Abstract
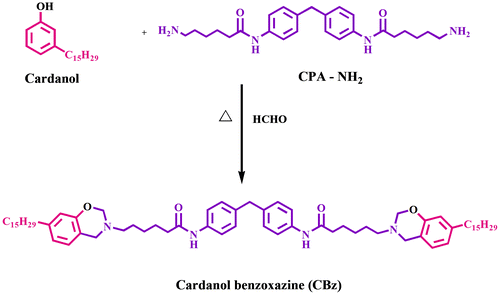
In the present work, benzoxazine was synthesized using caprolactam amine and cardanol, and its molecular structure was confirmed with FTIR and NMR spectroscopic techniques. Alumina (Al2O3) and titania (TiO2) were functionalized using 3-aminopropyltrimethoxysilane (APTMS). The nanocomposites were developed by reinforcing with varying weight (1, 3, 5, and 7) percentages of functionalized Al2O3 (F-Al2O3) and functionalized TiO2 (F-TiO2) with benzoxazine. The developed nanocomposites (F-Al2O3/CPBz and F-TiO2/CPBz) were characterized from their thermal, dielectric, and UV shielding studies. Data obtained from thermal studies show that 7 wt% of F-Al2O3-reinforced cardanol-based polybenzoxazine nanocomposite exhibits higher thermal stability than that of 7 wt% F-TiO2-reinforced cardanol-based polybenzoxazine nanocomposite. Dielectric and UV shielding studies show that 7 wt% F-TiO2 nanocomposite show higher value of dielectric constant (10.2) and UV shielding value (93%) than those of neat and other weight percentages of F-Al2O3 and F-TiO2 reinforced composites.
1. Introduction
Polybenzoxazine, a new class of phenolic resin-based thermosetting polymer, which has high glass transition temperature, high modulus, and dimensional stability. These materials can be used for structural, electronic engineering, and aerospace applications.[Citation1–4] Recently, the use of the benzoxazine resin as a matrix for highly filled composites has been achieved due to its low melt viscosity, which is one of the outstanding properties required for processing.[Citation5–9] An interesting dielectric behavior of benzoxazine has drawn a great research attention towards the fabrication of advanced integrated circuits (which have improved the functionality to enhance its dielectric property of polybenzoxazine blended with various inorganic materials for their synergetic effect). Thereby, enormous research has been focused on benzoxazine-based hybrid materials. Moreover, it is known that the modification in chemical structure could directly have an impact on their dielectric properties.[Citation10]
Polymers obtained from renewable resource materials have attracted the attention of both academic and industrial research. Cardanol is a well-known phenolic compound obtained as a renewable biosource and it is a byproduct of cashew nut shell. The chemically modified cardanol and its polymers exhibit an excellent performance and polymerization into specialty polymers.[Citation11–14] Cardanol-based polybenzoxazine have received much research attention because they can be used as diluent, and have good mechanical and dielectric properties, low viscosity, and hydrophobic behavior than the conventional phenolic resins.[Citation9,15–18]
Many researchers tried to improve the dielectric constant of the polybenzoxazine and polyethylene by incorporating TiO2 into the matrices. They have reported that TiO2 possesses an outstanding toughening, ability particularly with a rigid matrix such as found in many ceramics composite systems.[Citation19–23] Therefore, the incorporation of TiO2 into a polybenzoxazine matrix should render at least twofold benefits to the benzoxazine resin. Alumina is one of the most cost-effective and widely used materials in the family of ceramic engineering. and it has excellent properties which include higher hardness, wear resistance, resistance to strong acid, good dielectric properties, alkali attack at elevated temperatures, and high thermal conductivity.[Citation24–29] Many researchers used alumina and titania as reinforcements to improve the dielectric constant of polybenzoxazine matrix.
In the present work, a new type of cardanol-based benzoxazine (CBz) was synthesized using cardanol as renewable resource material with caprolactam amine (CPA-NH2). 3-aminopropyltrimethoxysilane (APTMS) functionalized alumina (Al2O3) and titanium dioxide (TiO2) were synthesized and varying weight percentages (1, 3, 5, and 7) of F-Al2O3 and F-TiO2 were reinforced into polybenzoxazine to obtain nanocomposites. Data obtained from thermal, dielectric, and UV shielding studies were discussed and reported.
2. Experimental
2.1. Materials
Alumina nanoparticles (Al2O3), APTMS and Titanium (IV) isopropoxide (TTIP) were purchased from Sigma Aldrich. Analytical grades of chloroform, Sodium hydroxide (NaOH), Sulphuric acid (H2SO4), Ethanol, and paraformaldehyde were purchased from SRL, India. 4, 4-diaminodiphenylmethane (DDM) was obtained from Ciba-Geigy Ltd., India. Cardanol was purchased from Satya Cashew Chemicals Pvt. Ltd. Chennai, India.
2.2. Synthesis of CBz
Caprolactam diamine was synthesized as per our earlier report [Citation30] and its molecular structure was confirmed with FTIR and NMR spectroscopy. To a solution of caprolactam diamine (5 g, 0.011 mol) in chloroform, paraformaldehyde (1.4 g, 0.047 mol) was added and stirred for 30 min at 0 °C. Subsequently, 7.42 g of cardanol (0.023 mol) was added to the reaction mixture and stirred overnight at 70 °C. After the completion of reaction, the reaction mixture was extracted with ethyl acetate and washed with 2 N NaOH, water, brine, and concentrated the organic layer to obtain product with 92% yield and the formation of benzoxazine was confirmed from 1H and 13C NMR [Citation31] studies (Scheme ).
