Abstract
Biodegradable amphiphilic triblock copolymer of PCL-b-PEG-b-PCL was synthesized by a simple, environmentally friendly and one-pot biocatalytic route that employs lipase Novozym-435 as a biocatalyst and poly (ethylene glycol) (PEG) as a macromolecular initiator to induce ring-opening polymerization of ε-caprolactone (ε-CL). The structure of the prepared copolymers was characterized by gel permeation chromatography, Fourier transform infrared spectrum and nuclear magnetic resonance spectrum. The crystalline behaviours of the PCL-b-PEG-b-PCL were investigated by differential scanning calorimetry and X-ray diffraction. The self-assembly behaviours of the amphiphilic PCL-b-PEG-b-PCL were studied by fluorescence technique, dynamic light scattering instrument and transmission electron microscopy. The structure analyses indicate that PCL-b-PEG-b-PCL with desired components can be synthesized by changing the molar ratio of ε-CL monomer and PEG macroinitiator. Interestingly, the crystalline structure of PCL-b-PEG-b-PCL is mainly determined by the crystallization of PCL block, suggesting that the crystallization of PEG block in the copolymer is inhibited by the PCL block. The self-assembly behaviours of PCL-b-PEG-b-PCL in water can be adjustable according to the molar ratio of ε-CL and PEG. Size of the formed PCL-b-PEG-b-PCL micelles increased and the critical micelle concentration decreased while the polycaprolactone chain length increased.
1. Introduction
Aliphatic polyesters, such as polylactic acid and polycaprolactone (PCL), have generated great interests for several decades because of their good biodegradability, biocompatibility and drug permeability, and thus have been widely used in biomedical applications.[Citation1–3] However, when used as drug carriers, the hydrophobic PCL nanoparticles can be easily adsorbed by proteins or be identified and captured by reticuloendothelial cells, so it is impossible to achieve the expected effect of targeting drug delivery and controlled drug release. Surface hydrophilic modification of PCL nanoparticles is a good way to solve this problem. Poly ethylene glycol (PEG) is a well-known non-ionic and water-soluble polymer with some unique properties, such as biological compatibility, non-toxicity and non-immunogenicity. It can be used as the hydrophilic part of linear amphiphilic block copolymers. The PCL-block-PEG (PCL-b-PEG) copolymer, produced by introducing PEG chains into PCL, can be made into nanoscale micelles in proper solvents.[Citation4–6] These drug-loaded nano-micelles can escape from being phagocytized by the reticuloendothelial system, retain for a long time in blood circulation and enrich continuously around the tumour location.[Citation1,2] As a result, they can improve the curative effect of drugs and reduce the toxicity to normal cells.[Citation1,2]
At present, aliphatic polyesters are mainly prepared by active ionic polymerization using traditional organometallic compounds’ catalysts, such as tetrabutyle titanate and stannous octoate and aluminium complexes.[Citation7,8] Unfortunately, even a tiny quantity of metal catalyst residues can cause serious toxicity problems when they are used as biomedical materials. Therefore, it is of great importance to find some highly efficient and non-toxic catalysts to synthesize the PCL-based copolymer. One possible method to solve this problem is to use enzyme-catalysed route instead of chemical catalysts. Klibanov et al. [Citation9,10] firstly reported the fairly high catalytic activity and heat stability of lipase in organic medium when used in ester synthesis reactions. This report opens up a new research area of non-aqueous phase enzymology and extends the application range of enzyme greatly, making a quite great development of enzyme application in organic synthesis. Thus, it is reasonable to use lipase to catalyse the ring-opening polymerization (ROP), esterification and transesterification of lactones. Enzyme is a kind of renewable and environmentally friendly resource, and the reactive conditions of enzyme-catalysed synthesis are mild and controllable. Furthermore, enzyme is easily removed and recycled from the reactive system after synthesis, so the purification of products is much simple. It has been indicated that the commercial lipase Novozym-435 is a kind of efficient biocatalyst in the ROP, esterification and transesterification of lactones.[Citation11,12] It is usually highly efficient for the preparation of polyesters in common solvents such as toluene and isooctane in the temperature range of 50–80 °C. In this work, the ROP of ε-caprolactone (ε-CL) was conducted in organic medium toluene using lipase Novozym-435 as a biocatalyst and PEG as a macromolecular initiator to synthesize a triblock copolymer of PCL-b-PEG-b-PCL. The structure of the prepared copolymer was characterized by gel permeation chromatography, Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopy. The crystalline behaviours of PCL-b-PEG-b-PCL were investigated by differential scanning calorimetry (DSC) and X-ray diffraction (XRD). The self-assembly properties of PCL-b-PEG-b-PCL in water were revealed by fluorescence technique, dynamic light scattering (DLS) and transmission electron microscopy (TEM).
