Abstract
In the present work calcium alginate/poly (sodium acrylate) composite beads have been prepared by in situ formation of cross-linked poly (sodium acrylate) network, within the calcium alginate (CA) beads. The CA/poly (SA) beads have been found to be stable for more than 48 h, in the physiological fluid (PF) of pH 7.4, while the plain alginate beads disintegrated within a couple of hours. The water uptake of beads was investigated under various composition parameters such as the amount of alginate, concentration of ionic cross-linker Ca++ ions, monomer sodium acrylate (SA) contents, and degree of cross-linking. The beads also exhibited fair stability in the media of varying pH. Finally the release of model drug methylene blue (MB) was investigated. It was found that plain CA and CA/poly (SA) composite beads exhibited different release mechanisms.
1. Introduction
Alginate is a polysaccharide which is extracted from brown algae and consists of 1,4-linked β-D-mannuronic (M) and α-L-guluronic acid(G) residues arranged randomly along the chains.[Citation1,2] It undergoes ionic gelatin in the presence of divalent ions such as Zn++, Ca++ and acquires a bead-like structure of cross-linked chains, usually explained on the basis of ‘egg-box’ model.[Citation3,4] Calcium alginate beads have been widely used for drug delivery applications mainly due to the fact that alginates are totally nontoxic biocompatible polymers and the preparation of cross-linked beads is carried out under normal conditions like aqueous medium, room temperature, and physiological pH; moreover, control over the size of beads and cost-effectiveness of the entrapment process are additional features.[Citation5,6] In addition to drug delivery, these beads have also been used for other biomedical applications such as enzyme immobilization, call-encapsulation, and bone tissue engineering, as scaffolds.[Citation7–10]
However calcium alginate beads do not exhibit fair stability in the physiological fluid or phosphate buffer of pH 7.4.[Citation11,12] The presence of counter ions in the swelling medium induces ion exchange between cross-linking Ca++ ions that are present within the so called ‘egg-box’ cavity, which finally results in the degradation of alginate beads within a course of few hours. In addition, the transition of drug-loaded calcium alginate beads from stomach to large intestine consists of passage of drug-loaded oral formulations via the simulating gastric fluid (SGF) of pH 1.0 for 2–3 h, followed by their transfer in simulating intestinal fluid (SIF) of pH 7.4. In case of calcium alginate beads, it has been reported that beads are degraded at a faster rate when they are transferred from SGF to SIF.[Citation13,14] This finally reduces the stability of beads in the SIF and the release of entrapped bioactive ingredient is limited to a shorter period. There have been sincere efforts to provide mechanical study on alginate-based beads, intended for various biomedical applications. For example, Maggie et al. have carried out in situ polymerization of N-vinyl pyrrolidone and sodium acrylate and used these modified microcapsules for long-term delivery of recombinant gene products in the cell-based method of gene therapy. Similarly, Khorram et al. have used calcium alginate beads as template or support to prepare well-shaped big-sized thermo-sensitive poly(N-isopropyl acrylamide) beads for faster release of entrapped drug as a function of temperature. To the best of our knowledge, no reports are available which describe enhanced stability of alginate-based composite beads along the gastrointestinal tract.[Citation15,16]
In light of above discussion, the present work is a sincere attempt to enhance the stability of the calcium alginate beads intended to be used for oral drug delivery. The vinyl monomer sodium acrylate (SA) has been polymerized in situ within the bead matrix so as to provide fair stability to the resulting beads. Moreover, the poly (acrylate) network produced within the beads enhances the pH-sensitivity of the resulting CA/poly(SA) beads.
2. Experimental
2.1. Materials
Sodium alginate (SA molecular weight and viscosity) was purchased from Hi Media Chemicals Mumbai, India. The vinyl sodium acrylate (SA), cross-linker N,Nʹ methylene bisacrylamide (MB), initiator potassium persulphate (KPS), ionic cross-linker CaCl2, and model drug Riboflavin were received from Merck Chemical Industries, India and were of analytical grade. The double-distilled water was used throughout the investigations.
