10% was determined as 30–40 min. However, co-polymers do not reach to sufficient molecular weight for precipitation as the quantity of ACTES in co-polymer feed increases. Since the molecular weight of the ACTES is much bigger relative to the comonomers, the quantity of the comonomers was not sufficient to obtain suitable reaction mixture. Thus, determination of reactivity ratios cannot determined gravimetric means. Instead, the conversions of monomers were determined via in situ NMR spectroscopy. In situ NMR technique offers the advantage of high sensitivity and real-time data acquiring. However, a few requirement should be met for this technique: (a) reaction rates of monomers should be lower than data acquiring rate, (b) growing polymers should not precipitate in NMR solvent, and (c) Signals of both reactants and products should not coincide.[Citation31]
Signals of vinyl protons of both ACTES and MMA are very close to each other thus imparting inconsistencies in determining conversion ratios violating the above conditions (Figure ). Styrene, on the other hand, has suitable proton signals (6.61, 5.61, and 5.18) that do not interfere with ACTES proton signals (6.48, 6.01 and 5.50 ppm). Conversions of monomers were obtained via the integration of aromatic signals of styrene between 7 and 7.5 ppm and tertiary butoxy methyl protons signals of ACTES at 1.3 ppm.
3.4. Reactivity ratios
Reactivity ratios of ACTES, styrene, and N-vinyl pyrrolidone were represented as rac, rst, and rnvp, respectively and are represented in Table . Reactivity ratios of ACTES and styrene monomer pair were found to be rst = 0.27 and rac = 0.44 (entry 4). For the ACTES and NVP monomer pair, these values appeared as rac=1.01 and rnvp=0.36 (entry 8). All values in all graphs and methods for both monomer pairs are linear. These linearities indicate that copolymerization reactions follows classical copolymerization kinetics and show that reactivities of polymeric radicals depend on the terminal monomer unit. The product of reactivity ratios of ACTES and styrene monomer pair (rac × rst = 0.12) indicates a much closer tendency towards alternation than those of ACTES and NVP pair (rac × rnvp = 0.36).
Table 1. Reactivity ratios of ACTES-ST and ACTES-NVP monomer pairs.
The transition probabilities for an almost equimolar monomer feed were given in Table . The formation probability of MactMact and MstMst blocks (pac,ac = 22% and pst,st = 31%) are less than the formation probability of cross propagation, MactMst, and/or MstMact. The number average uninterrupted monomer sequence length is 1.48 for ACTES and 1.23 for styrene. These information also support the tendency for alternation for this copolymerization.[Citation32] The probability of MnvpMnvp block formation was found to be 27%, a lower value than the probability of MactMact (pac,ac = 50%), MnvpMact (pnvp,act = 50%) and MactMnvp (pac,nvp = 73%) block formations. The block length (number average) of pure ACTES and NVP monomers is 2.2 and 1.3, respectively.
Table 2. Transition probabilities of ACTES-ST and ACTES-NVP monomer pairs.
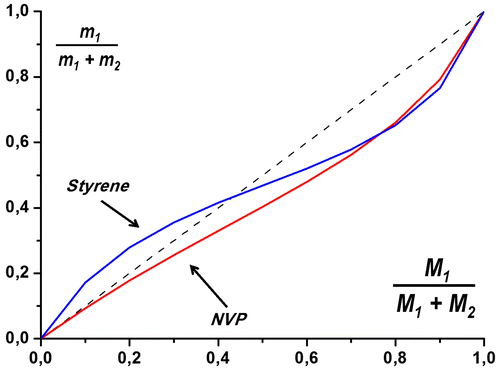
Figure shows monomer feed and co-polymer composition for styrene and ACTES monomer. The initial monomer feed ratio and monomer mole ratio in co-polymer composition is equal at the junction point. For styrene and dendronized polymer, this point (Mst = mst) is 0.45. Theoretical value was found as 0.44 with the formula below.
3.5. “Q” and “e” values
These values can be used as a method to determine the reactivities of the monomers. “Q” parameter is an indication of resonance and steric effect within the monomer, and “e” parameter represents the polarity. These parameters are useful in determining the polymerization behaviors of novel monomer pairs and determined by Alfrey-Price [Citation33] method via equations (supplementary info). The more the electron withdrawing character of the monomer is, the more the values of “e” and “Q” are and vice versa.
