Abstract
Diethyl-dithiocarbamic acid 2-[4-(2-diethylthiocarbamoylsulfanyl-2-phenyl-acetyl)-2,5-dioxo-piperazin-1-yl]-2-oxo-1-phenyl-ethyl ester as a novel di-functional reversible addition–fragmentation chain transfer (RAFT) agent was synthesized based on 2,5-diketopiperazine. The RAFT agent was designed based on the propagating core (R group) approach and characterized by 1H NMR, 13C NMR, FT-IR, elemental analysis, and melting point technique. Then, ethyl methacrylate was synthesized via free radical and RAFT polymerizations. To investigate the effect of the RAFT agent on the kinetic of polymerization, molecular weight, and polydispersity index (PDI) of polymers and also monomer conversion were monitored. Also, synthesized polymers were characterized by 1H NMR, 13C NMR, FT-IR, and TGA. Characterization analyses of synthesized RAFT agent were consistent with the structure. NMR and FTIR analyses confirmed end group incorporation of RAFT agent into polymer structure. According to results, poly(ethyl methacrylate) with low PDI (1.14) was obtained. Kinetic study indicated well-controlled polymerization of ethyl methacrylate by synthesized RAFT agent. TGA results showed that RAFT agent could reduce termination reactions and so reduce head-to-head bonds and chain-end unsaturation by keeping the concentration of radicals low enough.
1. Introduction
Synthesis of polymers with well-defined and pre-determined structure and functionality is a key way to develop advanced materials which are applicable in several fields.[Citation1–4] Controlled/‘living’ radical polymerization (CLRP) techniques based on the common concept of alternating activation/deactivation processes are successful and efficient mechanisms to design structure and functionality in polymers with controlled molecular weight and narrow dispersity.[Citation5–8] Among CLRP techniques, reversible addition–fragmentation chain transfer (RAFT) polymerization is one of the most versatile processes.[Citation9–11] Pre-equilibrium and main equilibrium reactions in RAFT polymerization result in well-defined polymers.[Citation12] It is able to readily polymerize a variety of monomers under mild reaction conditions.[Citation13–15] RAFT mechanism is compatible with a variety of functionalities in reaction components and a wide range of temperatures.[Citation11,12] The key to successful operation lies on the use of a highly efficient chain transfer agent (CTA), which is typically a thiocarbonylthio compound.[Citation16] By proceeding polymerization reaction, substituent groups next to the C=S moiety in CTA structure remain as end groups of the polymer backbone.[Citation17,18] Several researchers have used this concept as an interesting strategy to introduce bio-segments in the polymer backbone by direct coupling of RAFT agent to biomolecule which is further used in polymerization.[Citation19,20] Different in situ biomolecule insertions in polymers via this method are reported, such as insertion of phospholipids in polyacrylamide derivative and poly(N-acryloylmorpholine),[Citation20] proteins such as bovine serum albumin in poly(N-isopropylacrylamide),[Citation21] poly(oligo(ethylene glycol) acrylate) [Citation14] and soy protein in polyacrylamide and poly(acrylic acid).[Citation22] Also, in recent years, increasing interest has been paid to the incorporation of biodegradable and biocompatible moieties based on amino acids in synthetic polymers to allow good interactions of polymer with biological systems.[Citation23–27] These advanced synthetic polymers referred as synthetic co-polypeptides have attracted interest as models for natural polypeptides and proteins [Citation28] with applications from nanotechnology to tissue engineering.[Citation18]
In this work, a novel bifunctional cyclopeptide-based CTA was synthesized. The structure of final and intermediate products was characterized by elemental analysis (CHNS), Fourier transform infrared spectroscopy (FT-IR), proton nuclear magnetic resonance (1H NMR), carbon NMR (13C NMR), and melting point technique. Ethyl methacrylate (EMA) was polymerized via RAFT technique in the presence of synthesized RAFT agent. To investigate effect of synthesized CTA on the structure and properties of polymer, monomer conversion, molecular weight, molecular weight distribution, chemical structure, and thermal behavior of synthesized polymers via RAFT and TRP mechanisms were studied via FT-IR, 1H NMR, and 13C NMR, gel permeation chromatography (GPC), and thermogravimetric analysis (TGA).
2. Experimental section
2.1. Materials
Ethyl methacrylate (Aldrich, 99%, EMA), 2,5-diketopiperazine (Aldrich, ≥ 98%, DK), sodium dithiocarbamate trihydrate (Aldrich, SDTC), chloro trimethylsilane (Aldrich, ≥ 99%, TMSCl), α-chlorophenylacetyl chloride (Aldrich, 90%, CPACl), ethanol (Merck, ≥ 99.5%, EtOH), acetonitrile (Aldrich, 99.8%, ACN), acetone (Merck, ≥ 99.0%) were used as received. Azobisisobutyronitrile (Aldrich, 98%, AIBN) was recrystallized from ethanol before use. Triethylamine (Aldrich, ≥ 99%, TEA) and tetrahydrofurane (Aldrich, ≥ 99.9%, THF) were dried over calcium hydride and molecular sieve, respectively.