2. Experimental
2.1. Materials
Novozym-435 (immobilized lipase B from Candida antartica, the enzyme activity was 10,000 PLU/g) was purchased from Novozymes A/S. Both ε-CL (99%) and PEG (Mn = 2000, 4000, 6000 g/mol) were purchased from Shanghai Chemical Reagents (China). Toluene was dried over Na and further distilled before use. All other chemicals were used without further purification unless otherwise stated.
2.2. Lipase-catalysed synthesis of PCL-b-PEG-b-PCL
Synthetic route of the amphiphilic triblock copolymer of PCLN-b-PEGM-b-PCLN (M: polymerization degree (DP) of PEG block, N: DP of PCL block) is shown in Scheme . The dihydroxyl-terminated linear PEG with desired molecular weight is used as a macromolecular initiator and the chain length of PCL block can be controlled by changing the value of M/N ratio.
Novozym-435 (9%, wt%) was dried under vacuum at room temperature for 24 h and then transferred into a thoroughly dried flask containing ε-CL, PEG and toluene (2:1 ratio of volume/total weight of reactant, as solvent). The flask was then sealed with a glass stopper and immersed into an oil bath at 70 °C under stirring for a given time. The mixture was cooled to room temperature and then dichloromethane was added. The resulting mixture was then filtered to remove Novozym-435. The obtained filtrate was introduced into plenty of petroleum ether to remove unreacted ε-CL and undesired PCL homopolymer. The precipitate was re-dissolved in dichloromethane, and then the resulting solution was added into cold methanol by deposition to remove the unreacted PEG. Finally, the PCL-b-PEG-b-PCL was dried in a vacuum oven and obtained as a white solid.
2.3. Preparation of micellar solution
PCL-b-PEG-b-PCL triblock copolymer micelles were prepared by a dialysis method. One hundred milligrams of PCL-b-PEG-b-PCL were first dissolved in 25 mL of acetone, and the solution was added dropwise into 25 mL of distilled water under moderate stirring at 25 °C. The acetone in the obtained suspension was then removed by dialysis method for 48 h.
2.4. Characterization
2.4.1. Molecular weight determination
Molecular weight and polydispersity were determined by size exclusion chromatography (SEC). The SEC analysis was performed at Waters 2410 detector using THF as the solvent with a flow rate of 0.6 mL/min. Calibration was accomplished with polystyrene standards (Polysciences, USA).
2.4.2. Structural characterization
Structures of the as-prepared products were characterized using FTIR spectroscopy and NMR spectroscopy. The FTIR determination was carried out on a Bio-Rad FTS 3000 and obtained from 32 scans with a resolution of 2 cm−1. The polymer powder was mixed with KBr and pressed into a plate for measurement. NMR (400 MHz) spectra were recorded on a Bruker Ultra Shied 400 spectrometer using CDCl3 as the solvent and tetramethylsilane as the internal reference.