2.3. Synthesis of TiO2
Nine milliliters of titanium (IV) isopropoxide (TTIP) in 20 mL of ethanol was hydrolyzed through the sol–gel method using ethanol–water mixture (50:50 V/V ratio). The resulting slurry was stirred vigorously at room temperature to obtain a homogeneous product. The gelation process was completed by the dropwise addition of 1 M sulfuric acid (pH = 3.5). The gel was aged for 12 h at room temperature. The product obtained was filtered and washed several times with water and dried at 60 °C for 12 h. The dried sample was powdered and calcined at 400 °C for 4 h to remove the organic impurities in order to obtain TiO2 nanoparticle, and was confirmed by XRD.[Citation30,32]
2.4. Surface functionalization of Al2O3 and TiO2
Surface functionalization of nano alumina and TiO2 was carried out using APTMS as a coupling agent. Alumina (2 g) and titania (2 g) were separately dispersed in 95% ethanol and 5% deionized water through ultrasonication for 1 h and the pH was adjusted to 4.5 using acetic acid. Two milliliters of APTMS was then added drop by drop and the mixture was sonicated for 30 min, and further refluxed at 70 °C for 12 h under stirring. The resulting amine-functionalized alumina and titania was collected by filtration and dried under vacuum for 12 h and preserved for further use.[Citation33–35]
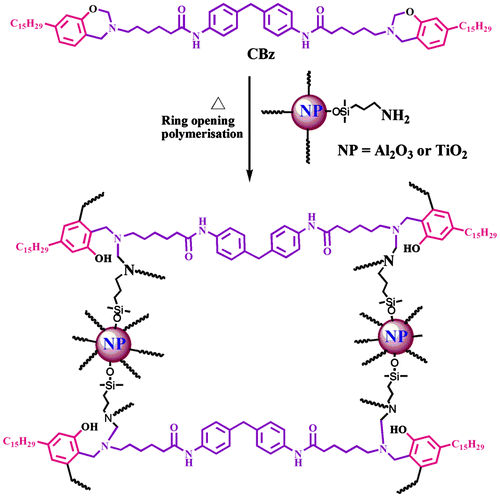
2.5. Preparation of functionalized Al2O3 and TiO2-reinforced polybenzoxazine nanocomposite
APTMS functionalized alumina and titanium dioxide reinforced polybenzoxazine composites were prepared by varying weight percentages of functionalized alumina and titania (0, 5, 10, 15, and 20 wt %) separately using chloroform as solvent to the CPBz monomer solution. The resulting product was poured into a Petri dish and the samples were cured at 100, 120, 160, 200, and 220 °C for each 1 h to get a functionalized alumina and titanium dioxide-reinforced film for further characterization and is shown in Scheme .
3. Characterization
Fourier transform infrared (FT-IR) spectra of the samples were recorded on a Perkin Elmer 6X FTIR spectrometer. The 1HNMR and 13CNMR spectra were recorded with a BRUCKER 300 MHz NMR spectrometer. Samples were diluted using deuterated chloroform. Thermogravemetric analysis (TGA) was carried out using the DSTA 409 PC analyzer (Netzsch Gerateban GmbH). The dielectric studies were carried out with the help of an impedance analyser, solartron impedance/gain phase analyzer 1260) at RT using platinum (Pt) electrode at 30 °C at a frequency of 1 MHz .This experiment was repeated for four times at the same conditions. The glass transition temperature (Tg) of the samples was determined using differential scanning calorimeter (DSC 200 PC, Netzsch Gerateban GmbH).
X-ray diffraction patterns were recorded at room temperature by monitoring the diffraction angle 2θ from 10° to 70° as the standard, on a Rich Seifert (Model 3000) X-ray powder diffractometer. A JEOL JSM-6360 field emission scanning electron microscope (SEM) was used for morphological studies. An analytical TEM (JEM-3010, JEOL, Tokyo, Japan), operating at 80 kV with a measured point-to-point resolution of 0.23 nm, was used to characterize the phase morphology of the nanocomposites developed in the present work. TEM samples were prepared by dissolving the composite samples in ethanol mounted on carbon-coated copper TEM grids and dried for 1 h at 70 °C to form a film of <100 nm. The dielectric constant (DC) of F- Al2O3 and F-TiO2 reinforced polybenzoxazine nanocomposites were determined with the help of an impedance analyzer (Solartron impedance/gain phase analyzer 1260) using Pt electrode at 40 °C in a frequency range of 1 MHz. The UV transmittance spectra were recorded in Jasco (V-650, Japan) spectrophotometer.