2.2. Instrumentation
The IR spectra were recorded on a Shimadzu FT-IR-8300 spectrophotometer within a range of 400−4000 cm−1. An average of 32 scans has been carried out for each sample. The samples were prepared on a KBr pellet in vacuum desiccators under a pressure of 0.01 Torr. 1H (250 MHz) and 13C NMR (62.9 MHz) spectra were recorded on a Bruker Avance spectrometer with tetramethylsilane (TMS) as an internal standard. The mass spectra were taken on a Shimadzu GC MS-QP 1000 EX apparatus. Elemental analysis (CHNS) was performed on a Perkin-Elmer 240-B micro-analyzer. The reaction monitoring was accomplished by thin layer chromatography on silica gel PolyGram SILG/UV254 plates. Column chromatography was carried out on columns of silica gel 60 (70–230 mesh). Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes. TGA curves were taken on a Mettler-Toledo TG-50 Thermal Analyzer;a sample weight of about 10 mg was used for all the measurements and nitrogen was used as the purging gas at a flow rate of 200 mL min−1. Thermograms were obtained with heating rate of 10 °C min−1 from ambient temperature to 650 °C. Average molecular weights and molecular weight distributions were measured by GPC technique. A Waters 2000 ALLIANCE with a set of three columns of pore sizes of 10,000, 1000, and 500 Å was utilized to determine polymer average molecular weight and polydispersity index (PDI). THF was used as the eluent at a flow rate of 1.0 mL/min, and the calibration was carried out using low dispersity PS standards.
2.3. Synthesis and characterization of RAFT agent
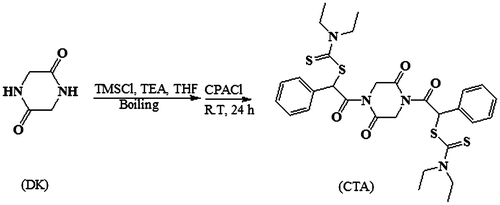
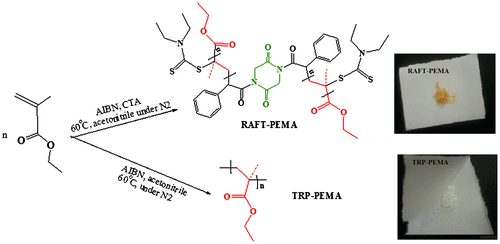
A mixture of DK (0.01 mol), TEA (0.02 eq), TMSCl (0.2 eq) and anhydrous THF (40 mL) was refluxed under N2 atmosphere for 30 h. Then, CPACl (0.02 mol) was added to mixture at 0 °C, and the mixture was stirred overnight at room temperature. After adding dichloromethane (20 mL), obtained pale brown mixture was washed with saturated sodium hydrogen carbonate and deionized water several times. The organic layer was then collected and dried over sodium sulfate, before filtration and removal of solvent under reduced pressure. The obtained product (compound (1)) was a beige crystal and was used without further purification. SDTC (0.02 mol) was mixed with compound (1) (0.01 mol) and anhydrous ethanol (150 mL). The reaction mixture was refluxed for 48 h. After adding deionized water (100 mL), the resulting precipitate was filtered off and washed by water several times. The product was dissolved in petroleum ether (200 mL), and the organic layer was washed several times with saturated sodium hydrogen carbonate and finally with deionized water. The organic layer was dried over sodium sulfate, and after removal of solvent, CTA (compound (2)) was obtained by flash chromatography on silica using ethyl acetate and petroleum ether (6:20) as eluent. The synthesis route is shown in Scheme .
2.4. Spectral analysis
Compound (1) 1H NMR (DMSO-d6, δH (ppm), Figure S1 (A)): 4.57 (s, 4H, a), 6.55 (s, 2H, b), 7.35–7.40 (m, 10H, c,d,e). 13C NMR (DMSO-d6, δc (ppm), Figure S1 (B)): 45.4 (s, 2C, a), 59.2 (s, 2C, b), 125–140 (s, 10C, e,f,g), 136.7 (s, 2C, d), 167.5 (s, 2C, h), 169.2 (s, 2C, i). Melting point: m.p. > 350 °C. FT-IR main absorption peaks (υ/cm−1, Figure S2 (B)): 1731 (stretching of C=O in imide and amide groups); 2800–3000 (stretching of CH2); 1456 (bending of CH2); 1420, 1456, 1641 (stretching of C=C in aromatic ring); 3010 (stretching of aromatic =C–H); 709, 717 (monosubstituted aromatic ring, (bifurcated peak)); 854 (bending of aromatic =C–H, out of plane); 1870–1958 (aromatic ring overtones, weak); 571 (stretching of C–Cl). CHNS results (C20H16Cl2N2O4, 419.26): Calculated (C: 57.30%, H: 3.85%, N: 6.68%), found (C: 57.30%, H: 3.85%, N: 6.68%).