2.4.3. Crystalline behaviours
Crystallization of the PCL-b-PEG-b-PCL was tested with DSC analysis on a Netzsch 204 F1, which was first heated to 130 °C under N2 at a speed of 10 °C/min before determining to eliminate the heat history. The sample was then cooled to −10 °C and heated to 130 °C again at the same heating speed.
The crystallization structure was determined with a Bruker D8 Advance X-ray diffractometer. The Cu–Kα radiation was used operating at 40 kV and 40 mA in the 2θ range from 18 to 25° in steps of 0.02°.
2.4.4. Self-assembly behaviours
2.4.4.1. Critical micelle concentration determination
Critical micelle concentration (CMC) of the PCL-b-PEG-b-PCL in aqueous solution was estimated by fluorescence spectroscopy using pyrene as a hydrophobic fluorescence probe. Samples of polymer solution with concentrations ranging from 1 × 10−5 to 5 mg/mL were prepared and then left to equilibrate with constant pyrene concentration of 2 × 10−6 M for 24 h at 25 °C. Fluorescence spectra of pyrene were recorded with a Hitachi F-4500 fluorescence spectrophotometer at room temperature. The excitation wavelength was 339 nm and the emission spectra were obtained from 300 to 460 nm. The CMC value was taken from the peak intensity ratio of I3/I1 (I1 at 373 nm and I3 at 384 nm) against the logarithm of polymer concentration.[Citation13]
2.4.4.2. Nanoparticle size and morphology observation
The micelles were characterized by DLS instrument and TEM. The size and its distribution were determined by DLS using a Malvern Nano-ZS90 laser nanogranulometric analysis instrument. Each measurement was averaged three times and the micellar solution was filtrated with a 0.45-µm microfilter before the determination. TEM image of the micelles was conducted on a JEM-100cX II instrument operating at an acceleration voltage of 80 kV. A drop of the micellar solution was deposited on carbon-coated copper grids for 1 min followed by staining with 1 wt% aqueous solution of phosphotungstic acid and then water was removed with a piece of filter paper.
3. Results and discussion
3.1. The lipase-catalysed synthesis of PCL-b-PEG-b-PCL and the structural characterization
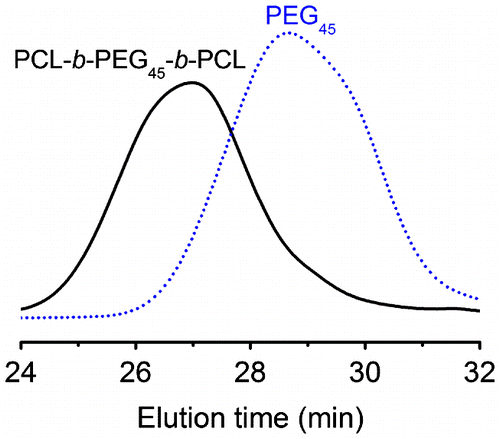
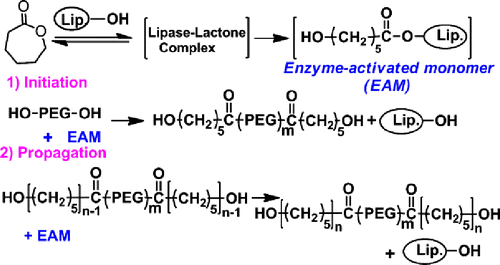
Lipase-catalysed ROP of lactones is viewed as a process via an acyl enzyme intermediate. The enzymatic ROP of ε-CL is explained by the mechanism described in Scheme .[Citation11] A key step is the ROP reaction of ε-CL with lipase to give an enzyme-activated monomer. Initiation is a nucleophilic attack of PEG onto the acyl carbon of the intermediate. Propagation reaction proceeds via the nucleophilic attack of intermediate by the terminal hydroxyl group of a propagating polymer. However, the PCL homopolymer is probably formed by the ROP of ε-CL itself. The length of PCL block can be determined by the molar ratio of ε-CL monomer with the PEG macroinitiator. The as-prepared block copolymer is denoted by PCLN-b-PEGM-b-PCLN, where M and N represent the DP of PEG block and PCL block, respectively. Typically, feeding PEG45 (Mn = 2000 g/mol) and ε-CL with N/M of 1 was employed for the Novozym-435-catalysed ROP of ε-CL to synthesize the PCL45-b-PEG45-b-PCL45. The molecular weight (Mn) of purified PCL45-b-PEG45-b-PCL45 was traced by SEC, which was shown in Figure . Clearly, a single peak of PCL45-b-PEG45-b-PCL45 was observed within the high molecular weight range, indicating that there is only one kind of polymer and no residual ε-CL. The SEC results show that the PCL45-b-PEG45-b-PCL45 has a Mn of 11,900 g/mol and a polydispersity index (PDI) of 1.28. This value close to the theoretical Mn of 12,200 g/mol and the narrow PDI both suggest the living polymerization character of this method. PCLN-b-PEGM-b-PCLN with various M/N values was prepared via this Novozym-435-catalysed method and all the results were summarized in Table .