4. Results and discussion
4.1. FTIR spectra
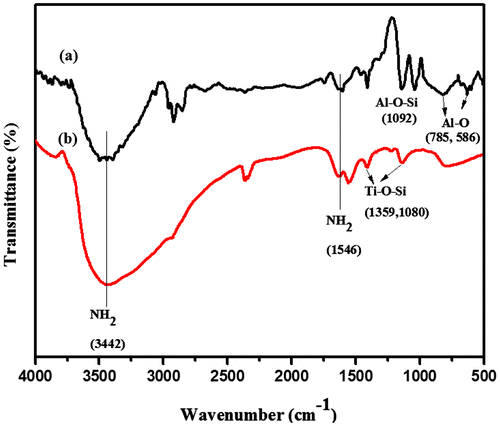
The formation of CBz monomer was supported by FTIR spectrum and is presented in (Figure S1, supporting information). The peak that appeared at 1528 cm−1 indicates the presence of trisubstituted benzene ring and the new peak that appeared at 936 cm−1 represents the stretching vibration of C–O of benzoxazine. Figure (a) shows the FTIR spectrum of amine-functionalized Al2O3 and TiO2. The peak appearing at 3442 cm−1 represents the stretching vibration of N–H bond of aminosilane. The peak appearing at 1092 cm−1 corresponds to the stretching vibration of Al–O–Si linkage and peaks that appeared at 785 and 586 cm−1 represent the Al–O linkage. In the FTIR spectra of F-TiO2, (Figure (b)) the peaks that appeared at 3442 and 1546 cm−1 are assigned to stretching and bending vibration of the N–H bond of aminosilane, respectively. The peaks that appeared at 1359 and 1080 cm−1 are assigned to the stretching vibrations of Ti–O–Si linkage.
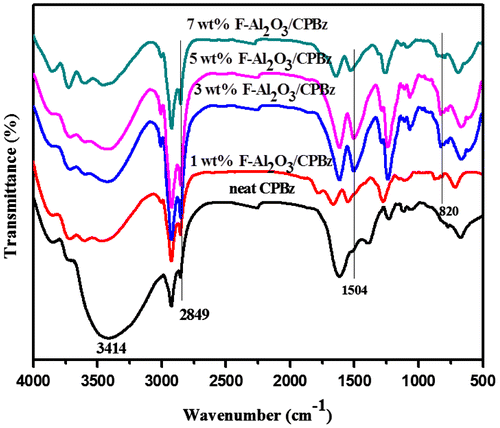
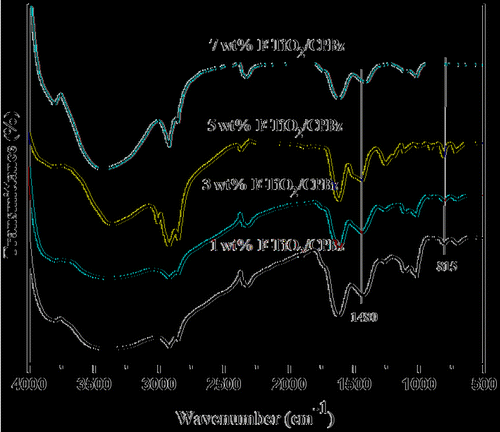
Figure illustrates the FTIR of neat polybenzoxazine and varying weight percentages of Al2O3.The disappearance of peaks at 1528 and 936 cm−1 is related to trisubstituted benzene ring and stretching vibration of C–O of benzoxazine, respectively, which indicates the ring-opening polymerization. The appearance of new peaks at 1504 cm−1 represents the tetra-substituted benzene ring and the peak appearing at 2850 cm−1 is attributed to O⋯H⋯N intra molecular hydrogen bonding of the polybenzoxazine. The peak appeared at 820 cm−1 represents Al–O linkage, which is absent in the FTIR spectrum of neat polybenzoxazine support the reinforcement of F-Al2O3 into the polybenzoxazine matrix. The FTIR spectra of varying weight percentages of F-TiO2-reinforced polybenzoxazine nanocomposites are shown in Figure . The disappearance of peak related to oxazine ring at 936 cm−1 infers the ring-opening polymerization and a new peak appeared at 1480 cm−1 represents the tetra-substituted benzene ring of polybenzoxazine. The additional peak that appeared at 815 cm−1 represents Ti–O–Si linkage.[Citation31,32]
4.2. NMR spectra
Figure S2 (supporting information) shows the 1H and 13C NMR spectra of CBz. The peaks that appeared at 6.64–7.34 ppm were assigned to the aromatic protons. The peaks that appeared at 4.55 and 5.29 ppm are assigned to –O–CH2–N– and Ar–CH2–N–, respectively. The peak that appeared at 5.36 ppm is due to the vinyl protons of long alkyl side chain present in the cardanol skeleton. In addition, all other aliphatic protons appeared from 0.75 to 2.78 ppm. Figure S3 shows 13C NMR spectra of cardanol benzoxazine, carbonyl carbons appeared at 180 ppm, aromatic carbons appeared from 115 to 144 ppm, and aliphatic carbon atoms appeared from 14 to 36 ppm. The peak appearing at 130 ppm represents vinyl carbon of long aliphatic side chain.[Citation31,32]
4.3. MALDI–Mass spectrum of CBz monomer
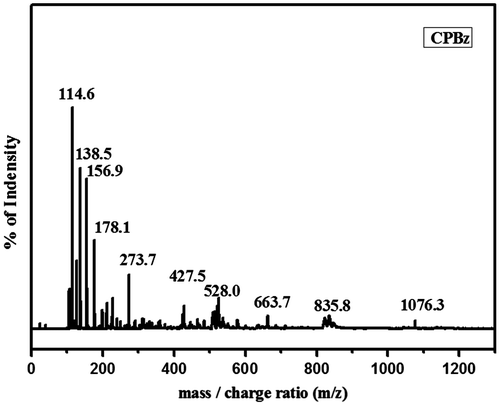
MALDI–Mass spectroscopy is an important technique used to determine the higher molecular weight of organic compounds. The formation as well as the molecular weight of CBz monomer is further confirmed by MALDI–Mass spectroscopy (Figure ). The spectral analysis shows the molecular weight of the benzoxazine monomer as 1076, which is in good agreement with the theoretically calculated molecular weight value. Hence, above results of FTIR, 1H & 13C NMR, and MALDI Mass confirm the successful formation of CBz monomer.[Citation31]
