Abstract
A new chiral diamine L-methyl-2-[(3,5-diaminobenzoyl)amino]-3-(1H-indol-3yl)propanoate was synthesized using L-tryptophan (essential amino acid) as starting material. The structure of the synthesized diamine was supported by Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (1H and 13C-NMR) and mass spectral techniques. The diamine was polymerized with 3,3’,4,4’-benzophenone tetracarboxylic dianhydride via thermal imidization method to produce thermally stable chiral polyimide (PI) with low dielectric constant. Additionally, polyimide nanocomposites were also prepared by incorporating amino functionalized polyhedral oligomeric silsesquioxane (POSS) into the PI matrix. The polyimide and PI/POSS nanocomposites were characterized by FT-IR spectroscopy. The PI was found to have specific optical rotation of –41.4°. The inherent viscosity was found to be 0.77 dLg−1 indicating that a high molecular weight PI was formed. Surface morphology of the neat PI and nanocomposites was studied by scanning electron microscopy, transmission electron microscopy and atomic force microscopy (AFM) that reveal uniform distribution of the nanoparticles in the PI matrix. DSC analysis indicates that the Tg of the PI and its nanocomposites are in the range of 222–250 °C. The T10% was found to be in the range of 402.4–470.5 °C for the PI and its nanocomposites. The dielectric constant values are in the range of 3.5–2.1.
Introduction
Over the past several decades, an increasing attention has been focused in the development of high-performance polymers as a replacement for ceramics and metals in the field of microelectronics, aerospace and automobile industries.[Citation1,2] To be more specific, different aromatic polyimides (PIs) were synthesized and their performance were studied. Though PIs possess good thermal, electrical and mechanical properties, the presence of dark colour (due to intramolecular charge transfer complex formation) and poor solubility (due to its rigid structure) restrict their applications in industries.[Citation3,4] Several attempts have been made to overcome these limitations by tailoring the structure of the PIs, i.e. using new diamines with different structures.
There are many approaches such as incorporating flexible linkages, non-coplanar conformation units and bulky pendant groups for solving the drawbacks without sacrificing their attractive properties.[Citation5,6] One which has been accepted widely is to increase the flexibility along the polymer chain by introducing flexible links, i.e. aliphatic methylene groups and the use of unsymmetrical or chiral molecules.
We are undeniably living with chiral world. Most of the naturally occurring macromolecules such as proteins and nucleic acids are chiral and optically active.[Citation7] One of the most useful and extensively accepted uses of chiral polymers is their application as chiral stationary phase in HPLC for the separation of racemic compounds.[Citation8] The synthesis and application of optically active polymers are topics that have been paid more attention recently because polymers with chiral structures are biologically very important. Naphthyl-based chiral polyimides have potential to be used as packing material in column chromatography.[Citation9]
Hence, it is proposed to prepare new optically active polyimides by introducing chiral diamines during the synthesis of PIs. The incorporation of chiral centre interrupts the π conjugation and intermolecular electron transfer resulting insulator behaviour.[Citation10] The dielectric constant of the system can be controlled by employing chiral centres. The asymmetric centres disfavour the planar confirmation that results in non-planar structure in the polymer architecture. This modification leads to irregularity, bulkiness and non-linearity of the polymer chains which restricts the close chain packing and hence a large decrease in crystallinity. The effects of crystallinity on the electronic properties of the poly(dimethyltin esters) is remarkable one and reported in Ref [Citation11]. The chiral polymers find applications in the non-linear optical devices, optical switches and liquid crystals, etc. In the preparation of chiral diamine, we used amino acids as chiral inducting agents. These materials are naturally existing compounds, and therefore, synthetic polymers based on amino acids are expected to be biodegradable and biocompatible.[Citation12] In the present investigation, novel optically active polyimide containing L-tryptophan moiety was prepared for the first time. L-tryptophan, an essential amino acid, is found in Bjerkandera odusta (wood decay fungi). L-tryptophan has an aliphatic substitution which increases the flexibility and decreases the colour in the polymer backbone. The presence of α-amino acid, i.e. chiral units [Citation13] in the polymer matrix helps it to have enhanced biodegradability. L-tryptophan amide covalently bonded to β-cyclodextrin is used as chiral stationary phase in one of the most active research groups.[Citation14]
Polyimide nanocomposites have become a rapidly growing field of research due to their different applications. It is possible to readily tune the properties of the polymers by making nanocomposites with suitable fillers which play an important role in the synthesis of organic/inorganic hybrid composite material.[Citation15] From this viewpoint, polyhedral oligomeric silsesquioxane (POSS) is attractive and ideal building block for the synthesis of new organic inorganic hybrid materials. Embedding functionalized POSS in the polymer matrix not only leads to the enhancement of electrical properties [Citation16] and thermal stability [Citation17] due to their unique characteristics, but also improves their biocompatibility as well [Citation18–20]. Functionalization of POSS also leads to better distribution in the polymer by covalent interactions, a higher miscibility with polymer and disperses very well in the cured network which improves the properties of the polymers significantly.[Citation21–23]
In this study, we focus on the effect of incorporation of L-tryptophan in the polyimide matrix on the thermal and electrical properties. Hence, we report the synthesis of chiral polyimide using L-tryptophan-based diamine and BTDA as raw materials. The thermal, electrical and morphological properties of the above-mentioned polyimide and its POSS nanocomposites with respect to their chemical structure are also reported.