2.2. Method
2.2.1. Preparation of calcium alginate/poly (SA) composite beads
The cross-linked poly (sodium acrylate) network has been formed in situ within the calcium alginate beads. In brief, a pre-calculated quantity of sodium alginate (SA) was dissolved in distilled water under gentle stirring to ensure complete dissolution. To this, definite amounts of monomer SA, cross-linker MB, and initiator KPS were added and the resulting solution was kept at 15 °C, so as to prevent any polymerization reaction in the solution. Now this solution was added dropwise, using a syringe with an internal diameter of 0.5 mm, to CaCl2 solution of a definite concentration, maintained at 15 °C. The alginate solution was stirred gently to prevent agglomeration of beads. The cross-linking time was 5 min. Now the freshly prepared beads were placed in electric oven (Temp. Star, India) at 60 °C, for a period of 1 h; after the in situ polymerization within alginate beads was over, the beads were taken out and allowed to dry in a vacuum chamber till they attained constant weight. In all, nine bead samples were prepared. The compositions of various samples, plain as well as composite beads, are given in Table .
Table 1. Composition of various CA/poly (SA) composite beads.Table Footnote*
2.2.2. Preparation of drug-loaded beads
The model drug methylene blue (MB)-loaded CA/Poly (SA) beads were prepared by a similar method. A pre-determined quantity of drug was also added to the sodium alginate solution before making the dropwise addition to CaCl2 solution.
2.3. Characterization of beads
The FTIR spectrum of synthesized beads was recorded on FTIR spectrophotometer (Shimadzu, 8400S) using KBr for this; dry beads were grinded and mixed with KBr, the scans recorded were the average of 100 scans and spectral range selected was 400–4000 cm−1.
The scanning electron microscope (SEM) images were recorded using a JEOL 6400F microscope operated with an accelerating voltage of 2 kV and a working distance of 4.4 mm. 50-μL sediment suspensions (0.01 wt%) were dropped onto clean silicon wafers followed by air-drying for 24 h, and then sputtered with an approximately 6-nm layer of gold/palladium.
The thermogravimetric analysis was performed using a thermogravimetric analyzer (Mettler, Toledo TGA/SDTA 851, and Switzerland). A definite quantity of grinded sample was placed in a ceramic crucible and analyzed over the temperature range of 30–800 °C, at the heating rate of 100 °C min−1, under the constant flow of N2 at the rate of 30 mL min−1.
The X-ray diffraction patterns were recorded with the diffractometer (X-PERT-PRO) equipped with a PW 3050/60, channel control goniometry and proportional counter. Radiation was generated from a copper anode tube (Cu Kα 1.54060 A) using an X-ray generator operated at 40 kV and 30 mA. UV–vis spectroscopy was carried out using JASCO-UV–vis–NIR Spectrophotometer with bare glass slide as reference.
2.4. Swelling measurements of beads
The water absorption behavior of CA/poly (SA) beads was studied gravimetrically.[Citation17] The pre-weighed beads were placed in phosphate buffer solution of pH 7.4, under sink conditions at 37 °C. The beads were taken out at regular time intervals, wiped superficially to remove loosely bound surface water, weighed accurately and then again put in the swelling medium. The mass measurement process continued until the swollen beads could be weighed properly. The percent swelling (PS) was determined using the following expression:(1)
where M0 and Mt are the initial dry mass and mass at time ‘t’ respectively. All the experiments were carried out in triplicate and average values are reported in the data.
2.5. Drug release study
The pre-weighed drug loaded CA/poly (SA) beads were placed in 100 mL of release medium (i.e. physiological fluid PF of pH 7.4 at 37 °C). After regular time intervals, 2 mL of the aliquot was taken out and its absorbance was measured spectrophotometrically at 610 nm. The quantity of drug released was calculated using Lambert–Beer’s plot obtained for drug solutions of known concentrations. The release experiments were continued till the two successive measurements yielded almost the same values.