4.4. TGA analysis
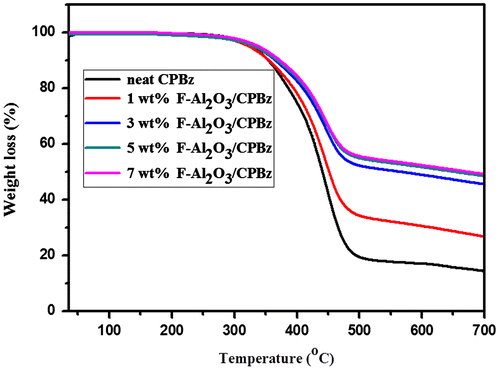
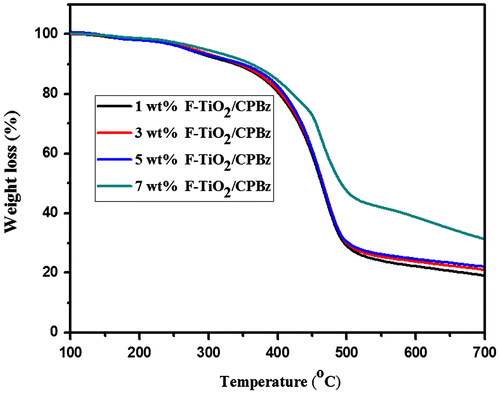
TGA thermograms of the neat polybenzoxazine and varying weight percentages of functionalized alumina and titanium dioxide-reinforced polybenzoxazine composites are presented in Figures and and Table . The percentage char yield value of neat polybenzoxazine is 13, whereas that of 1, 3, 5, and 7 wt% F-Al2O3 are 27, 44, 46, and 48, respectively, and for F-TiO2 are 17, 19, 22, and 31, respectively. From the TGA analysis it is inferred that, as the weight percentages of the reinforcement increases, the thermal stability was also increased.[Citation21] This is due to the presence of alumina and titania in the nanocomposite, that contributes to additional heat capacity and restricts the chain mobility of polybenzoxazine segments resulting from good adhesion between the nanoparticles and the surrounding polymer matrix, which stabilized the materials against thermal decomposition. Among the two reinforcements, the F-Al2O3 reinforced polybenzoxazine, show higher char yield at 700 °C. This is due to higher thermal stability of TiO2 than that of alumina.[Citation26,36]
Table 1. Thermal and dielectric properties of neat CPBz matrix and varying weight percentages of F-Al2O3 and F-TiO2-reinforced polybenzoxazine nanocomposites.
4.5. DSC analysis
DSC data of neat polybenzoxazine matrix and different weight percentages of alumina and titania-reinforced polybenzoxazine nanocomposites are presented in Table . The Tg of neat polybenzoxazine and 1, 3, 5, and 7 wt% of alumina-reinforced nanocomposites are 95, 113, 124, 133, and 137 °C, respectively. When increasing the weight percentages of functionalized alumina, the Tg also increases. There are many factors which alter the Tg value of the polymer, among them one of the most important factors are molecular chain segment mobility, free volume, and cross linking density. As the weight percentages of alumina increases, the interaction between functionalized alumina nanoparticles and polybenzoxazine matrix increased, and in turn reduced the free volume. Hence, 7 wt% of F-Al2O3-reinforced polybenzoxazine shows higher Tg value than that of other samples. In the case of F-TiO2 reinforced polybenzoxazine, the same phenomena were obtained. 7 wt% of F-TiO2-reinforced polybenzoxazine shows the higher value of Tg (176 °C). Among the two reinforcements used for benzoxazine, the F-TiO2-based polybenzoxazine nanocomposites possess the higher value of Tg than that of F-Al2O3-based polybenzoxazine nanocomposites.[Citation26,36]
4.6. XRD analysis
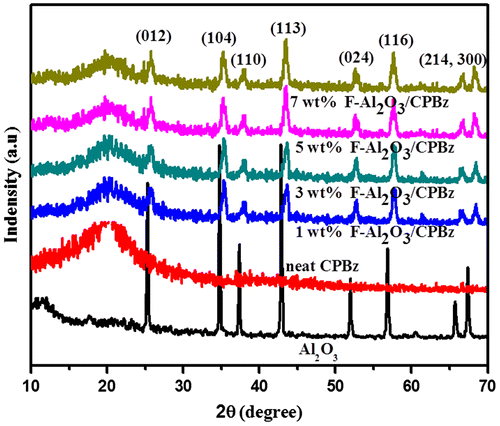
XRD spectra of alumina, benzoxazine, and varying weight percentages of F-Al2O3 reinforced polybenzoxazine are shown in Figure . The XRD spectrum of Al2O3 shows the 2θ peaks at 25.31°, 34.89°, 37.43°, 42.84°, 52.12°, 56.88°, 65.81°, and 67.41° with orientation along (0 1 2), (1 0 4), (1 1 0), (1 1 3), (0 2 4), (2 0 4), (1 1 6), and (2 1 5) plane, respectively, confirming the existence of α-Al2O3. The neat polybenzoxazine displayed only a broad amorphous peak at 2θ = 19.61°, indicating the existence of amorphous phase. On the other hand, the diffraction pattern of F-Al2O3-reinforced polybenzoxazine witnessed a sharp and strong crystalline peaks corresponding to F-Al2O3 in the polybenzoxazine nanocomposites, and this confirmed the reinforcement of F-Al2O3 into polybenzoxazine.