Experimental
Materials
L-tryptophan, hydrazine mono hydrate and thionyl chloride were purchased from Spectrochem Pvt. Ltd, India. 3,3’,4,4’-benzophenone tetracarboxylic dianhydride (BTDA) was purchased from Thermo Fisher Scientific India Pvt. Ltd. Dichloromethane, N-methyl pyrrolidone, N,N-dimethyl acetamide and triethylamine were purchased from E-Merck Limited, India. Ethanol was purchased from A.R. Life science, India. Phenyltrichlorosilane (>97%, density 1.321 g/mL), Pd/C and 3,5 dinitrobenzoyl chloride were purchased from Sigma Aldrich, USA. Benzyl trimethyl ammonium hydroxide 40% in methanol solution was procured from Alfa Aesar (Johnson Mathew Company), USA. Triethylamine was distilled over KOH/CaH2 under N2 atmosphere immediately prior to use. N-methyl pyrrolidone (NMP) was purified by distillation under nitrogen atmosphere. All the reagents were used as received unless specified.
Characterization
FT-IR spectra were recorded on a ABB Bomem (Model MB 3000) spectrometer to characterize the precursors, monomer and the polymers. The samples were made into thin pellets using fresh KBr (E-Merck, India, IR Grade). 1H and 13C-NMR (400 MHz and 300 MHz) spectra were recorded with Bruker instrument, using DMSO-d6 and deuterated chloroform as solvent and TMS as the reference. Mass spectra were recorded on Jeol GC mate III, Japan. Specific optical rotations (SOR) were measured using an advanced research instrument PA-1R model polarimeter. Inherent viscosity, at a concentration of (0.5 dLg−1) of polymers, was measured by a standard procedure using Ubbelohde viscometer at 25 °C using NMP as the solvent. DSC analyses were performed on TA instruments Q10 model, under nitrogen atmosphere using 5–10 mg of the sample at a heating rate of 10 °C/min from ambient to 350 °C with a nitrogen flow of 60 mL/min. TGA curves were recorded on a (TA Q 600 thermal analyzer) at a heating rate of 20 °C min−1 under N2 atmosphere. Samples were analysed in open silicon pan up to a maximum temperature of 800 °C. The gold-palladium-coated fracture surface of PI and PI/POSS nanocomposites were photographed by VEGA3 TESCAN scanning electron microscopy (SEM). The surface topology of the fractured surface was investigated by means of atomic force microscopy (AFM) Seiko SPI3800 N, series SPA-400 (Tokyo, Japan). High-resolution transmission electron microscopic (HR-TEM) images were recorded using JEOL, JEM 2100 electron microscope operated at 200 kV. The dielectric properties of neat PI and the PI/POSS nanocomposites were tested with an impedance analyzer (Solatron 1260 Impedance/Gain-phase Analyzer) at room temperature using a platinum electrode sandwich model at a frequency range of 1 KHz–1 MHz. Thin-layer chromatography (TLC) was performed on pre-coated Silica gel 60 F254 plates (E-Merck).
Synthesis of monomer
L-Methyl 2-amino-3-(1H-indol-3-yl)propanoate [L-tryptophan methyl ester]
In a 250-mL two-necked round-bottomed flask, equipped with a reflux condenser, L-tryptophan (2.04 g, 0.01 mol) and 50 ml of methanol were taken in an ice bath and cooled at 0–5 °C. A known volume of 2.38 g (0.02 mol) of thionyl chloride was added dropwise to this solution. The resultant mixture was heated to reflux and stirred for 8 h at reflux temperature. Unreacted SOCl2 was removed by distillation under reduced pressure. The crude product of L-tryptophan methyl ester, obtained as green coloured solid, was separated by filtration and washed with 25 mL of diethyl ether twice. The material which became white in colour was dried at 60–65 °C for 10 h in air oven. Yield (95%), m.p. 139 °C. FT-IR (KBr, cm−1): 1751 cm−1 (asymmetric stretching vibrations of the carbonyl group in the ester).