3. Results and discussion
3.1. Preparation of and drug-loaded CA/poly(SA) beads
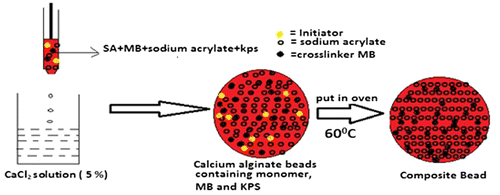
The in situ polymerization of monomer SA within the calcium alginate beads has been a unique approach to render stability to the alginate beads, so that they can withstand the physiological environment against disintegration during their journey from mouth to colon. The overall scheme for the formation of CA/poly (SA) beads has been illustrated in the Figure , and may be described as follows: when the polymerization mixture containing sodium alginate solution is allowed to be dropped into CaCl2 solution the Ca(II) ions instantaneously enter into the bead matrix and cross-link alginate chains via ‘egg-box’ formation. Within these beads, molecules of monomer SA, cross-linker MB and initiator KPS are present and evenly distributed.
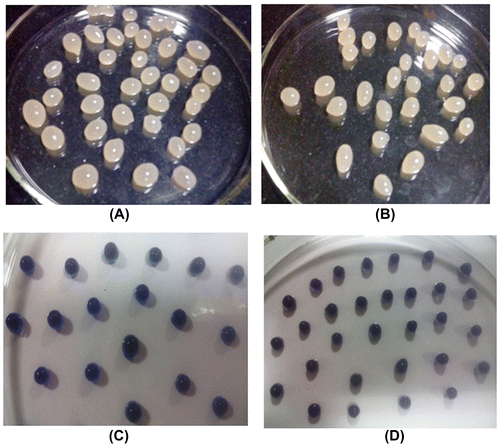
Now these beads are kept in electric oven at 60 °C. The free radical-induced polymerization of SA begins within the beads matrices and cross-linked poly (SA) chains are produced. In this way the calcium alginate beads contain uniformly distributed polymer network of cross-linked poly (SA) chains and these chains are expected to enhance the overall stability of beads against dissolution/disintegration in PF. The drug MB-loaded beads have also been prepared in similar way. The optical images of plain CA, CA/poly (SA), and drug MB-loaded beads are shown in Figure .
3.2. Characterization of beads
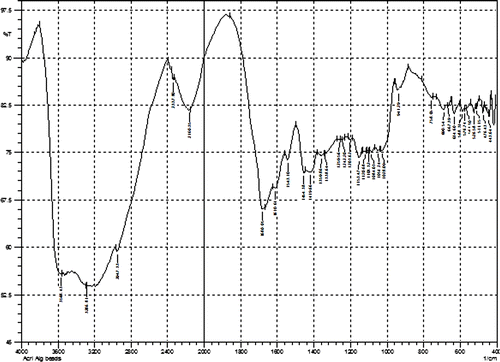
The in situ formation of poly (sodium acrylate) chains within the calcium alginate beads was confirmed by FTIR spectroscopy. The FTIR spectrum of CA/poly (SA) composite beads is shown in Figure .
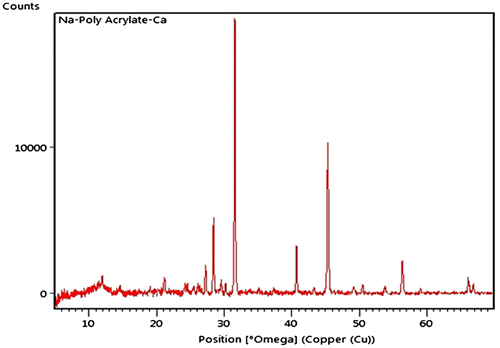
The spectrum contains all the characteristic peaks of calcium alginate and poly (SA). In brief, a relatively sharp peak around 3416 cm−1 is attributable to stretching vibrations of the hydroxyl groups (band of free –OH groups and hydrogen bonds) coupled with –NH stretching from amide of N,Nʹ methylene bisacrylamide. Characteristic absorption band at 1649 cm−1 corresponds to deformation vibrations C–OH and band at 1566 cm−1is typical for symmetrical stretching vibrations of carboxylate anion –COO−.A small peak around 2949 cm−1 indicates –CH stretching. The spectrum also contains a band around 1026 cm−1 (C–O–C stretching) which is characteristic of the saccharide structure of alginate. In addition, a peak at 1419 cm−1 may be assigned to symmetric stretching of carboxylate salt groups. The XRD pattern of CA/poly (SA) is shown in Figure .