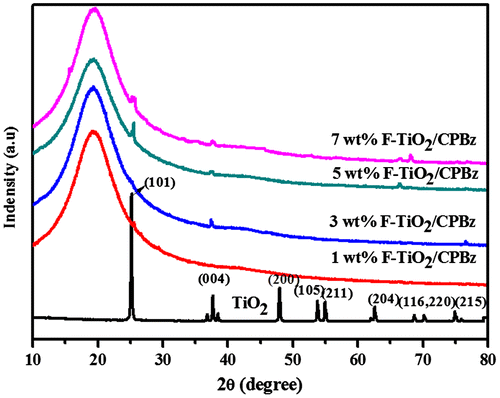
Figure illustrates the XRD pattern of TiO2 and F-TiO2-reinforced polybenzoxazine nanocomposites. In the XRD of TiO2, the 2θ peaks appeared at 25.49°, 37.98°, 48.20°, 54.01°, 55.33°,62.71°, 68.73°, 70.2°, and 75.26° with orientation along (101), (004), (2 0 0), (1 0 5), (2 1 1), (2 0 4), (1 1 6), (2 2 0), and (2 1 5), respectively, confirm the existence of anatase phase of TiO2.[Citation30,33] The average crystallite size of about 35 nm of TiO2 was calculated using Scherer’s formula. In Figure , the diffraction pattern of F-TiO2-reinforced polybenzoxazine nanocomposites system shows the appearance of sharp and strong peak at 2θ = 19.29°, and this suggests the complete dispersion of F-TiO2 reinforced into the polybenzoxazine nanocomposites (Figure ). The XRD pattern of all the F-TiO2-reinforced polybenzoxazine nanocomposites samples show a similar pattern, which implies that F-TiO2 is uniformly distributed in the polybenzoxazine nanocomposites.
4.7. Morphological studies
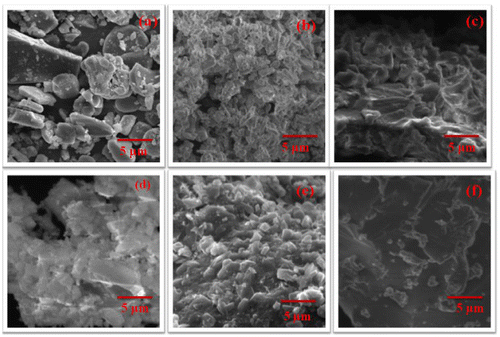
Figure shows the SEM micrograph of alumina and titania nanoparticles ((a) and (d)), F- Al2O3 and F-TiO2 ((b) and (e)), 5 wt% of F-Al2O3 and F-TiO2-reinforced polybenzoxazine nanocomposites ((c) and (e)). The SEM images of alumina and titania shows irregular shape, whereas, the surface of the functionalized alumina and titania shows the different morphology from neat nanoparticles, where neat nanoparticles are covered by APTMS. [Citation26] The images of F-Al2O3 and F-TiO2 reinforced polybenzoxazine nanocomposites shows the homogeneous nanoparticles at the nanoscale level in the polybenzoxazine matrix.