L-Methyl-2-[(3,5-dinitrobenzoyl)amino]-3-(1H-indol-3yl)propanoate (MDNP)
A mixture of L-tryptophan ester (2.53 g, 0.01 mol) and triethylamine (7.5 mL) in 20 ml of CH2Cl2 (MDC) was taken in a two-necked round-bottomed flask and cooled in an ice bath. The fresh 3,5 dinitro benzoylchloride (2.30 g, 0.01 mol) dissolved in 10 mL of CH2Cl2 was added dropwise to this solution. The resultant mixture was stirred for 2 h at room temperature (25–30 °C). A yellow coloured solid was formed. The course of the reaction was monitored using TLC plate with EtOAc : Hexane mixture (3:1) as the mobile phase. The solid product was filtered and washed with MDC to remove impurities. The product (MDNP) was dried in an air oven at 50–55 °C. Yield (97%), m.p. 171 °C. FT-IR (KBr, cm−1): 1543 and 1342 cm−1 (asymmetric and symmetric stretching vibrations of the nitro group), 1636 cm−1 (stretching vibrations of the C=O group), 2932 and 2901 cm−1 (asymmetric and symmetric stretching vibrations of the methylene group). 1H-NMR (CDCl3, ppm): 10.8 (s, 1H, Ha), 9.6 (s, 1H, Hb), 9.0 (s, 2H, Hc), 8.9 (1H, Hd), 6.9–7.5 (5H, Ar–H, H(e-i)), 4.8 (s, 1H, Hj), 3.6 (3H, Hk), 2.7 (2H, Hl). 13C-NMR (CDCl3, ppm): C1–172.3, C2–162.9, C3–148.6, C4–136.5, C5–128.6, C6–128.1, C7–127.4, C8–124.1, C9–121.5, C10–121.5, C11–118.9, C12–118.4, C13–111.9, C14–110.0, C15–54.7, C16–52.6, C17–27.0. EI-MS (m/z): [M+] calculated for C19H16N4O7, 412; Found 412.
L-Methyl-2-[(3,5-diaminobenzoyl)amino]-3-(1H-indol-3yl)propanoate (MDAP)
A 250-mL two-necked round-bottomed flask equipped with magnetic stirrer and a reflux condenser was charged with 4.1 g (0.01 mol) of MDNP, 0.05 g of 10% palladium on activated carbon and 50 mL of ethanol. The mixture was heated to reflux, and then, 8 mL of hydrazine mono hydrate was added slowly and refluxed for 10 h. The course of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was filtered hot to remove the catalyst. The filtrate was cooled to precipitate the product. The crystals formed in ethanol were collected by filtration and dried in a vacuum oven at 50–55 °C. Yield 80%, m.p. 155 °C. FT-IR (KBr, cm−1): 3448 and 3356 cm−1 (asymmetric and symmetric stretching vibrations of the primary amino group), 3055 and 3024 cm−1 (asymmetric and symmetric stretching vibrations of the aromatic C–H bond). 1H-NMR (CDCl3, ppm): 10.7 (s, 1H, Ha), 9.2 (s, 1H, Hb), 7.9–6.9 (H, Hc-g), 6.2 (s, 2H, Hh), 5.9 (1H, Hi), 4.8 (4H, Hj), 4.6 (1H, Hk), 3.6 (3H, Hl), 3.1 (d, 2H, Hm). 13C-NMR (CDCl3, ppm): C1–171.6, C2–167.7, C3–149.4, C4–136.5, C5–136.1, C6–127.8, C7–123.9, C8–121.3, C9–118.9, C10–118.7, C11–111.7, C12–110.7, C13–105.6, C14–102.5, C15–52.9, C16–51.1, C17–28.4. EI-MS (m/z): [M+] calculated for [C19H21N4O3], 353; Found 353.