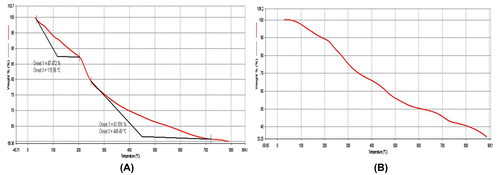
There exist two typical peaks around 14° and 22.5° of 2θ which are related to the lateral packing among alginate molecular chains and the layer spacing along the molecular chain direction.[Citation18,19] The amorphous poly (SA) does not make any contribution toward the crystallinity of the XRD pattern. The thermal stability of pure CA and CA/poly (SA) beads are shown in Figure .
The initial weight loss up to 200 °C, in both the beads is due to moisture loss. Later on, there is continuous weight loss in both of the samples due to de-polymerization up to 500 °C. The plain CA beads have lost almost 50%, of their initial weight whereas the CA/poly (SA) beads suffer a weight loss of only 33%. This indicates an improved thermal stability of CA/poly (SA) beads due to the presence of poly (SA) segments. Finally the total weight loss suffered by plain CA beads up to 700 °C is 70%, while the CA/poly (SA) beads loose only 40%, of the total weight. In this way it may be concluded that CA/poly (SA) beads are more stable than plain CA beads.
The surface morphology of the CA/poly (SA) beads is shown in Figure . The Figure (A) with a magnification of 250×, indicates a drastic change in the surface morphology of the composite beads as compared to the plain CA beads. Here, the surface looks smoother and the previously observed entangled network of alginate chains disappears completely thus suggesting that poly (SA) has been formed throughout the bead’s surface. In a 1000× magnified view (Figure (B)), there can be observed some cracks that are usually observed when a cross-linked matrix undergoes drying. In addition, some impurities like CaCL2 and unreacted salts are also visible. Finally in a 4000× magnified view (Figure (C)) small pits are also visible throughout the surface, with some depositions also.
3.3. Swelling behavior analysis
3.3.1. Swelling behavior of plain CA and CA/poly (SA) beads in pH 7.4
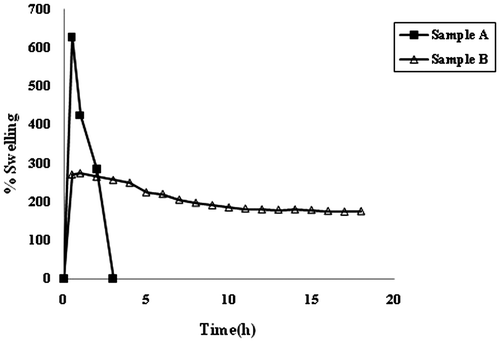
The swelling behavior of samples CA and CA/poly (SA) in the swelling medium of pH 7.4, phosphate buffer is shown in Figure . It can be seen clearly that the plain calcium alginate beads i.e. sample A, exhibit a maximal percent swelling of 626%, in 30 min, and then they begin to disintegrate and after 3 h, they dissolve completely in the swelling medium. However, the CA/poly (SA) beads sample B, shows almost different behavior. The beads achieve a maximum percent swelling of 273%, in 1 h and then the percent swelling decreases gradually to 174%, extended over a total period of 18 h. In other words, after acquiring a maximum swelling of 273%, in 1 h the swelling decreases by 40%, in next 17 h. The difference in the swelling behavior of samples A and B may be explained as follows:
When CA beads are put in PF, Na+ ions present in the swelling medium enter into the bead matrix and undergo ion exchange with Ca++ ions, which are attached to –COO− groups of M blocks. This results in the relaxation of M chains along with increased swelling. Later on, the external Na+ ions enter in to ‘eggbox’ cavities and replace the already existing Ca++ ions.[Citation20] This also results in enhanced percent swelling. Now this fully hydrated structure begins to lose its structural integrity due to disruption of ‘egg-box’ cavities and the alginate chains begin to disintegrate and dissolve. However the sample B, i.e. CA/poly (SA) beads shows enhanced stability in the PF. After achieving maximum percent swelling of 273%, the Na+–Ca++ ion-exchange process becomes operative as mentioned above. This causes alginate chains to dissolve. But in spite of the dissolution of alginate chains the cross-linked poly (SA) network present within the beads continues to maintain the overall structural integrity of the composite beads. We observed that even after a period of 36 h, the beads were in existence. In this way, the stability of the composite beads is very high as compared to the plain CA beads.