Compound (2) (RAFT agent) 1H NMR (DMSO-d6, δH (ppm), Figure S3 (A)): 0.96 (s, 12H, a), 3.99 (s, 4H, b), 4.1–4.17 (m, 8H, c), 5.86 (s, 2H, d), 7.18–7.40 (m, 10H, e, f, g). 13C NMR (DMSO-d6, δc (ppm), Figure S3 (B)): 11.5 (s, 4C, a), 47.0 (s, 4C, b), 49.5 (s, 2C, c), 58.8 (s, 2C, d), 128.8 (s, 10C, e,f,g), 133.8 (s, 2C, h), 174.0 (m, 4C, i,j), 193.0 (s, 2C, k). Melting point: m.p. 87.5 ± 2.5 °C. FT-IR main absorption peaks (υ/cm−1, Figure S2 (C)): 2965, 3983 (stretching of CH2); 1450–1480 (bending of CH2); 1358 (rocking of CH2); 1492, 1666 (stretching of aromatic C=C in ring); 3065 (stretching of aromatic =C–H); 699, 736 (mono-substituted aromatic ring); 836 (bending of aromatic =C–H, out of plane); 1929, 1957 (aromatic ring overtones, weak); 1146, 1209, 1279 (C–S, C=S). CHNS results (C30H36N4O4S4, 644.89): Calcd (C%: 55.87, H%: 5.63, N%: 8.69, S%: 19.89), found (C%: 55.00, H%: 5.75, N%: 8.20, S%: 19.72).
2.5. Procedure of ethyl methacrylate (EMA) polymerization
A stock solution of EMA (0.87 mol L−1) as monomer, AIBN (6.09 mmol L−1) as initiator, RAFT agent (0.0155 mol L−1 for RAFT polymerization) and acetonitrile as solvent was mixed in degassed test tubes sealed with rubber septas (suba seal). The solutions were deoxygenated by nitrogen for 5 min and subsequently transferred to an oil bath heated at 60 °C. The test tubes were removed at predetermined intervals and polymerizations stopped by placing test tubes in ice water and adding cold acetone. Conversion was calculated gravimetrically and purification was performed by precipitation into cold acetone followed by drying under vacuum.
3. Results and discussion
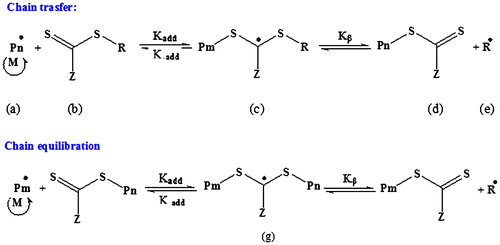
RAFT agents have the generic structure of S=C(Z)S–R. According to the mechanism of RAFT polymerization (Scheme ), the R group must form a radical stable enough to favor rapid fragmentation from the dithioester radical intermediate of (c).[Citation29] The chosen R group with phenylacetyl moiety is already known to be very effective in the controlled polymerization of methacrylate monomers.[Citation30] –XCHPh group that X is carbonyl group, is suitable R group with a high fragmentation rate for methacrylate monomers. Carbonyl group acts as a suitable electron withdrawing group.[Citation29,30] In addition, the R group increases fragmentation rate by its spatial hindrance. The new bifunctional RAFT agent has central R group containing diketopiperazine cycle and fragmentation/reinitiation occurs from two sides of RAFT agent. Also, synthesized RAFT agent structure presents carbonyl (C=O) groups of imide functionality that could affect the stabilization of (e) and results in higher Kß/K-add in chain transfer step. Successful conversion of DK to compound (1) via silylation reaction was proved by spectral analysis. This method led to direct access to the desired product (compound (1)) without any by-products. So, need to purification steps was eliminated via this approach. Creating two stiffening imide groups in the compound (1) increases H-bonding ability, rigidity, and so its heat of fusion (>350 °C) compared to primary compound (DK) with m.p. of 95 °C. 1H NMR spectra of compound (1) (Figure S1) and comparison of FT-IR spectra of compound (1) with that of DK (Figure S2) confirms substitution of H of amide –NH– group in DK with phenylacetyl group in compound (1). Then, Cl groups of compound (1) were substituted with thiocarbamate functionality in sodium diethyl dithiocarbamate salt. Nucleophilic substitution of bis(chloro) compound (1) with sodium diethyl dithiocarbamate salt increased flexibility of final product (new bifunctional RAFT agent) and so decreased m.p. to 87.5 ± 2.5 °C. Comparison of NMR and FTIR spectra of compounds (1) and (2) (Figures S1–S3) confirms the success of this reaction.
3.1. Polymerization of EMA via RAFT and free radical polymerization
To investigate the effect of synthesized RAFT agent on the polymerization of EMA, reaction was carried out via free radical and RAFT mechanisms as depicted in Scheme . The first main difference between polymers synthesized by means of two methods was the color of obtained polymer. Poly(ethyl methacrylate) that synthesized via free radical mechanism (TRP-PEMA) had no specific color, while polymer obtained via RAFT polymerization (RAFT-PEMA) was orange due to the attachment of RAFT agent to the chain ends.