Synthesis of MDAP/BTDA–PI
The MDAP/BTDA–PI was synthesized by thermal imidization method by reacting the synthesized diamine (MDAP) with BTDA. In a completely dried 25-mL three-necked round-bottomed flask equipped with a stirring bar under nitrogen atmosphere, 1.03 g (0.003 mol) of MDAP and 10 mL of N-methyl pyrrolidone were taken and stirred until a clear solution was obtained. A known volume of 0.96 g (0.003 mol) of BTDA was added and stirred for 12 h in nitrogen atmosphere at room temperature. Yellow coloured viscous solution of poly(amic acid) (PAA) obtained was then cast on a glass substrate and thermally treated at 100 °C for 6 h, 150 °C for 4 h, 250 °C for 2 h and 300 °C for 2 h. The yellow transparent film was removed from the glass substrate with the aid of deionized water and dried at 100 ◦C in a vacuum oven.
Poly(amic acid) (PAA)
FT-IR (KBr, cm−1): 3433 cm−1 (s, asymmetric stretching vibrations of the carboxylic OH group), 2924 and 2850 cm−1 (w, asymmetric and symmetric stretching vibrations of the methylene group), 1653 cm−1 (m, C=O stretching vibrations of the amide group).
Polyimide (MDAP/BTDA–PI)
FT-IR (KBr, cm−1): 3070 cm−1 (w, stretching vibrations of aromatic C–H group), 2922 cm−1 (w, asymmetric stretching vibrations of the methylene group) and 2853 cm−1 (w, symmetric stretching vibrations of the methylene group), 1665 cm−1 (aromatic C=C stretching), 1783 and 1725 cm−1 (asymmetric and symmetric stretching vibrations of the imide carbonyl group), 1380 cm−1 (C–N–C stretching vibrations of the imide ring), 716 cm−1 (C–N–C bending vibrations), Yield: 85%, Inherent viscosity: 0.77 dLg−1, SOR = –41.4°.
Synthesis of POSS
The amine-functionalized POSS named as octa (aminophenyl) silsesquioxane (OAPS) was synthesized by the following methods reported in the previous literature.[Citation24,25]
Synthesis of polyimide/POSS nanocomposites
The PI/POSS nanocomposites were prepared using the conventional procedure depicted in Scheme . A three-necked round-bottomed flask was first purged with nitrogen gas to remove the moisture. MDAP (10 mmol, 4.15 g)/NMP (40 mL) solution was added to the flask, and then, BTDA (10.5 mmol, 3.38 g) was added in three portions. The mixture was stirred under nitrogen at room temperature for 6 h, and a viscous poly(amic acid) (PAA) solution was obtained. At this point, POSS (1 wt%, 0.04 g) was added and the mixture was stirred at room temperature for an additional 2 h to give a transparent solution. The obtained polyamic acid/POSS solution was then cast on a glass substrate and thermally treated at 100 °C for 6 h, 150 °C for 4 h, 250 °C for 2 h and 300 °C for 2 h. The films were removed from the glass substrates with the aid of deionized water and dried at 100 °C in a vacuum oven. The thickness of the films was about 0.3 mm. Using this general approach, a series of hybrids having POSS composition of 1, 3 and 5 wt% were prepared.
Results and discussion
Synthesis and characterization of the diamine MDAP
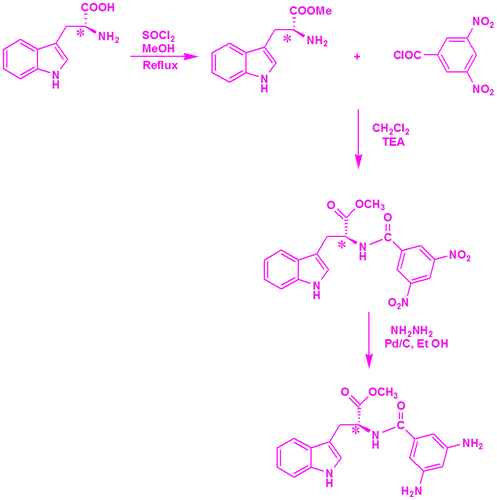
L-Tryptophan methyl ester was prepared by reacting the amino acid L-tryptophan with thionyl chloride and methanol. MDNP was prepared by reacting the L-tryptophan methyl ester with 3,5 dinitrobenzoyl chloride in methylene dichloride (MDC) in the presence of triethylamine under ice-cold conditions. The dinitro compound was then reduced to the diamine MDAP using palladized carbon (Scheme ). The structure of the prepared precursors and diamine was confirmed using FT-IR, 1H and 13C-NMR and mass spectral techniques.