3.3.2. Swelling in media of continuous varying pH (1.0 and 7.4)
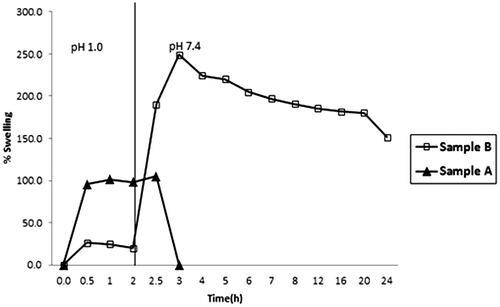
When a drug formulation is taken orally it travels along the gastrointestinal (GI) tract under the environments of varying pH. Initially it goes to stomach where it may reside for 1–3 h, depending on the physiological conditions of the individual. Later on, it passes on to small intestine and then to large intestine where the pH of the environment is almost 7.4. If the drug carrier polymer is pH-sensitive then it becomes essential to investigate the swelling behavior of polymeric drug carrier in the media of varying pH.
The results are shown in Figure . It can be seen that the plain CA beads remain quite stable in the SGF and acquire a percent swelling of 110%. However when they are transferred into SIF, they begin to disintegrate rapidly and within next 30 min, the beads get almost dissolved in the medium. On the other hand the CA/poly (SA) beads attain a maximal swelling of 23%, in the first 2 h, the low percent of swelling is attributable to the fact that –COOH groups of poly (SA) chains present within the beads’ matrix remain as un-dissociated and they remain in collapsed state due to strong inter and intra molecular bonding within the bead matrix. This accounts for lower degree of swelling of these beads. Now as soon as the beads are transferred into SIF of pH 7.4, the beads exhibit a total percent swelling of around 248%.
In this way an 11-fold increase in the percent swelling is observed. Later on the beads begin to lose their weight slightly probably due to the dissolution of alginate chains. However, the presence of poly (SA) within the matrix enhances the overall stability of the beads and the beads do not dissolve/disintegrate till 48 h. This indicates that the CA/poly (SA) beads are fairly stable in the media of varying pH and therefore they are expected to release the entrapped biologically active ingredient for a longer duration in colon.
3.4. Drug release studies
3.4.1. MB release in medium of pH 7.4
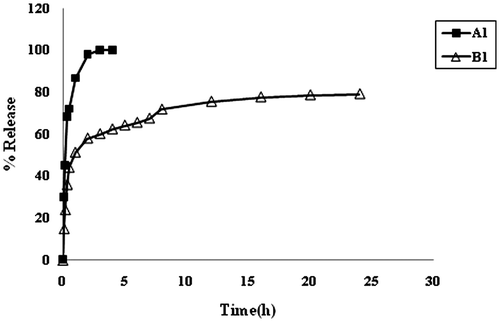
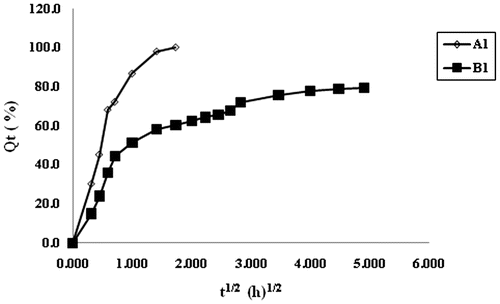
From the above discussion, it is clear that plain CA beads are not very stable in the physiological pH 7.4, and disintegrate within a short period of 2–3 h. On the other hand, CA/poly (SA) beads exhibit a fair stability, and hence they are expected to release the entrapped drug over a long time period. The dynamic release of model drug MB from the samples A1 and B1 is shown in Figure . It can be seen that the plain CA beads sample A1 demonstrates an almost 100% release in a duration of 2 h. On the other hand, the sample B1 i.e. CA/poly(SA) beads exhibits a relatively slower release extended over a time span of 20 h. In this way, it is clear that the introduction of poly (SA) chains within the calcium alginate beads matrix results in a slower release with an extended time period.