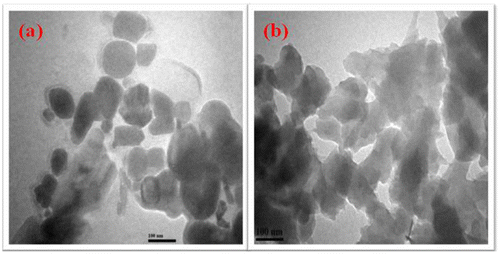
Figure ((a) and (b)) shows the TEM images of 5 wt% of F-Al2O3 and F-TiO2-reinforced polybenzoxazine nanocomposites. The images show uniform dispersion and dimension of nano-sized materials embedded within a polybenzoxazine matrix. This further confirmed that the hybrid films showed good interfacial interaction between the reinforcements (F-Al2O3 and F-TiO2) and the polybenzoxazine matrix. The nanometer-level dispersion of F-Al2O3 and F-TiO2 within the polybenzoxazine matrix was also ascertains the thermal stability of the resulting hybrid materials.[Citation26,31,35,37]
4.8. Dielectric constant
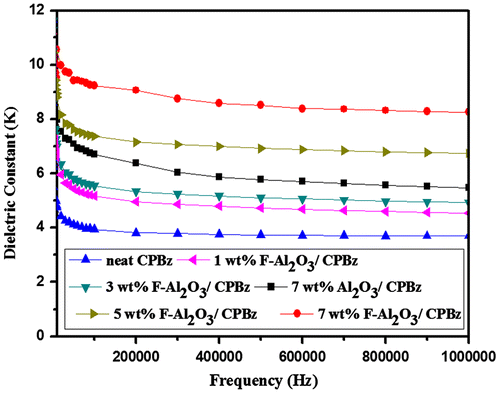
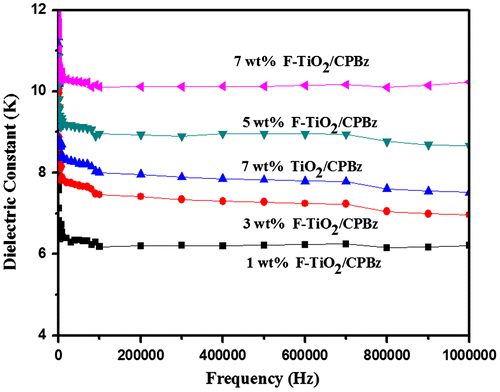
Figures and and Table presents the dielectric properties of neat polybenzoxazine, 7 wt% alumina and titania-reinforced polybenzoxazine nanocomposites and varying weight percentages of F-Al2O3 and F-TiO2-reinforced polybenzoxazine nanocomposites. The value of dielectric constant of neat polybenzoxazine is 3.6, whereas the reinforcement of alumina and varying weight percentages F-Al2O3-reinforced polybenzoxazine nanocomposites are 4.1, 5.2, 5.6, 6.5, and 8.1, respectively. The value of dielectric constant of F-Al2O3 reinforcement nanocomposites is higher than that of neat polybenzoxazine and alumina. This is due to the polar groups present in the polybenzoxazine and can orient themselves, resulting in a higher value of dielectric constant. When the frequency was increased above 1 MHz, the polarization groups fails to settle itself and the values of dielectric constant of nanocomposites begins to decrease at increasing frequencies. The value of dielectric constant of titania and varying weight percentages F-TiO2-reinforced polybenzoxazine nanocomposites are 7.5, 6.1, 6.9, 8.6, and 10.2, respectively. The value of dielelectric constant increases with increasing weight percentages of F-TiO2 due to high polarizability of TiO2, when compared to that of Al2O3.[Citation22,26–28,38–42]
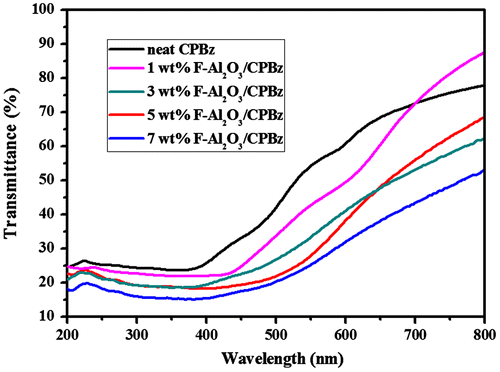
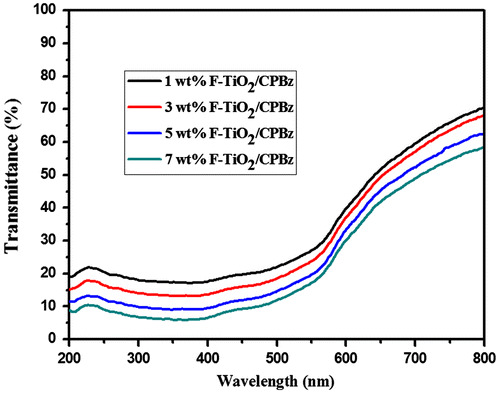
4.9. Optical properties
UV transmittance of F-Al2O3 and F-TiO2-reinforced polybenzoxazine nanocomposites are shown in Figures and , respectively. The UV-shielding efficiency of the nanocomposites increases as the weight % of F-Al2O3 and F-TiO2 are increased. The UV-shielding values of neat PBz, 1, 3, 5 & 7 wt% F-Al2O3-reinforced polybenzoxazine nanocomposites are 76, 78, 80, 82, and 85%, respectively, and that of 1, 3, 5 & 7 wt% of F-TiO2-reinforced polybenzoxazine nanocomposites are 82, 86, 90, and 93%, respectively. Among the varying weight percentages of F-Al2O3 and F-TiO2 reinforced nanocomposites, 7 wt % of F-TiO2/PBZ shows the superior UV-shielding properties (93%) than that of F-Al2O3-reinforced polybenzoxazine nanocomposites. It is proposed that UV-shielding properties of F-TiO2-reinforced polybenzoxazine nanocomposites was higher, as compared to that of F-Al2O3-reinforced polybenzoxazine nanocomposites. As reported from the previous literatures, the band gap of Al2O3 and TiO2 are 8.28 and 3.2 eV, respectively.[Citation27,28] From the solar spectrum, the UV region was covered as near UV (315–400 nm) and far UV (280–380 nm), respectively. Based on the band gap illustration of the Al2O3 have lower shielding efficiency, whereas the TiO2 possesses higher refractive index and higher shielding efficiency.[Citation42,43]
5. Conclusion
The varying weight percentages of F-TiO2 and F-Al2O3-reinforced polybenzoxazine nanocomposites (1, 3, 5 & 7 wt%) were developed and their thermal, dielectric, and UV shielding properties were studied. 7 wt% F-Al2O3-reinforced nanocomposites possesses improved thermal properties due to effective interaction between the polybenzoxazine and F-Al2O3. The homogenous level of dispersion of the composites was confirmed by SEM, TEM, and XRD analysis. Data obtained from dielectric and UV shielding studies show that 7 wt% F-TiO2-reinforced composites possesses higher value of dielectric constant (10.2) and UV shielding behaviour (93%) than those of 7 wt% F-Al2O3-reinforced nanocomposites.