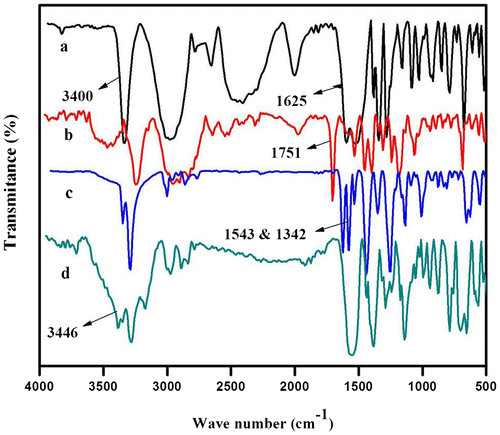
The FT-IR spectra of L-tryptophan, L-tryptophan ester, MDNP and MDAP are illustrated in Figure . The FT-IR spectrum of L-tryptophan (Figure (a)) shows absorption band due to the N–H stretching vibrations of the indole ring at 3400 cm−1 apart from the absorption bands due to aliphatic C–H bond and C=O group of carboxylic acid group at 2900 and 1600 cm−1, respectively. The carbonyl absorption of the ester group in L-tryptophan methyl ester appears at 1751 cm−1 as shown in Figure (b). The presence of NO2 group in MDNP was proved by the presence of absorption bands at 1543 and 1342 cm−1 (asymmetric and symmetric stretching vibrations of the nitro group); (Figure (c)). The absence of –NO2 group and the presence of primary amine are confirmed by the disappearance of bands at 1543 and 1342 cm−1 and the appearance of bands at 3446 and 3352 cm−1 due to asymmetric and symmetric stretching vibrations of the NH2 group (Figure (d)).
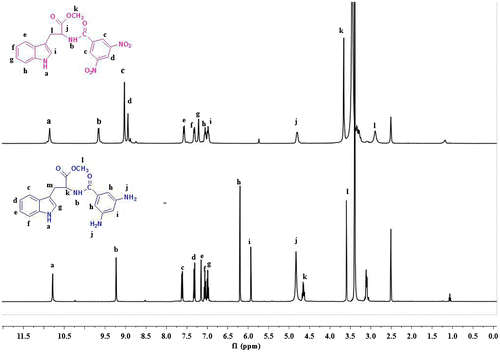
Figure (a) presents the 1H-NMR spectrum of the intermediate MDNP. The characteristic peaks due to the NH proton of the indole ring and the amide proton CO–NH appear at 10.6 and 9.5 ppm, respectively. The two ortho protons of the aromatic ring attached to the amide carbonyl carbon and other proton in the same ring resonate at 9.0 ppm and 8.9 ppm, respectively. These farthest downfield shifts can be explained by the inductive effect of electron withdrawing carbonyl and nitro groups. The singlet observed at 4.7 ppm can be assigned to the C–H proton of the chiral centre of the amino acid moiety, while the methylene protons resonate at 2.7 ppm. Aromatic protons of the indole ring appear between 7.0 and 7.5 ppm.
Figure (b) confirms the conversion of the nitro group into amino group by the singlet at 4.8 ppm corresponding to the protons of the primary amino group. The doublet at 3.1 ppm can be assigned to the methylene protons. The CH proton deshielded by two amino groups appears as singlet at 5.9 ppm. The two ortho protons appear as singlet at 6.2 ppm.
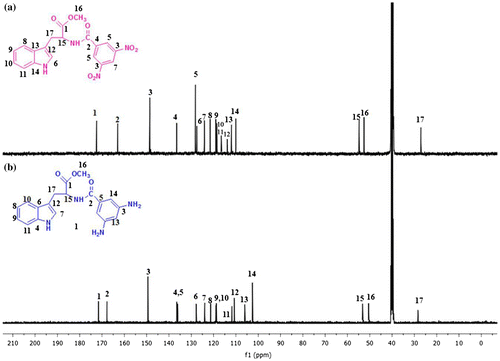
Figure (a) presents the 13C-NMR spectrum of the MDNP. The peak at 162.9 ppm is assigned to the amide carbon (HN–C=O). The methylene carbon and the carbon attached to the amide nitrogen appear at 27.0 and 54.7 ppm, respectively. The peak at 52.6 ppm may be attributed to the methyl carbon of the ester group (COO–CH3). Aromatic carbons appear between 110.0 and 136.5 ppm. The characteristic peak at 148.6 ppm is attributed to the aromatic carbon attached to the nitro group (Ar–NO2). 13C-NMR spectrum of the MDAP is given in Figure (b). The carbon attached to the amino group (Ar–NH2) of the MDAP appears at 149.4 ppm. The alkyl carbons appear between 28.4 and 52.9 ppm, and the aromatic carbons appear between 102.5 and 136.5 ppm.