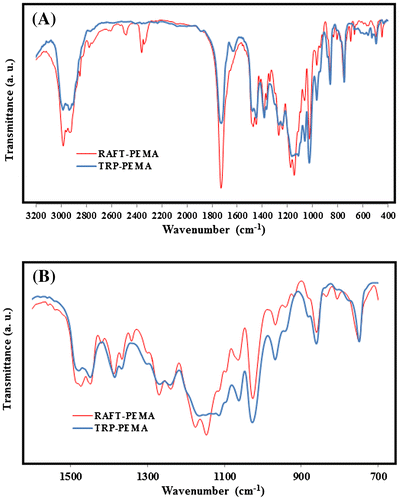
Figure shows FT-IR spectra of TRP-PEMA and RAFT-PEMA in which there are some common characteristic peaks. Absorptions at 2900–3000 cm−1, about 1700 cm−1, and 1000–1300 cm−1 are ascribed to the C–H of alkyl groups, carbonyl stretching vibration, and C–O stretching, respectively. Also, disappearance of specific peaks related to EMA confirmed successful conversion of monomer to polymer. The C=C bond stretching and the C–H bonding in =CH2 group of EMA are commonly observed between 1640–1680 cm−1 and 650–1000 cm−1, respectively, that disappears after polymerization. The main difference between FT-IR spectra of TRP-PEMA and RAFT-PEMA is the presence of peaks referred to the functional groups of CTA. This confirms entering of R and Z groups of new CTA in polymer backbone as follows: 1427 and 1572 cm−1 (stretching of aromatic C=C in ring), 3010 cm−1 (stretching of aromatic =C–H weak), 807 cm−1 and 836 cm−1 (bending of aromatic =C–H, out of plane), 671 and 701 cm−1 (monosubstituted aromatic ring), 1150 and 1178 cm−1 (C–S, C=S), 2850–2880 cm−1 (stretching of aliphatic CH2), 1385 and 1448 cm−1 (symmetric and asymmetric bending of CH3 and CH2, respectively), 2812 and 2869 cm−1 (stretching of CH3).
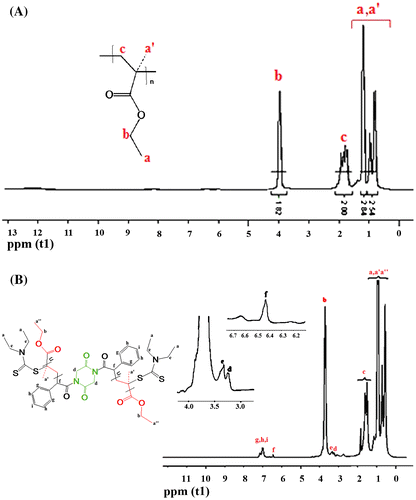
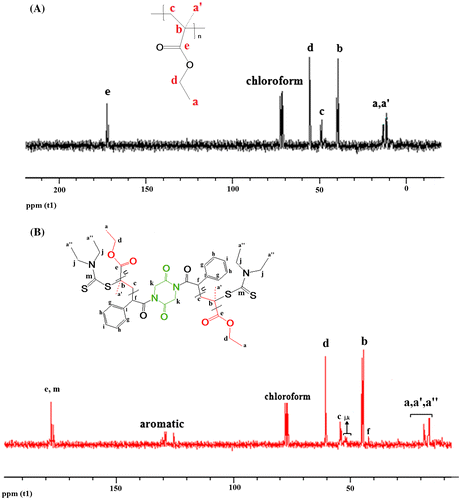
Also, NMR spectra of RAFT-PEMA confirmed successful conjugating RAFT agent to polymer backbone. 1H NMR of TRP-PEMA in Figure (A) shows a peak at 0.8 ppm which is due to α-CH3 and at 1.0 ppm due to methyl (CH3) pendant group of EMA. A peak at 1.8 ppm is ascribed to CH2 protons in the polymeric chain. The prominent peak at 3.9 ppm is observed due to the presence of –OCH2 in the PEMA. The 13C NMR spectrum of TRP-PEMA in Figure (A) shows a peak at 13.8 and 11.8 ppm due to the presence of α-methyl carbon (CH3) and pendant methyl carbon (CH3) in the polymer, respectively. A peak at 39.0 ppm is attributed to quaternary carbon in the polymeric chain, while methylene carbon (–CH2–) of the polymeric chain appears at 49.0 ppm. The alkoxy carbon (OCH2) appears at 56.0 ppm and carbonyl carbon (C=O) appears at 172.0 ppm. NMR spectra of RAFT-PEMA showed similar peaks of TRP-PEMA in the same area. In addition, the presence of peaks referred to functional groups of CTA confirmed entering of RAFT agent in the polymer backbone. The peaks of RAFT agent in 1H NMR spectra of RAFT-PEMA (Figure (B)) are as follows: peaks at 3.36 ppm (w, 4H, d), 3.39 ppm (w, 8H, e), 6.43 ppm (w, 2H, f), 7–8 ppm (w, 10H, g,h,i), peak related to CH3 protons are presented along with CH3 protons of PEMA at ~0.94 ppm. The peaks of RAFT agent in 13C NMR spectra of RAFT-PEMA (Figure (B)) include the following: 16.5, 18.5 ppm (m, 4C, a″), 42 ppm (m, 2C, f), 50–52 ppm (m, 6C, j,k), 125–132 ppm (m, 10C, aromatic), 175–180 ppm (m, 2C, m).
3.2. Kinetic study
In this work, a novel bifunctional dithiocarbamate CTA was synthesized and used in controlled/‘living’ polymerization of ethyl methacrylate. To this end, as kinetics results, conversion, molecular weight, and dispersity were investigated during reaction progression. Figure (A) shows the conversion of monomer vs. time for free radical and RAFT polymerization systems. According to results (Figure (B)), free radical polymerization follows a non-linear increment of conversion vs. time. However, adding RAFT agent in the same system results in a significant induction time after that linear pseudo first-order kinetic is observed. This indicates that the concentration of active species is stable during long times of polymerization and a well-controlled behavior of polymerization has been mediated by CTA via RAFT mechanism. Also, RAFT polymerization results in lower final conversion value with respect to free radical polymerization as it decreases from ~86.5 to ~63.6% as stated in other research works.[Citation31,32] Slow growth of all chains in RAFT polymerization is due to equilibrium between active and dormant species that keeps concentration of radicals low enough to minimize termination reactions.