Supplementary material
The supplementary material for this paper is available online at http://dx.doi.10.1080/15685551.2015.1092014.
Supplemental_Data.pdf
Download PDF (250.7 KB)Acknowledgments
The authors also thank R.Sasikumar, M.Ariraman, Polymer Composite Lab, Department of Chemical Engineering, Anna University, H. Madhesan, School of Advanced Science, VIT University, Vellore and Rama Subramanian, MFL, Chennai for their support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Yagci Y, Kiskan B, Ghosh NN. Recent advancement on polybenzoxazine-a newly developed high performance thermoset. J. Polym. Sci. Polym. Chem A. 2009;47:5565–5576.10.1002/pola.v47:21
- Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines -new high performance thermosetting resins: synthesis and properties. Prog. Polym. Sci. 2007;32:1344–1391.10.1016/j.progpolymsci.2007.07.002
- Kiskan B, Ghosh NN, Yagci Y. Polybenzoxazine-based composites as high-performancematerials, Polym. Int. 2011;60:167–177.10.1002/pi.v60.2
- Ishida, H, Agag T. Hand book of benzoxazine resins. Amsterdam: Elsevier; 2011. p. 621–648.
- Nair CPR. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 2004;29:401–498.10.1016/j.progpolymsci.2004.01.004
- Wang Y, Liu XW, Zhang HF, et al. Fabrication of super-hydrophobic surfaces on aluminum alloy substrates by RF-sputtered polytetrafluoroethylene coatings, AIP Adv. 2014;4: 031323–5.
- Callies M, Quéré D. On water repellency. Soft Matter. 2005;1:55–61.10.1039/b501657f
- Gogoia N, Rastogib D, Jassala M, et al. Low-surface-energy materials based on polybenzoxazines for surface modification of textiles. J. Text. Inst. 2014;11:1212–1220.
- Vengatesan MRDevaraju S, Dinakaran K, et al. SBA-15 filled polybenzoxazine nanocomposites for low-k dielectric applications. J. Mater. Chem. 2012;22:7559–7566.10.1039/c2jm16566j
- Guo L, Yuan W, Li J, et al. Stable superhydrophobic surfaces over a wide pH range. Appl. Surf. Sci. 2008;254:2158–2161.10.1016/j.apsusc.2007.08.089
- Huang K, Zhang Y, Li M, et al. Preparation of a light color cardanol-based curing agent and epoxy resin composite: cure-induced phase separation and its effect on properties. Prog. Org. Coat. 2012;74:240–247.10.1016/j.porgcoat.2011.12.015
- Sultania M, Rai JSP, Srivastava D. Process modeling, optimization and analysis of esterification reaction of cashew nut shell liquid (CNSL)-derived epoxy resin using response surface methodology. J. Hazard. Mater. 2011;185:1198–1204.10.1016/j.jhazmat.2010.10.031
- Ikeda R, Tanaka H, Uyama H, et al. Synthesis and curing behaviors of a crosslinkable polymer from cashew nut shell liquid. Polymer. 2002;43:3475–3481.10.1016/S0032-3861(02)00062-9
- Balgude D, Sabnis AS. CNSL: an environment friendly alternative for the modern coating industry. J. Coat. Technol. Res. 2014;11:169–183.10.1007/s11998-013-9521-3
- Xu G, Shi T, Liu J, et al. Preparation of a liquid benzoxazine based on cardanol and the thermal stability of its graphene oxide composites. J. Appl. Polym. Sci. 2014;131:40353.