3.4.2. Modeling of the release data
The Higuchi model was initially developed for the planar systems but later on extended to systems with different geometries. It is based on the following assumptions (1) initial drug concentration in the matrix is much higher than its solubility, (2) thickness of drug particles is much smaller than the thickness of the matrix, (3) drug diffusivity is constant, and (4) perfect sink conditions are always maintained. The most simplified form of this model is given as:(2)
where F may be taken as the fractional release and KH is the Higuchi constant.
The major benefits of this equation include the possibility to: (i) facilitate device optimization and (ii) to better understand the underlying drug release mechanisms. The equation can also be applied to other types of drug delivery systems than thin ointment films e.g. controlled release transdermal patches or films for oral controlled drug delivery.
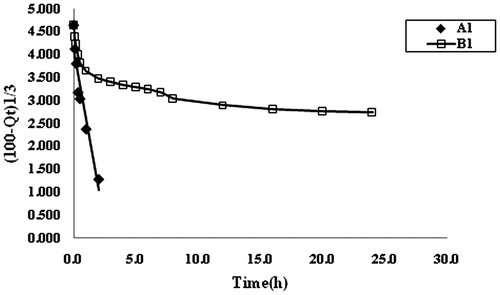
The dynamic release data shown in Figure , were used to draw plots between F and t1/2. It can be seen that the plots are not linear thus indicating the unsuitability of the Higuchi model in the present study. This suggests that release of drug MB from the bead samples A1 and B1, does not follow the diffusion-controlled mechanism. It may probably be due to the fact that both of the samples namely A1 and B1, contain ionizable groups (alginates and alginates/poly (sodium acrylate) respectively) and so the chain relaxation of polymeric segments predominates in the release mechanism may be governed by the chain relaxation process. The release data were also applied on the well-known Hixon–Crowel model,[Citation10] according to which the cube root of percentage drug remaining vs. time is correlated with the release from systems with polymer erosion/dissolution resulting in a change in the surface area and diameter of polymeric drug carrier beads or tablets. This model is given as,(3)
where Qt is the amount of drug remained in the polymeric carrier at time t, Q0 is the initial amount of the drug in the dosage form and kH·t is the rate constant for the Hixson-Crowell rate equation. A graphical representation of the cube root of the amount remaining vs. time will be linear if the equilibrium conditions are not reached and if the geometrical shape of the dosage form diminishes proportionally overtime (Cube root of initial drug load minus cube root of percent drug remaining) are plotted against time (hours) to demonstrate the Hixson Crowell plot. The plots for this model are shown in Figure , for release kinetic data obtained for the samples A1 and B1. It is clear that the sample A1 consisting of calcium alginate beads shows fair fitness with a regression value of 0.9647, while the sample B1 i.e. CA/poly (SA) beads exhibit very poor regression and do not show a fair fitness. The suitability of Hixon–Crowell model for the sample A1 is attributable to the fact that calcium alginate beads undergo erosion in the phosphate buffer medium of pH 7.4, as discussed previously. On the other hand, the sample B1 is fairly stable due to the presence of poly (sodium acrylate) network within the alginate beads and therefore they do not fit in the criterion of Hixon–Crowel model.