Table 1. Molecular weight of the as-prepared PCLN-b-PEGM-b-PCLN copolymers determined by SEC and 1H NMR.
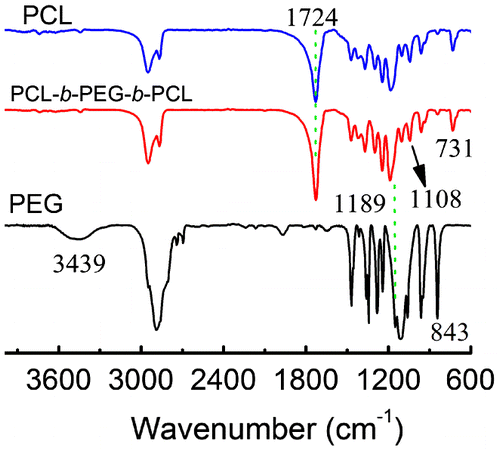
The structure of PCL45-b-PEG45-b-PCL45 has been further confirmed by the combination of FTIR and 1H NMR. Figure represents the FTIR spectra of PCL45-b-PEG45-b-PCL45, PCL and PEG45 homopolymers. As shown in Figure , the characteristic absorption peak of terminated –OH in PEG45 was visible at 3439 cm−1, but it was very weak for the PCL45-b-PEG45-b-PCL45. This difference implies that the PCL45-b-PEG45-b-PCL45 has a higher Mn than PEG homopolymer, so the very low content of terminated –OH in the triblock copolymer makes its absorption so weak that it cannot be observed any more by FTIR. The absorption attributed to –C=O of PCL block at 1724 cm−1, characteristic C–O–C stretching vibrations due to the repeated –OCH2CH2 units at 1108 cm−1 and C–O stretching vibrations at 1189 cm−1 in PEG block were also detected by the FTIR spectra. Obviously, all these typical absorptions show that they should come from the PCL45-b-PEG45-b-PCL45 and not be caused by the mixed homopolymers of PCL and PEG because the SEC trace suggests the existence of only one kind of polymer. It is worth noting that we could not find the typical absorption peaks of PEG around 843 cm−1, which is well known to be the characteristic of the crystalline phase of PEG,[Citation14] suggesting that no PEG crystals are formed in the copolymers. But the peak at 731 cm−1, resulting from crystalline PCL, suggests the presence of some PCL crystalline phases in the copolymers.[Citation14]
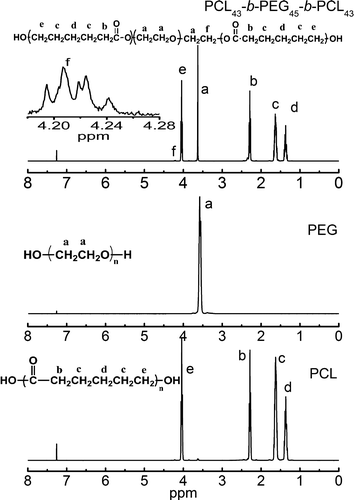
The structure of the PCL45-b-PEG45-b-PCL45 has been further determined by 1H NMR spectroscopy, which is illustrated in Figure and compared with PEG and PCL homopolymers at the same time. As shown in Figure , the sharp peak at 3.64 ppm (peak a in the top and middle) is attributed to the repeated methylene protons of the PEG blocks. The small peak centred at 4.22 ppm (f in inset) is attributed to the ended methylene proton of PEG in the copolymer of PCL45-b-PEG45-b-PCL45. In addition, peaks at 1.38, 1.64, 2.30 and 4.05 ppm (d, c, b and e) are ascribed to methylene protons in PCL blocks, respectively. These results are consistent with those triblock copolymers of PCL-b-PEO-b-PCL prepared from the ROP of ε-CL with alkali–metal alkoxide derivatives of PEG.[Citation15] Since the Mn of the PEG block has been given already, the Mn of PCL block can be calculated according to the area ratio of peak a at 3.64 ppm and peak b (or c, d and e) at 2.30 ppm. Then, the obtained Mn of PCL block is 12,100 g/mol by 1H NMR, very close to the theoretically designed Mn and practically measured value, suggesting the living polymerization character of this lipase-catalysed synthesis again. Note that these peaks should be from PCL45-b-PEG45-b-PCL45 but not from the mixture of PCL and PEG because there was a single peak in the SEC trace and the newly generated proton signal of peak f was observed at 4.22 ppm.