Figure 4. Monomer conversion (A) and Ln([M]0/[M]) (B) vs. TRP and RAFT polymerization time of EMA at 60 °C.
![Figure 4. Monomer conversion (A) and Ln([M]0/[M]) (B) vs. TRP and RAFT polymerization time of EMA at 60 °C.](/cms/asset/cd73d859-8a92-44b0-a94e-758aa5e25ec2/tdmp_a_1092013_f0004_oc.gif)
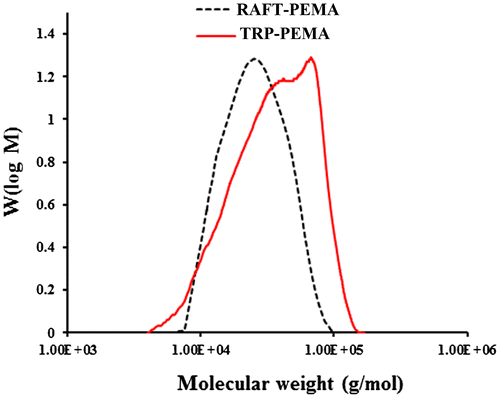
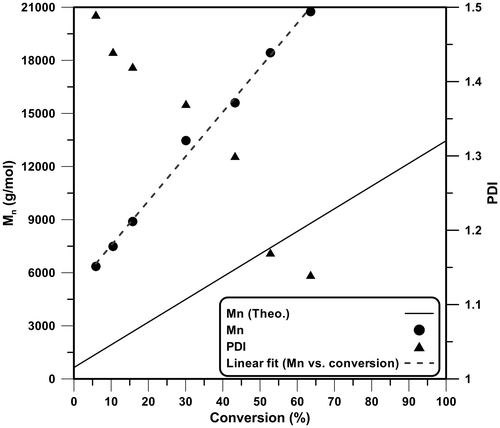
To investigate the effect of RAFT agent on molecular weight distribution of PEMA, we compared GPC results of polymers synthesized with and without CTA after 72 h (Figure ). Polymer synthesized via TRP mechanism showed a bimodal distribution with Mn = 33,327 g/mol and PDI = 1.60. Introducing of CTA in the reaction media changed molecular weight distribution to a narrow unimodal pattern with PDI = 1.14. RAFT mechanism produced polymer chains with lower average molecular weight of Mn = 20,754 g/mol rather than to TRP mechanism. These results are regular for RAFT polymerization and indicate that the new CTA could control EMA polymerization successfully. In addition, a bifunctional CTA leads to polymer chains with higher molecular weight and also increase of polymerization rate.[Citation33,34] Also, to further prove the success of RAFT agent in controlling polymerization, Mn and PDI values were investigated during polymerization (Figure ) and Mn was compared to theoretical Mn value with Equation (Equation1(1) ) [Citation35]:
(1)
Molecular weight values showed some deviation from theoretical values originated from early stages of polymerization where polymerization is in its unsteady state and low control could be performed on the polymerization. Subsequently, higher molecular weight values are obtained. However, Mn increases linearly with increasing conversion that is the best justification of controlling polymerization by means of synthesized RAFT agent. Also, all measured PDI values are lower than 1.5, and with polymerization progression, PDI decreases until it reaches 1.14 at conversion of 63.6%.
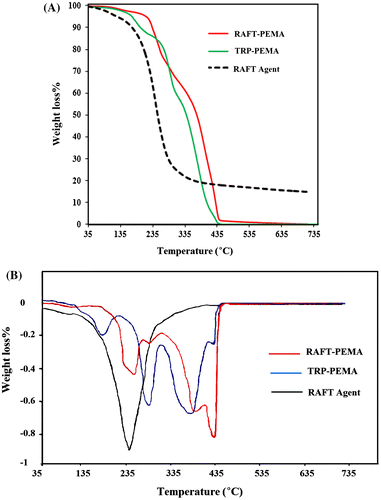
3.3. Thermogravimetric analysis (TGA)
Understanding thermal stability and thermal decomposition kinetics of polymers makes it possible to apply them in various industrial fields.[Citation36] TGA is a simple and useful method to characterize thermal decomposition behavior of materials.[Citation37–41] Figure depicts TGA and DTGA patterns of CTA and synthesized polymers via TRP and RAFT polymerization systems. According to the DTGA curves, TRP-PEMA presented three stages of degradation; such a thermal behavior has been observed formerly in TGA analysis of poly(methacrylate)s prepared via traditional radical polymerization with different heating rates.[Citation36,42–45] Three stages of TRP-PEMA degradation include Td,max of 180, 290, and 385 °C related to thermal degradation of head-to-head linkages, unsaturated end groups, and random chain scission phenomena, respectively.[Citation43,46] In RAFT polymerization mechanism (Scheme ), rapid equilibrium between active propagating radicals ( and
in structure (g)) and the dormant polymeric thiocarbonylthio compound via chain transfer reactions avoids chains to be rapidly terminated. Therefore, thermally weak linkages such as head-to-head bond and chain-end unsaturation are severely reduced. The isolated polymer is dormant with the RAFT agent attached to the ends of the chains.[Citation47] Therefore, as shown in Figure , two first steps in the degradation of TRP-PEMA were eliminated in TGA of RAFT-PEMA. However, the weight loss mechanism and profile observed by the TGA of polymers synthesized via RAFT mechanism has been found to depend strongly on both the RAFT agent and polymer type.[Citation17,45] RAFT-PEMA degraded thermally via two main steps: the first between 200 and 263 °C and another beginning at 325 °C. Two-stage degradation has been reported for poly(methacrylate)s prepared via RAFT polymerization and attributed to chain-anchored RAFT agents and random chain scission.[Citation45,48–50] According to DTGA thermogram, degradation of CTA was occurred in one step between 135 and 340 °C. So, the first step in degradation of RAFT-PEMA coincides with degradation of the CTA. As also, reported for thermal degradation of analogous compounds to the CTA, this associates to degradation of S–C=S groups of thiocarbamate end-functionality of polymer chains.