The reference values for styrene are Q1 = 1 and e1 = −0.80. The Q and e value for ACTES (Table ) with the reference as styrene were found to be 1.152 and 0.66, respectively. The higher Q value of ACTES in table can be regarded as that steric effect is more predominant with respect to the octadecyl derivative with long alkyl chain. ACTES does not have significant difference in terms of polarity from acrylamide but less polarity than methylol and octadecyl derivatives according to e value.
Table 3. “Q” and “e” values of ACTES and its analog.
4. Conclusion
Acrylated behera amine, ACTES, a polymerizable g[1] dendron, was successfully homopolymerized. ACTES was also copolymerized with commodity monomers such as styrene, methyl methacrylate, and N-vinyl pyrrolidone. Reactivity ratios of ACTES-MMA pair could not be obtained due to the overlap of vinyl proton signals. Reactivity ratios of ACTES-ST and ACTES-NVP monomer pairs were determined via NMR spectroscopy. Copolymerization diagram for ACTES-ST and ACTES-NVP monomer pairs was established. “Q” and “e” values of ACTES were also compared with its analog.
Supplemental data
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/15685551.2015.1070506.
Supplementary_material.doc
Download MS Word (2.3 MB)Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Xiong X, Chen Y. Tailoring dendronized polymers. Chem. Commun. 2010;46:5049–5060.
- Frauenrath H. Dendronized polymers – building a new bridge from molecules to nanoscopic objects. Prog. Polym. Sci. 2005;30:325–384.10.1016/j.progpolymsci.2005.01.011
- Carlmark A, Hawker CJ, Hult A, Malkoch M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009;38:352–362.10.1039/B711745K
- Zhang A, Shu L, Bo Z, Schlüter AD. Dendronized polymers: recent progress in synthesis. Macromol. Chem. Phys. 2003;204:328–339.10.1002/macp.200290086
- Welch PM, Welch CF. The effects of crowding in dendronized polymers. Nano Lett. 2006;6:1922–1927.10.1021/nl061036d
- Wall FT. The structure of copolymers. II 1. J. Am. Chem. Soc. 1944 [cited 1944 Dec 1];66:2050–2057.10.1021/ja01240a014
- Odian G. Principles of polymerization. 4th ed. New York (NY): Wiley; 2004.10.1002/047147875X
- Krebs A, Bruchmann B, Müller-Cristadoro A, Al-Hellani R, Schlüter AD. Copolymerization of a dendronized monomer with styrene and different acrylates: determination of reactivity ratios. J. Polym. Sci. A Polym. Chem. 2012;51:1372–1377.
- Rimondino GN, Strumia MC, Martinelli M. Synthesis and characterization of water-soluble dendronized chitosan using Newkome-type dendrons. ACS Sustainable Chem. Eng. 2014;2:2582–2587.
- Schönamsgruber J, Zeininger L, Hirsch A. Grafting perylenes to ZnO nanoparticles. Chem. Eur. J. 2014;20:2529–2536.10.1002/chem.v20.9
- Schönamsgruber J, Schade B, Kirschbaum R, et al. Synthesis and aggregation properties of polycationic perylenetetracarboxylic acid diimides. Eur. J. Org. Chem. 2012;2012:6179–6186.10.1002/ejoc.201201062
- Jung H, Carberry TP, Weck M. Synthesis of first- and second-generation poly(amide)-dendronized polymers via ring-opening metathesis polymerization. Macromolecules. 2011;44:9075–9083.10.1021/ma2016375
- Kostiainen MA, Szilvay GR, Lehtinen J, et al. Precisely defined protein–polymer conjugates: construction of synthetic DNA binding domains on proteins by using multivalent dendrons. ACS Nano. 2007;1:103–113.10.1021/nn700053y
- Hardy JG, Kostiainen MA, Smith DK, Gabrielson NP, Pack DW. Dendrons with spermine surface groups as potential building blocks for nonviral vectors in gene therapy. Bioconjugate Chem. 2006;17:172–178.10.1021/bc050212r
- Love CS, Ashworth I, Brennan C, Chechik V, Smith DK. Dendron-protected Au nanoparticles – effect of dendritic structure on chemical stability. J. Colloid Interface Sci. 2006;302:178–186.10.1016/j.jcis.2006.05.064