A series of copolymers with various length of PEG blocks was synthesized by the Novozym-435-catalysed ROP of ε-CL monomer. The reaction was theoretically designed with DPPCL/DPPEG of 1.0 when the Mn of PEG macroinitiator was changed from 2000 to 6000 g/mol under the same conditions, and the results were also listed in Table . The results indicate that the determined Mn of the obtained triblock copolymers increased with increase in the length of PEG chains. The determined Mn increased to 19,000 g/mol when the Mn of PEG changed from 2000 to 6000 g/mol, but these results were deviated from the desired values. However, the obtained Mn of PCL-b-PEG-b-PCL was close to the designed value when the PEG was fixed with Mn of 2000 g/mol. Obviously, the activity of the terminal –OH of PEG was dropped with an increase in the length of PEG chains, which indicates that the polymerization is only living when the PEG chains are short. Hence, the final Mn of PCL-b-PEG-b-PCL hardly exceeds 20,000 g/mol when the length of PEG chains is long. The viscosity of the reaction system may be prodigious when the Mn reaches 20,000 g/mol, and thus the diffusion rates of PEG and the intermediated copolymers to the active site of the enzyme were restricted, which is consistent with the results reported previously.[Citation11]
3.2. Crystallization behaviours of PCL-b-PEG-b-PCL
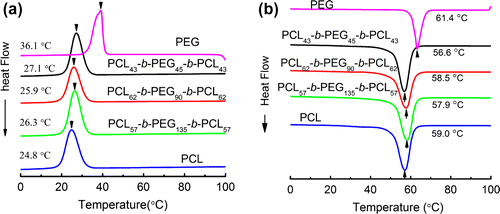
Both PEG and PCL are semi-crystalline polymers, and thus PCL-b-PEG-b-PCL also is a kind of typical semi-crystallization block copolymer. Additionally, both crystallization temperature (Tc) and melting temperature (Tm) for the PEG and PCL homopolymer are adjacent to each other.[Citation16] Also, both PEG and PCL show good drug permeability and biocompatibility, so they are widely used for biomedical applications. However, the drug permeability and biocompatibility are possibly affected by the crystallization behaviours of copolymers. Figure (a) and (b) represents the cold crystallization and melting processes of PCL-b-PEG-b-PCL revealed by DSC analysis, respectively, while PEG and PCL homopolymers are also used for a comparison. Clearly, all the DSC plots in Figure (a) and (b) showed the only detectable crystallization and melting processes of the PCL blocks in the copolymers, indicating that the crystallization ability of PEG blocks is inhibited by the PCL blocks. The crystallization behaviours of the triblock copolymer are mainly inflected by the crystallization of PCL blocks. The Tm and Tc of PCL-b-PEG-b-PCL are very much close to the pure PCL. The resulting Tm, Tc and their enthalpy variations (H, J/g) of PCL-b-PEG-b-PCL are summarized in Table . These results suggest that the crystallization behaviours are similar to that of PCL homopolymer. The PEG chain motions are disturbed by the PCL crystallization process, probably because the PCL contents are much higher than the PEG content, as evidenced by the Table . Interestingly, these observations are consistent with the results of FTIR, where the characteristic absorption of PEG crystallization is absent at 843 cm−1.