[Citation12,51,52] However, weight loss at this stage (~18%) is much greater than that expected for the degradation of RAFT agents. In this step, end group loss is accompanied by some polymer degradation and lowering of molecular weight by degradation of weak bonds such as head-to-head linkages and unsaturated end groups. These bonds can be attributed to thermolysis mechanisms of RAFT agent terminated poly(methacrylate)s,[Citation45] or may also be partly due to some deviation from ideal controlled/living polymerization and termination of macro-radicals in early stages of polymerization.[Citation49] The thermolysis of RAFT agent produces macro-radicals, which then subjected to unzipping and de-propagation of polymeric chains and consequent weight loss of polymer that can be stopped via termination reactions. The small peak in the range of 265–300 °C (~5% weight loss) could be ascribed to the degradation of remaining part of the CTA that contains cyclic imide groups and aromatic moiety. Random chain scission of RAFT-PEMA has occurred in the second step between 325 and 440 °C.
4. Conclusion
Novel peptide-based RAFT agent was synthesized via an efficient method with high purity and quantitative yield. It was successfully applied to the straightforward synthesis of well-defined peptide-conjugated poly(ethyl methacrylate). The synthesized RAFT agent allows for a unique control over the architecture of the polymer–peptide conjugates, with a live end group functionality and narrow dispersity. Results showed that the presence of RAFT agent leads to poly(ethyl methacrylate) with well-controlled molecular weight and low PDI (1.14). Reversible equilibrium reactions of RAFT agent with propagating radicals reduces concentration of active radicals and so termination reactions. Thermal stability of polymer also confirmed decrease in radical termination reactions in the presence of RAFT agent.
Supplemental data
The supplemental data for this paper is available online at http://dx.doi.org/10.1080/15685551.2015.1092013.
Supplementary_material.zip
Download Zip (2.3 MB)Acknowledgements
The authors wish to thank Iran National Science Foundation (INSF) of I.R. Iran, Shiraz University, Amirkabir University of Technology, and Tehran University of Medical Sciences for the financial and instrumental support of this research.
Funding
This work was supported by Iran National Science Foundation (INSF) of I.R. Iran, Shiraz University, Amirkabir University of Technology, and Tehran University of Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Knight AS, Zhou EY, Francis MB, et al. Sequence programmable peptoid polymers for diverse materials applications. Adv. Mater. 2015. doi:10.1002/adma.201500275.
- Torkpur-Biglarianzadeh M, Salami-Kalajahi M. Multilayer fluorescent magnetic nanoparticles with dual thermoresponsive and pH-sensitive polymeric nanolayers as anti-cancer drug carriers. RSC. Adv. 2015;5:29653–29662.10.1039/C5RA01444A
- Husseman M, Malmström EE, McNamara M, et al. Controlled synthesis of polymer brushes by “living” free radical polymerization techniques. Macromolecules. 1999;32:1424–1431.10.1021/ma981290v
- Banaei M, Salami-Kalajahi M. Synthesis of poly(2-hydroxyethyl methacrylate)-grafted poly(aminoamide) dendrimers as polymeric nanostructures. Colloid Polym. Sci. 2015;293:1553–1559.10.1007/s00396-015-3559-y
- Zhang H. Controlled/“living” radical precipitation polymerization: a versatile polymerization technique for advanced functional polymers. Eur. Polym. J. 2013;49:579–600.10.1016/j.eurpolymj.2012.12.016
- Mahjub A, Mohammadi H, Salami-Kalajahi M, et al. Simulation of reversible chain transfer catalyzed polymerization (RTCP): effect of different iodide based catalysts. J. Polym. Res. 2012;19:9740–9748.10.1007/s10965-011-9740-1
- Nikdel M, Salami-Kalajahi M, Hosseini MS. Dual thermo- and pH-sensitive poly(2-hydroxyethyl methacrylate-co-acrylic acid)-grafted graphene oxide. Colloid Polym. Sci. 2014;292:2599–2610.10.1007/s00396-014-3313-x
- Le Droumaguet B, Nicolas J. Recent advances in the design of bioconjugates from controlled/living radical polymerization. Polym. Chem. 2010;1:563–598.10.1039/b9py00363k
- Panahian P, Salami-Kalajahi M, Salami Hosseini M. Synthesis of dual thermosensitive and pH-sensitive hollow nanospheres based on poly (acrylic acid-b-2-hydroxyethyl methacrylate) via an atom transfer reversible addition–fragmentation radical process. Ind. Eng. Chem. Res. 2014;53:8079–8086.10.1021/ie500892b
- Nikdel M, Salami-Kalajahi M, Salami Hosseini MS. Synthesis of poly(2-hydroxyethyl methacrylate-co-acrylic acid)-grafted graphene oxide nanosheets via reversible addition–fragmentation chain transfer polymerization. RSC Adv. 2014;4:16743–16750.10.1039/c4ra01701c
- Salami-Kalajahi M, Haddadi-Asl V, Behboodi-Sadabad F, et al. Effect of silica nanoparticle loading and surface modification on the kinetics of RAFT polymerization. J. Polym. Eng. 2012;32:13–22.