- Jubsilp C, Takeichi T, Hiziroglu S, et al. High performance wood composites based on benzoxazine-epoxy alloys. Bioresour. Technol. 2008;99:8880–8886.10.1016/j.biortech.2008.04.057
- Jubsilp C, Takeichi T, Rimdusit S. Effect of novel benzoxazine reactive diluent on processability and thermomechanical characteristics of bi-functional polybenzoxazine. J. Appl. Polym. Sci. 2007;104:2928–2938.10.1002/(ISSN)1097-4628
- Ishida H, Ohba S. Thermal analysis and mechanical characterization of maleimide-functionalized benzoxazine/epoxy copolymers. J. Appl. Polym. Sci. 2006;101:1670–1677.10.1002/(ISSN)1097-4628
- Liu L, Hu JM, Leng WH, et al. Novel bis-silane/TiO2 bifunctional hybrid films for metal corrosion protection both under ultraviolet irradiation and in the dark. Scr. Mater. 2007;57:549–552.10.1016/j.scriptamat.2007.04.044
- Shen GX, Chen YC, Lin CJ. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films. 2005;489:130–136.10.1016/j.tsf.2005.05.016
- Agag T, Tsuchiya H, Takeichi T. Novel organic–inorganic hybrids prepared from polybenzoxazine and titania using sol–gel process. Polymer. 2004;45:7903–7910.10.1016/j.polymer.2004.09.022
- Shiv Prakash S, Karan P, Anal T, et al. Effects of SiO2 and TiO2 fillers on thermal and dielectric properties of eco-friendly bismuth glass microcomposites of plasma display panels. Bull. Mater. Sci. 2010;33:33–41.10.1007/s12034-010-0005-0
- Alam MJ, Cameron DC. Preparation and characterization of TiO2 thin films by Sol–Gel method. J. Sol-Gel Sci. Technol. 2002;25:137–145.10.1023/A:1019912312654
- Xu Y, Chung DDL, Mroz C. Thermally conducting aluminum nitride polymer-matrix composites. Composites Part A. 2001;32:1749–1757.10.1016/S1359-835X(01)00023-9
- Gaber AAAA, Ibrahim DM, Abd-AImohsen FF, et al. Synthesis of alumina, titania, and alumina-titania hydrophobic membranes via sol–gel polymeric route. J. Anal. Sci. Technol. 2013;4:1–20.
- Kajohnchaiyagual J, Jubsilp C, Dueramae I, et al. Thermal and mechanical properties enhancement obtained in highly filled alumina-polybenzoxazine composites. Polym. Compos. 2014;35:2269–2279.10.1002/pc.v35.11
- Mohanty A, Srivastava VK. Dielectric breakdown performance of alumina/epoxy resin nanocomposites under high voltage application. Mater. Des. 2013;47:711–716.10.1016/j.matdes.2012.12.052
- Mohanty A, Srivastava VK. Compressive failure analysis of alumina nanoparticles dispersed short glass/carbon fiber reinforced epoxy hybrid composites. Int. J. Sci. Eng. Res. 2012;3:1–7.
- Wu J, Yang S, Gao S, et al. Preparation, morphology and properties of nano-sized Al2O3/polyimide hybrid films. Eur. Polym. J. 2005;41:73–81.10.1016/j.eurpolymj.2004.08.014
- Lakshmikandhan T, Alagar M. Development and characterization of functionalized TiO2-reinforced Schiff base epoxy nanocomposites. High Perform. Polym. 2014. doi:10.1177/0954008314563057.
- Selvaraj V, Jayanthi KP, Lakshmikandhan T, et al. Development of polybenzoxazine/TSBA-15 composite from renewable resource cardanol for low k applications. RSC Adv. 2015;5:48898–48907.
- Sethuraman K, Alagar M. Thermo-mechanical and dielectric properties of graphene reinforced caprolactam cardanol based benzoxazine-epoxy nanocomposites. RSC Adv. 2014;5:9607–9617.
- Kavitha M, Gopinathan C, Pandi P. Synthesis and characterization of TiO2 nanopowders in hydrothermal and Sol–Gel method. Int. J. Adv. Res. Technol. 2013; 2:102–108.
- Tasviri M, Rafiee Pour HA, Ghourchian H, et al. Amine functionalized TiO2–carbon nanotube composite: synthesis, characterization and application to glucose biosensing. Appl. Nanosci. 2011;1:189–195.10.1007/s13204-011-0025-0
- Mandhakini M, Chandramohan A, Alagar M. Nanoindentation studies of nano alumina-reinforced ether-linked bismaleimide toughened epoxy-based nanocomposites. Polym. Plast. Technol. Eng. 2014;53:975–989.
- Zhou W, Yu D. Fabrication, thermal, and dielectric properties of self-passivated Al/epoxy nanocomposites. J. Mater. Sci. 2013;48:7960–7968.10.1007/s10853-013-7606-0
- Mandhakini M, Lakshmikandhan T, Chandramohan A, et al. Effect of nanoalumina on the tribology performance of C4-ether-linked bismaleimide-toughened epoxy nanocomposites. Tribol. Lett. 2014;54:67–79.10.1007/s11249-014-0309-0
- Zhao H, Li RKY. Effect of water absorption on the mechanical and dielectric properties of nano-alumina filled epoxy nanocomposites. Composites Part A. 2008;39:602–611.10.1016/j.compositesa.2007.07.006
- Zhou W, Yu D. Fabrication, thermal, and dielectric properties of self-passivated Al/epoxy nanocomposites. J. Mater. Sci. 2013;48:7960–7968.10.1007/s10853-013-7606-0
- Reddy KM, Manorama SV, Reddy AR. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2002;78:239–245.
- Litter M. Heterogeneous photocatalysis transition metal ions in photocatalytic systems. Appl. Catal., B. 1999;23:89–114.10.1016/S0926-3373(99)00069-7
- Venkatachalam N, Palanichamy M, Murugesan V. Sol–Gel preparation and characterization of nanosize TiO2: its photocatalytic performance. Mater. Chem. Phys. 2007;104:454–459.10.1016/j.matchemphys.2007.04.003
- Giancaterina S, Ben Amor S, Baud G, et al. Photoprotective ceramic coatings on poly(ether ether ketone). Polymer. 2002;43:6397–6405.10.1016/S0032-3861(02)00499-8