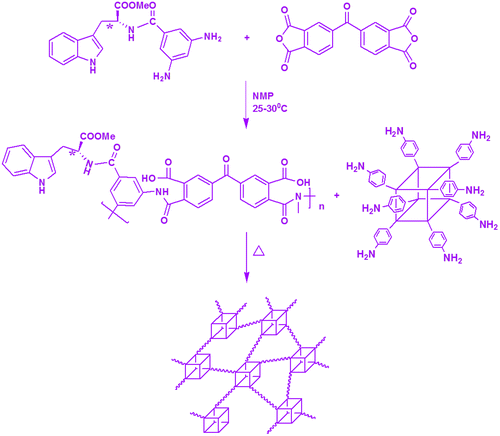
Figure 5. SEM Images of (a) neat MDAP/BTDA–PI (b) MDAP/BTDA–PI/POSS (1 wt%) (c) MDAP/BTDA–PI/POSS (3 wt%) (d) MDAP/BTDA–PI/POSS (5 wt%).
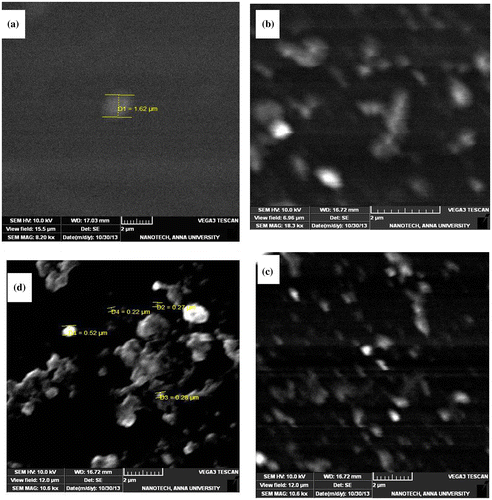
Figure 7. AFM Images of (a) MDAP/BTDA–PI/POSS (1 wt%) (b) MDAP/BTDA–PI/POSS (3 wt%) (c) MDAP/BTDA–PI/POSS (5 wt%).

Figure 8. DSC curves of (a) neat MDAP/BTDA–PI (b) MDAP/BTDA–PI/POSS (1 wt%) (c) MDAP/BTDA–PI/POSS (3 wt%) (d) MDAP/BTDA–PI/POSS (5 wt%).
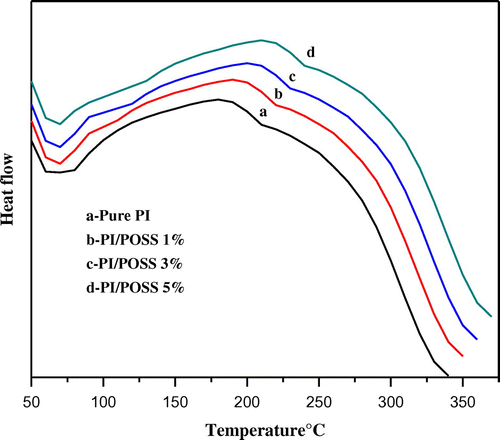
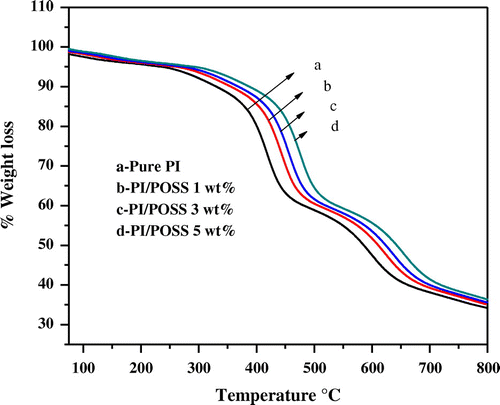
Figure 9. TGA curves of (a) neat MDAP/BTDA–PI (b) MDAP/BTDA–PI/POSS (1 wt%) (c) MDAP/BTDA–PI/POSS (3 wt%) (d) MDAP/BTDA–PI/POSS (5 wt%).