- Ganjeh-Anzabi P, Haddadi-Asl V, Salami-Kalajahi M, et al. Kinetic investigation of the reversible addition-fragmentation chain transfer polymerization of 1, 3-butadiene. J. Polym. Res. 2013;20:248–255.10.1007/s10965-013-0248-8
- Benaglia M, Chiefari J, Chong YK, et al. Universal (switchable) RAFT agents. J. Am. Chem. Soc. 2009;131:6914–6915.10.1021/ja901955n
- Liu J, Bulmus V, Herlambang DL, et al. In situ formation of protein–polymer conjugates through reversible addition fragmentation chain transfer polymerization. Angew. Chem. Int. Ed. 2007;46:3099–3103.10.1002/(ISSN)1521-3773
- Sarsabili M, Parvini M, Salami‐Kalajahi M, et al. In situ reversible addition–fragmentation chain transfer polymerization of styrene in the presence of mcm‐41 nanoparticles: comparing “grafting from” and “grafting through” approaches. Adv. Polym. Tech. 2013;32. doi: 10.1002/adv.21372.
- Zhou G, Harruna II. Synthesis and characterization of bis (2, 2ʹ: 6ʹ, 2″-terpyridine) ruthenium (II)-connected diblock polymers via RAFT polymerization. Macromolecules. 2005;38:4114–4123.10.1021/ma047955c
- Willcock H, O’Reilly RK. End group removal and modification of RAFT polymers. Polym. Chem. 2010;1:149–157.10.1039/B9PY00340A
- Zhao Y, Perrier S. Synthesis of well-defined conjugated copolymers by RAFT polymerization using cysteine and glutathione-based chain transfer agents. Chem. Commun. 2007;41:4294–4296.10.1039/b708293b
- Hentschel J, Bleek K, Ernst O, et al. Easy access to bioactive peptide−polymer conjugates via RAFT. Macromolecules. 2008;41:1073–1075.10.1021/ma8000934
- Bathfield M, Daviot D, D’Agosto F, et al. Synthesis of lipid-α-end-functionalized chains by RAFT polymerization. Stabilization of lipid/polymer particle assemblies. Macromolecules. 2008;41:8346–8353.
- De P, Li M, Gondi SR, et al. Temperature-regulated activity of responsive polymer−protein conjugates prepared by grafting-from via RAFT polymerization. J. Am. Chem. Soc. 2008;130:11288–11289.10.1021/ja804495v
- Bhattacharjee S, Bong D. Protein-polymer grafts via a soy protein derived macro-RAFT chain transfer agent. J. Polym. Environ. 2011;19:203–208.10.1007/s10924-010-0264-2
- Wakayama M, Katsuno Y, Hayashi S, et al. Cloning and sequencing of a gene encoding D-aminoacylase from Alcaligenes xylosoxydans subsp. Xylosoxydans A-6 and expression of the gene in Escherichia coli. Biosci. Biotechnol. Biochem. 1995;59:2115–2119.10.1271/bbb.59.2115
- Bauri K, Roy SG, Pant S, et al. Controlled synthesis of amino acid-based pH-responsive chiral polymers and self-assembly of their block copolymers. Langmuir. 2013;29:2764–2774.10.1021/la304918s
- Dutta P, Dey J. Drug solubilization by amino acid based polymeric nanoparticles: characterization and biocompatibility studies. Int. J. Pharm. 2011;421:353–363.10.1016/j.ijpharm.2011.10.011
- Ali SA. Novel cross-linked polymers having pH-responsive amino acid residues for the removal of Cu2+ from aqueous solution at low concentrations. J. Hazard. Mater. 2013;248–249:47–58.10.1016/j.jhazmat.2012.12.052
- Mallakpour S, Dinari M. Progress in synthetic polymers based on natural amino acids. J. Macromol. Sci. Part A. 2011;48:644–679.10.1080/15226514.2011.586289
- Dmitrovic V, Habraken GJ, Hendrix MM, et al. Random poly(amino acid)s synthesized by ring opening polymerization as additives in the biomimetic mineralization of CaCO3. Polymers. 2012;4:1195–1210.10.3390/polym4021195
- Moad G, Rizzardo E, Thang SH. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005;58:379–410.10.1071/CH05072
- Legge TM, Slark AT, Perrier S. Novel difunctional reversible addition fragmentation chain transfer (RAFT) agent for the synthesis of telechelic and ABA triblock methacrylate and acrylate copolymers. Macromolecules. 2007;40:2318–2326.10.1021/ma061372g
- Rahimi-Razin S, Haddadi-Asl V, Salami-Kalajahi M, et al. Matrix-grafted multiwalled carbon nanotubes/poly(methyl methacrylate) nanocomposites synthesized by in situ RAFT polymerization: a kinetic study. Int. J. Chem. Kinet. 2012;44:555–569.10.1002/kin.v44.8
- Sobani M, Haddadi-Asl V, Mirshafiei-Langari S-A, et al. A kinetics study on the in situ reversible addition–fragmentation chain transfer and free radical polymerization of styrene in presence of silica aerogel nanoporous particles. Des. Monomers Polym. 2014;17:245–254.10.1080/15685551.2013.840496
- Fallahi H, Koohmareh GA. Preparation of polystyrene/MMT nanocomposite through in situ RAFT polymerization by new chain transfer agent derived from bisphenol A. J. Appl. Polym. Sci. 2013;127:523–529.10.1002/app.37833
- Otsu T, Matsunaga T, Kuriyama A, et al. Living radical polymerization through the use of iniferters: controlled synthesis of polymers. Eur. Polym. J. 1989;25:643–650.10.1016/0014-3057(89)90023-2
- Sarsabili MR, Parvini M, Salami-Kalajahi M, et al. Effect of MCM-41 nanoparticles on the kinetics of free radical and RAFT polymerization of styrene. Iran. Polym. J. 2013;22:155–163.10.1007/s13726-012-0114-2
- Fares S. Influence of gamma-ray irradiation on optical and thermal degradation of poly (ethyl-methacrylate)(PEMA) polymer. Nat. Sci. 2012;4:499–507.