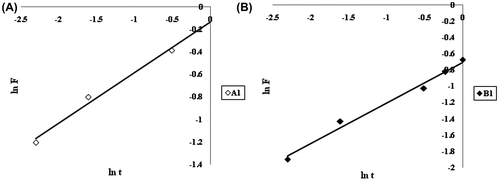
Finally the Ritger–Peppas model was applied on the kinetic release data to investigate the nature of the release process. Ritger and Peppas (1987) developed an empirical equation to analyze both Fickian and non-Fickian release of drug from swelling as well as non-swelling polymeric delivery systems. The equation is represented as:(4)
The logarithm form of Equation (Equation5(5) ) could be written as:
(5)
According to this model, the release exponent ‘n’ lies between 0.43 and 0.85, for the release mechanism to be anomalous or non-Fickian. The ln F vs. ln t plots for initial 60% data for samples A1 and A2 are shown in Figure (A) and (B), respectively. It can be seen that values of release exponent ‘n’ for the samples A1 and B1 are 0.44 and 0.55 respectively, thus indicating non-Fickian behavior of release process for both the samples.
3.4.3. Release in media of varying pH
The release of model drug MB from the samples A1 and B1 is shown in Figure . It is noticed that the plain CA beads (i.e. sample A1) exhibit almost 78% drug release in the SGF of pH 1.0 in first 2 h. Later on as the beads are transferred into SIF of pH 7.4, the beads begin to disintegrate/dissolve and remaining drug is released in next 30 min. In this way it takes almost two and a half hours for the plain CA beads to release the whole quantity of the entrapped drug. On the other hand sample B1 (i.e. CA/poly (SA) beads show almost different behavior in the media of varying pH. These beads release nearly 28% of the entrapped drug in first 2 h, in SGF of pH 1.0. A low extent of release is attributable to the presence of cross-linked poly (SA) chains within the beads, which contain –COOH groups along the polymer segments and produce additional physical cross-links through H-bonding interactions. It is also worth mentioning here that the plain sample A1 and the composite beads sample B1 exhibit nearly 78 and 28% of the total drug entrapped in first 2 h, in SGF of pH 1.0.This indicates that as compared to plain alginate bead sample A1, the composite beads release 50% less drug in first 2 h in SGF. It means that the presence of cross-linked poly (SA) network within the alginate matrix imparts excellent pH-sensitivity to the sample B1.
Figure 13. Comparative depiction of release profiles of samples A1 and B1 in the media of varying pH at 37 °C.
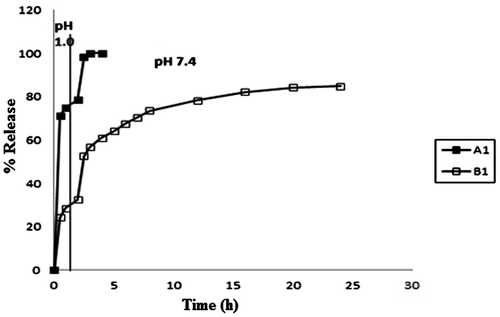
Later on when the composite beads are transferred into SIF of pH 7.4, the ionization of –COOH groups causes the acrylate chains to undergo relaxation due to repulsion among similar charges and this results in a relatively fast release of the entrapped drug. Thus in the next 22 h nearly 55%, of the entrapped drug is released. In this way there is release of almost 80% of the entrapped drug in a total period of 24 h. Therefore, it may be concluded that sample B1 is fairly stable in the media of varying pH.
4. Conclusion
From this study it is concluded that in situ polymerization of poly (SA) within the calcium alginate beads not only enhances the water absorbency of the resulting composite beads, but it also increases their stability with maintained structural integrity. These beads, in further work, shall be loaded with the anti-diabetic drug Glyclazide and shall be studied in vitro and in vivo to test their effectiveness in controlling the sugar level.