Table 2. The thermal parameters of PCL-b-PEG-b-PCL determined by DSC.
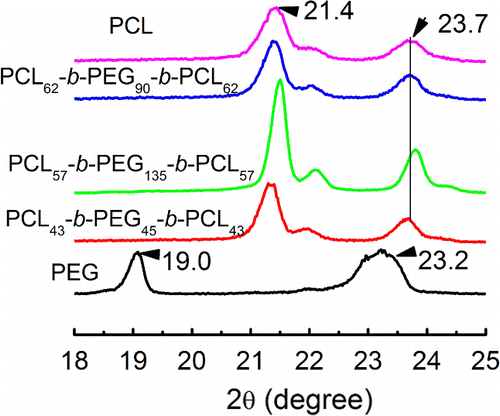
XRD diffraction method has been used to investigate the crystallization structure of PCL-b-PEG-b-PCL, which is presented in Figure . Obviously, the semi-crystalline PCL has two diffraction peaks at 21.4° and 23.4°, and PEG also has two diffraction peaks at 19.0° and 23.2°, respectively. They are the characteristic diffraction peaks for the PCL and PEG homopolymer.[Citation17] The triblock copolymers prepared in this work have sharp diffraction peaks near 21.4° and 23.7°, which means that the PCL-b-PEG-b-PCL also is a kind of semi-crystalline copolymer and has short-range ordered crystalline structure. However, the two diffraction peaks are close to the diffraction peak of PCL homopolymer. Again, it indicates that the crystallization of middle block of PEG is restricted by the outer PCL block, and the latter is easier to form ordered structure in the copolymers. Specially, PCL-b-PEG-b-PCL with the longest PEG chain (DP = 135) among the as-prepared triblock copolymers is still not able to show any PEG crystallization, even when longer PEG has commonly exhibited very strong crystalline ability. It means that the chain motions of PEG are strongly disturbed by the PCL block. The crystalline properties of PCL-b-PEG-b-PCL studied by XRD are the same to the results of FTIR and DSC analyses.