- Cho TJ, Zangmeister RA, MacCuspie RI, Patri AK, Hackley VA. Newkome-type dendron-stabilized gold nanoparticles: synthesis, reactivity, and stability. Chem. Mater. 2011;23:2665–2676.10.1021/cm200591h
- Chen YC, Wu TF, Jiang L, et al. Synthesis of dendritic catalysts and application in asymmetric transfer hydrogenation. J. Org. Chem. 2005;70:1006–1010.10.1021/jo048317v
- Cuggino JC, Calderón M, Alvarez CI, et al. New dendronized polymers from acrylate Behera amine and their ability to produce visco-elastic structured fluids when mixed with CTAT worm-like micelles. J. Colloid Interface Sci. 2011;357:147–156.10.1016/j.jcis.2011.01.075
- Zhou T, Kong XL, Liu ZD, Liu DY, Hider RC. Synthesis and Iron(III)-chelating properties of novel 3-hydroxypyridin-4-one hexadentate ligand-containing copolymers. Biomacromolecules. 2008;9:1372–1380.10.1021/bm701122u
- Newkome GR, Kotta KK, Moorefield CN. Convenient synthesis of 1 → 3 C-branched dendrons. J. Org. Chem. 2005 [cited 2005 June 1];70:4893–4896.10.1021/jo0504518
- Fineman M, Ross SD. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950;5:259–262.10.1002/pol.1950.120050210
- Kelen T, TÜdös F. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J. Macromol. Sci. Part A Chem. 1975;9:1–27.10.1080/00222337508068644
- Percec V, Schlueter D. Mechanistic investigations on the formation of supramolecular cylindrical shaped oligomers and polymers by living ring opening metathesis polymerization of a 7-oxanorbornene monomer substituted with two tapered monodendrons. Macromolecules. 1997;30:5783–5790.10.1021/ma970157k
- Chen Y-M, Chen C-F, Liu W-H, Li Y-F, Xi F. Poly(methacrylates) with different kinds of dendritic moieties as side groups. Macromol. Rapid Commun. 1996;17:401–407.10.1002/marc.1996.030170606
- Draheim G, Ritter H. Polymerizable dendrimers. Synthesis of a symmetrically branched methacryl derivative bearing eight ester groups. Macromol. Chem. Phys. 1995;196:2211–2222.10.1002/macp.1995.021960711
- Neubert I, Klopsch R, Claussen W, Schlüter AD. Polymerization of styrenes and acrylates carrying dendrons of the first and second generation. Acta Polym. 1996;47:455–459.10.1002/actp.1996.010471006
- Percec V, Ahn CH, Barboiu B. Self-encapsulation, acceleration and control in the radical polymerization of monodendritic monomers via self-assembly. J. Am. Chem. Soc. 1997;119:12978–12979.10.1021/ja9727878
- Zhang A, Zhang B, Wächtersbach E, Schmidt M, Schlüter AD. Efficient synthesis of high molar mass, first- to fourth-generation distributed dendronized polymers by the macromonomer approach. Chem. Eur. J. 2003;9:6083–6092.10.1002/(ISSN)1521-3765
- Zhang A, Okrasa L, Pakula T, Schlüter AD. Homologous series of dendronized polymethacrylates with a methyleneoxycarbonyl spacer between the backbone and dendritic side chain: synthesis, characterization, and some bulk properties. J. Am. Chem. Soc. 2004;126:6658–6666.10.1021/ja0494205
- Kasëmi E, Schlüter AD. An easy accessible homologous set of first to fifth generation dendritic methacrylic macromonomers and their polymerizations. New J. Chem. 2007;31:1313–1320.10.1039/b617989d
- Mahdavian AR, Abdollahi M, Mokhtabad L, Reza Bijanzadeh H, Ziaee F. Kinetic study of radical polymerization. IV. Determination of reactivity ratio in copolymerization of styrene and itaconic acid by 1H-NMR. J. Appl. Polym. Sci. 2006;101:2062–2069.10.1002/(ISSN)1097-4628
- Krebs A, Bruchmann B, Müller-Cristadoro A, Al-Hellani R, Schlüter AD. Copolymerization of a dendronized monomer with styrene and different acrylates: determination of reactivity ratios. J. Polym. Sci. Part A Polym. Chem. 2013;51:1372–1377.10.1002/pola.26504
- Alfrey C, Price C. Relative reactivities in vinyl copolymerization. J. Polym. Sci. 1947;A2:101–106.
- Brandrup J, Immergut EH, Grulke EA. Polymer Handbook. New york (NY): Wiley&Sons; 1989.