Polyimide and polyimide nanocomposites
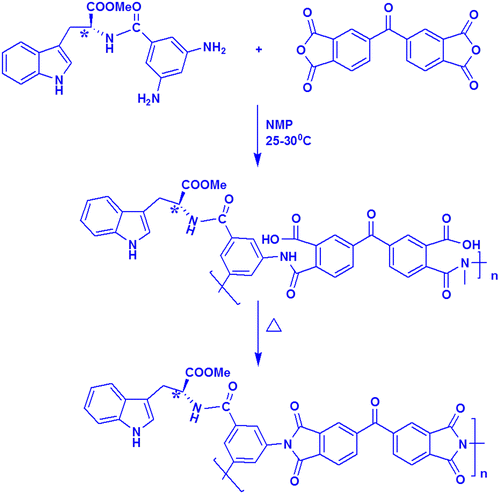
An optically active PI was synthesized by the condensation polymerization of an equimolar mixture of MDAP with BTDA in dry NMP. First, the polyamic acid was prepared by reacting the diamine MDAP with equimolar quantities of the dianhydride BTDA in dry NMP at 25 °C. To prepare the polyimide film (Scheme ), the PAA solution was coated onto a glass plate to obtain an uniform film by thermal imidization reaction by sequential heating (100 °C for 6 h, 150 ◦C for 4 h, 250 °C for 2 h and 300 ◦C for 2 h). The inherent viscosity of the synthesized PI was found to be 0.77 dL/g indicating the formation of high molecular weight polymer and the yield was 90%. The specific rotation of this polyimide is [α]D25 = −41.2°, indicating that the polyimide is optically active and chirality is introduced into the backbone of the polymer. Further, nanocomposites were prepared by adding different ratios of POSS into the PAA solution. The amino group of the functionalized POSS could interact chemically with the PAA matrix to produce thermally stable PI/POSS nanocomposites.
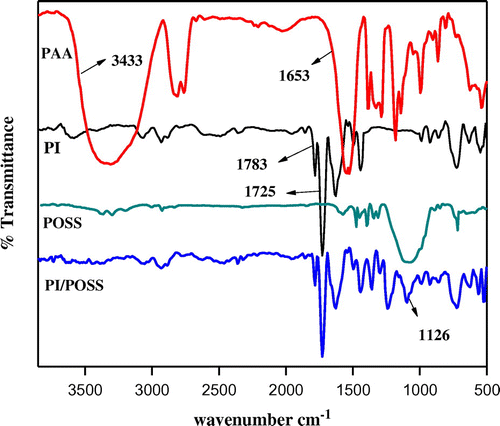
Chemical structure of the PAA, PI and PI/POSS nanocomposite was studied by FT-IR spectroscopy. Figure shows the FTIR spectra of the PAA, polyimide, POSS and polyimide/POSS nanocomposite (with 3 wt% of POSS). The spectrum shows absorption bands due to the stretching vibrations of the O–H bond of the carboxylic acid in PAA appearing at 3433 cm−1. The formation of polyimide was confirmed by the characteristic absorption bands of the imide group around 1783 and 1725 cm−1 due to the asymmetric and symmetric carbonyl stretching vibrations of the imide ring. The bands around 1380 and 716 cm−1 are ascribed to the stretching and bending vibrations of C–N–C in the imide ring (Figure ). The characteristic absorption of the amide carbonyl at 1653 cm−1 did not appear in the spectra of the PI and PI/POSS nanocomposites showing that the imidization reaction was completed. Typical absorption bands for Si-O-Si network vibrations observed at 1126 cm−1 and the absence of the broad and strong band at 3433 cm−1 in PAA due to the stretching vibrations of the COOH confirm the formation of PI/POSS nanocomposites. These bands were not observed in the FTIR spectrum of the pure PI (MDAP/BTDA–PI).