3.3. Self-assembly behaviours in water
Amphiphilic block copolymers consisting of a hydrophilic PEG block and a hydrophobic PCL block can form various micelles.[Citation4–6] In an aqueous system, the hydrophobic block aggregates to form a micellar core and the hydrophilic block constitutes the outer shell.[Citation4–6] Fluorescence spectra based on selective emission of pyrene in hydrophobic phase against aqueous phase have been used with great success for the measurement of CMC.[Citation13,18,19] The CMC is an effective parameter of micellar stability, and a low critical value is desired for stable micelles. In the emission spectra of pyrene, the emission intensity increases with increase in polymer concentration due to the fact that pyrene is transferred into the formed hydrophobic core of micelles and further aggregates there once the polymer micelles are produced. The fluorescence spectra of pyrene in water with changed concentration of PCL43-b-PEG45-b-PCL43 are shown in Figure (a). The third emission peak of I3 at 384 nm and the first emission peak of I1 at 373 nm are important since their intensity ratio of I3/I1 can be used as an indicator to represent the CMC value.[Citation13] Figure (b) represents the I3/I1 values plotted as a function of copolymer concentration (Log C). The CMC values for all the prepared copolymers of PCL-b-PEG-b-PCL are summarized in Table , and the CMC value of PCL43-b-PEG45-b-PCL43 determined by this method is 1.42 × 10−3 g/L. All these triblock copolymers have lower CMC values, providing evidence for an apparent stability of micelles and allowing their use in very dilute aqueous solutions such as body fluid. For the copolymer composed of PEG45 block, the CMC value decreased with the increase in PCL block mass fraction, indicating that the micelles having relative shorter PCL chains are more stable. Interestingly, the CMC value was enhanced when the chain length ratio of PEG/PCL (LPEG/LPCL) increased from 0.30 to 1.18, implying that the CMC mainly depends on the length of hydrophobic PCL block. The sizes of PCL-b-PEG-b-PCL micelles were measured by means of DLS method, and the results were also listed in the Table . The hydrodynamic diameters (DH) of the as-prepared micelles were in the range of 80–160 nm, and their size distributions (PDI) were relative narrow, less than 0.15. For the copolymer composed of PEG90 block, the DH of micelles increased as the mass fraction of PEG block decreased, which mainly originated from the increase in hydrophobic property caused by the longer hydrophobic PCL chain.
Figure 6. (a) Fluorescence emission spectra of pyrene in water with changed concentration of PCL43-b-PEG45-b-PCL43; (b) plot of Log C versus emission intensity ratio of I3/I1.
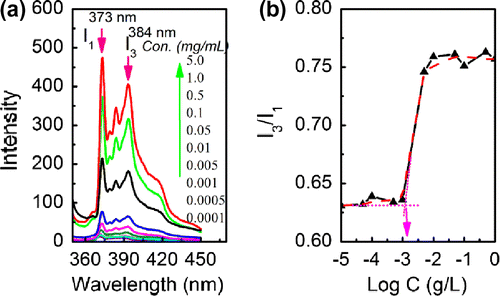
Table 3. The CMC and DH of PCL-b-PEG-b-PCL micelles with changing chain lengths of PEG and PCL.
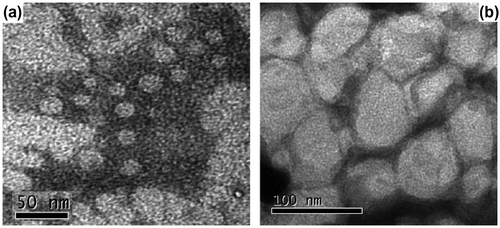
Morphologies of PCL-b-PEG-b-PCL micelles have been observed by TEM in Figure . The micelles formed by both PCL57-b-PEG135-b-PCL57 and PCL62-b-PEG90-b-PCL62 show spherical shape. The sizes estimated by TEM are less than those obtained from the DLS method in Table because the micelles determined by DLS were swollen in aqueous solution, whereas they were in a dried state with TEM.