- Maitra S, Bandyopadhyay N, Das S, et al. Non-isothermal decomposition kinetics of alkaline earth metal carbonates. J. Am. Ceram. Soc. 2007;90:1299–1303.10.1111/jace.2007.90.issue-4
- Friedman HL. Kinetics of thermal degradation of char‐forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Polym. Symp. 1964;6:183–195.
- Nam Jd, Seferis JC. A composite methodology for multistage degradation of polymers. J. Polym. Sci., Part B: Polym. Phys. 1991;29:601–608.10.1002/polb.1991.090290509
- Anderson DA, Freeman ES. The kinetics of the thermal degradation of polystyrene and polyethylene. J. Polym. Sci. 1961;54:253–260.10.1002/pol.1961.1205415920
- Radhakrishnan T. New method for evaluation of kinetic parameters and mechanism of degradation from pyrolysis-GC studies: thermal degradation of polydimethylsiloxanes. J. Appl. Polym. Sci. 1999;73:441–450.10.1002/(ISSN)1097-4628
- Salami-Kalajahi M, Haddadi-Asl V, Behboodi-Sadabad F, et al. Properties of PMMA/carbon nanotubes nanocomposites prepared by “grafting through” method. Polym. Compos. 2012;33:215–224.10.1002/pc.v33.2
- Salami-Kalajahi M, Haddadi-Asl V, Rahimi-Razin S, et al. A study on the properties of PMMA/silica nanocomposites prepared via RAFT polymerization. J. Polym. Res. 2012;19:9793–97103.10.1007/s10965-011-9793-1
- Kurt A, Koca M. Blending of poly(ethyl methacrylate) with poly(2-hydroxy-3-phenoxypropyl methacrylate): thermal and optical properties. Arabian J. Sci. Eng. 2014;39:5413–5420.10.1007/s13369-014-1103-x
- Chong B, Moad G, Rizzardo E, et al. Thermolysis of RAFT-synthesized poly(methyl methacrylate). Aust. J. Chem. 2006;59:755–762.10.1071/CH06229
- Costache MC, Wang D, Heidecker MJ, et al. The thermal degradation of poly(methyl methacrylate) nanocomposites with montmorillonite, layered double hydroxides and carbon nanotubes. Polym. Adv. Technol. 2006;17:272–280.10.1002/(ISSN)1099-1581
- Katsikas L, Avramovic M, Cortés BDR, et al. The thermal stability of poly(methyl methacrylate) prepared by raft polymerisation. J. Serb. Chem. Soc. 2008;73:915–921.10.2298/JSC0809915K
- Zhao Y, Perrier S. Synthesis of poly (methyl acrylate) grafted onto silica particles by Z-supported RAFT polymerization. Macromol. Symp. 2007;248:94–103.
- Rahimi-Razin S, Haddadi-Asl V, Salami-Kalajahi M, et al. Properties of matrix-grafted multi-walled carbon nanotube/poly(methyl methacrylate) nanocomposites synthesized by in situ reversible addition-fragmentation chain transfer polymerization. J. Iran. Chem. Soc. 2012;9:877–887.10.1007/s13738-012-0104-5
- Rahimi-Razin S, Salami-Kalajahi M, Haddadi-Asl V, et al. Effect of different modified nanoclays on the kinetics of preparation and properties of polymer-based nanocomposites. J. Polym. Res. 2012;19:9954–9969.10.1007/s10965-012-9954-x
- Rekik W, Naïli H, Bataille T, et al. Supramolecular networks of transition metal sulfates templated by piperazine. Inorg. Chim. Acta. 2006;359:3954–3962.10.1016/j.ica.2006.05.030
- Lee H-Y, Kim B. Grafting of molecularly imprinted polymers on iniferter-modified carbon nanotube. Biosens. Bioelectron. 2009;25:587–591.10.1016/j.bios.2009.03.040