Acknowledgments
The authors thank Dr O.P. Sharma, Head of the Department of Chemistry, Govt. Model Science College Jabalpur, MP, for his kind and unconditional support. This Research received no specific grant in the public, commercial, or the profit sector. We also thank ISER Bhopal for the analysis of samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mori M, Rossi S, Bonferoni MC, et al. Calcium alginate particles for the combined delivery of platelet lysate and vancomycin hydrochloride in chronic skin ulcers. Int. J. Pharm. 2014;461:505–513.10.1016/j.ijpharm.2013.12.020
- Araujo V, Gamboa A, Caro N, et al. Release of prednisolone and inulin from a new calcium-alginate chitosan-coated matrix system for colonic delivery. J. Pharm. Sci. 2013;102:2748–2759.10.1002/jps.23656
- Liakos I, Rizzello L, Bayer IS, et al. Controlled antiseptic release by alginate polymer films and beads. Carbohydr. Polym. 2013;92:176–183.
- Singh I, Kumar Pak P. Formulation and optimization of tramadol loaded alginate beads using response surface methodology. J. Pharm. Sci. 2012;25:741–749.
- Hunt NC, Grover LM. Encapsulation and culture of mammalian cells including corneal cells in alginate hydrogels. Methods Mol. Biol. 2013;1014:201–210.10.1007/978-1-62703-432-6
- Tran NM, Dufresne M, Duverlie G, et al. An appropriate selection of a 3D alginate culture model for hepatic Huh-7 cell line encapsulation intended for viral studies.Tissue Eng. Part A. 2013;19:103–113.10.1089/ten.tea.2012.0139
- Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10:1646–1662.10.1016/j.actbio.2013.12.006
- Wei X, Xi T, Gu Q, et al. Progress in alginate-based biomedical materials. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:1015–1020.
- Katsen-Globa A, Meiser I, Petrenko YA, et al. Towards ready-to-use 3-D scaffolds for regenerative medicine: adhesion-based cryopreservation of human mesenchymal stem cells attached and spread within alginategelatin cryogel scaffolds. J. Mater. Sci. Mater. Med. 2014;25:857–871.10.1007/s10856-013-5108-x
- Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind. Eng. Chem. 1931;23:923–931.10.1021/ie50260a018
- Kumaravel V, Gopal SR. Immobilization of Bacillus amyloliquefaciens MBL27 cells for enhanced antimicrobial protein production using calcium alginate beads. Biotechnol. Appl. Biochem. 2010;57:97–103.
- Sambu S, Xu X, Ye H, et al. Predicting the survival rate of mouse embryonic stem cells cryopreserved in alginate beads. Proceedings of the Institution of Mechanical Engineers. Bone Tissue Eng. 2011;225:1092–1107.
- Matsuno T, Hashimoto Y, Adachi S, et al. Preparation of injectable 3D-formed beta-tricalcium phosphate bead/alginate composite for bone tissue engineering. J. Bone Tissue Eng. 2008;27:827–834.
- Duggal S, Frønsdal KB, Szöke K, et al. Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds. Tissue Eng. Part A. 2009;15:1763–1773.10.1089/ten.tea.2008.0306
- Wang MS, Childs RF, Chang PL. A novel method to enhance the stability of alginate-poly-L-lysine-alginate microcapsules. J. Biomater. Sci. Polym. Ed. 2005;16:89–111.10.1163/1568562052843302
- Khorram M, Vasheghani-Farahani E, Ebrahimi NG. Fast responsive thermosensitive hydrogels as drug delivery systems. Iran. Polym. J. 2003;12:315–322.
- Pierre A, Hanna S, Gad H, et al. Optimization of gabapentin release and targeting absorption, through Incorporation into alginate beads. Br. J. Pharm. Res. 2013;3:597–616.
- Malesu VK, Sahoo D, Nayak PL. Chitosan-sodium alginate nanocomposites blended with cloisite 30B as a novel drug delivery system for anticancer drug curcumin. Int. J. Appl. Biol. Pharm. Technol. 2011;2:402–411.
- Li LB, Fang YP, Vreeker R, et al. Multiple steps and critical behaviors of the binding of calcium to alginate. Biomacromolecules 2007;8:464–468.10.1021/bm060550a
- Kikuchi A, Kawabuchi M, Watanabe A, et al. Effect of Ca+-alginate gel dissolution on release of dextran with different molecular weights. J. Controlled Release 1999;58:21–28.10.1016/S0168-3659(98)00141-2