4. Conclusions
A simple, environmentally friendly, one-pot biocatalytic route to amphiphilic triblock copolymers of PCL-b-PEG-b-PCL is achieved via lipase Novozym-435-catalysed ring-opening polymerization of ε-caprolactone with PEG as a macroinitiator. The structure of PCL-b-PEG-b-PCL can be tuned by changing the molar ratio of ε-CL and PEG. This lipase-catalysed polymerization of ε-CL is living when the PEG initiator has low molecular weight. Interestingly, PCL-b-PEG-b-PCL is a semi-crystalline copolymer, but its crystalline structure is caused by the crystalline PCL block, suggesting that the crystallization of PEG block in the copolymer is inhibited by the PCL block. The amphiphilic PCL-b-PEG-b-PCL can form micelles in water, while both size and CMC of the micelles are adjustable according to the feeding molar ratio of ε-CL and PEG, and the micelles can be spherical in shape. The size of micelle increased with increase in PCL chain length, but the CMC decreases as the PCL chain length increased.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Woodruff MA, Hutmacher DW. The return of a forgotten polymer – polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256.10.1016/j.progpolymsci.2010.04.002
- Dash TK, Konkimalla VB. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: a review. J. Control Release 2012;158:15–33.10.1016/j.jconrel.2011.09.064
- Tian H, Tang ZH, Zhuang XL, Chen XS, Jing XB. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012;37:237–280.10.1016/j.progpolymsci.2011.06.004
- Du ZX, Xu JT, Fan ZQ. Micellar morphologies of poly(є-caprolactone)-b-poly(ethylene oxide) block copolymers in water with a crystalline core. Macromolecules. 2007;40:7633–7637.10.1021/ma070977p
- Fairley N, Hoang B, Allen C. Morphological control of poly(ethylene glycol)-block-poly(ε-caprolactone) copolymer aggregates in aqueous solution. Biomacromolecules. 2008;9:2283–2291.10.1021/bm800572p
- Yang JX, He WN, Xu JT, Du Fan ZQ. Influence of different inorganic salts on crystallization-driven morphological transformation of PCL-b-PEO micelles in aqueous solutions. Chin. J. Polym. Sci. 2014;32:1128–1138.10.1007/s10118-014-1512-z
- Kukut M, Yilmaz OK, Yagcia Y. Synthesis, characterization, and hydrolytic degradation of graft copolymers of polystyrene and aliphatic polyesters. Des. Monomers Polym. 2013;16:233–240.10.1080/15685551.2012.725214
- Ruan JM, Xiao AG, Wu HW, Yang HL. Review-recent development of ring-opening polymerization of cyclic esters using aluminum complexes. Des. Monomers Polym. 2014;17:345–355.
- Zaks A, Klibanov AM. Enzymatic catalysis in organic media at 100 °C. Science. 1984;224:1249–1251.10.1126/science.6729453
- Klibanov AM. Improving enzymes by using them in organic solvents. Nature. 2001;409:241–246.10.1038/35051719
- Kobayshi S. Enzymatic polymerization: a new method of polymer synthesis. J. Polym. Sci. Part A: Polym. Chem. 1999;37:3041–3056.10.1002/(ISSN)1099-0518
- Zhang JX, Shi H, Wu D, et al. Recent developments in lipase-catalyzed synthesis of polymeric materials. Process Biochem. 2014;49:797–806.10.1016/j.procbio.2014.02.006
- Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977;99:2039–2044.10.1021/ja00449a004
- He CL, Sun JR, Zhao T, et al. Formation of a unique crystal morphology for the poly(ethylene glycol)-poly (ε-caprolactone) diblock copolymer. Biomacromolecules. 2006;7:252–258.10.1021/bm050627f
- Zhu ZX, Xiong CD, Zhang LL, Deng XM. Synthesis and characterization of poly (e-caprolactone) poly(ethylene glycol) block copolymer. J. Polym. Sci. Part A: Polym. Chem. 1997;35:709–714.10.1002/(ISSN)1099-0518
- Smith BER. Polymers: a property database. New York (NY): CRS Press; 2009.
- He CL, Sun JR, Deng C, et al. Study of the synthesis, crystallization, and morphology of poly(ethylene glycol)-poly(ε-caprolactone) diblock copolymers. Biomacromolecules. 2004;5:2042–2047.10.1021/bm049720e
- Tang WT, Hadziioannou G, Smith BA, Frank CW. Facile method for labelling polystyrene with various fluorescent dyes. Polymer. 1988;29:1313–1317.10.1016/0032-3861(88)90062-6
- Wilhelm M, Zhao CL, Wang YC, et al. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe study. Macromolecules. 1991;24:1033–1040.10.1021/ma00005a010