Microstructural analysis
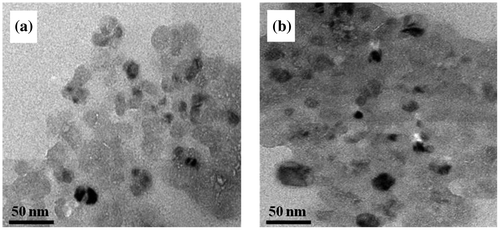
The SEM micrographs show the surface morphology of the neat PI and PI/POSS nanocomposites. The SEM image of neat PI film shows a non-porous and uniform surface (Figure (a)) indicating that the PI film has formed without any defects or cracks. The SEM images of PI/POSS nanocomposites show that the POSS nanoparticles are dispersed homogeneously in the PI matrix. Spherical POSS particles are well dispersed in the PI matrix at the ratio of 1 and 3 wt% (Figure (b) and (c)). It is very interesting to note that the POSS is distributed uniformly in the chiral polyimide matrix also like in optically inactive PIs. Beyond 3%, slight agglomeration of the POSS particles was observed (Figure (d)). The morphology of the nanocomposites was studied further by transmission electron microscopy (TEM) analysis. TEM study of the PI–POSS nanocomposites confirmed that the particles have almost spherical shape. TEM micrographs of PI/POSS nanocomposites (with 3 and 5 wt% of POSS) are shown in Figure (a) and (b). These images indicate that the dispersion of POSS nanoparticles is quite good. In this image, POSS particles can be seen in the form of black spots and particle size is found to be in the range of 30–50 nm. The improvement in the properties of the polymer filled with nanoparticles depends on the available polymer–filler interfacial surface area, which is affected by both the degree of loading and the size of the filler. The particle size of POSS being in the 30–50 nm range, in the PI/POSS nanocomposite is expected to have a large interfacial surface area and hence better properties than neat PI.[Citation26]
AFM was used to further characterize the surface morphology of the PI–POSS hybrids. AFM images of the PI/POSS nanocomposites are shown in Figure . There are many POSS particles (30–50 nm) uniformly dispersed in the polyimide matrix. Further, the AFM images of the PI/POSS nanocomposites also indicate that the size of the nodules formed by the PI/POSS particles are of uniform dimension, showing uniform distribution as evidenced from SEM and TEM images.
Thermal analysis
The DSC curves of the PIs and its nanocomposites with various compositions of POSS are given in Figure . The glass transition temperature of the polyimide and their nanocomposites were observed in the range of 222–250 °C. The prepared neat PI (MDAP/BTDA–PI), having methylene and ester group, displays lower Tg (222 °C) due to enhanced molecular segmental mobility. But in the case of all nanocomposites, increase in Tg can be explained by rigid and symmetrical nature of the silica unit in POSS.[Citation27]
The temperature of 10% weight loss and char yield at 800 °C were obtained from the thermograms, and used as criteria for the evaluation of thermal stability of the polymers. PI/POSS nanocomposite (5 wt%) shows better thermal stability than those containing 1 and 3 wt% POSS. Figure shows the thermal degradation behaviour of the hybrid films and neat polyimide film under nitrogen atmosphere. The results of the TGA for neat polyimide and polyimide hybrid films with different weight percentages of POSS are shown in Table . From the results, it can be seen that the addition of POSS significantly increases the 10% weight loss temperatures than the neat PI. The initial decomposition temperature of the neat PI was about 315.0 °C while it was higher for the obtained nanocomposites. This can be explained by high heat resistance exerted by the POSS nanoparticles. This material acts as thermal barrier heat sink and protects the polymer from undergoing decomposition at normal temperature.[Citation28]
Table 1. TGA, DSC and dielectric constant values of neat polyimide and PI/POSS nanocomposites.
Flame retardancy
The non-flammability of neat PI and PI/POSS nanocomposites is explained in terms of the value of limiting oxygen index (LOI). The LOI values were calculated from the char yield obtained from TGA analysis using Van Krevelan and Hofytzer equation as shown below,
where LOI = limiting oxygen index; CY = char yield. As the POSS content was increased in the nanocomposite films, char yields were also increased from 31.2 to 36.7%. This indicates that the incorporation of POSS in the PI enhances the T10% and char yield. The LOI values of the nanocomposites are in the range of 29–32.1% which is above the threshold value of 26. It shows that all the PI/POSS nanocomposites could be classified as the self-extinguishing and flame-retardant materials.[Citation29]
Dielectric constant
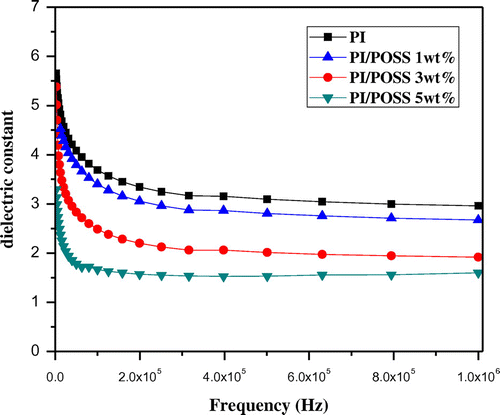
The dielectric constant of PI/POSS nanocomposite films in the frequency range of 0–1 MHz is shown in Figure . The dielectric constant of the hybrid materials decreases as the amount of POSS in the nanocomposites is increased. The low dielectric constant can be attributed to the cubic silica core with higher free volume and lower polarity nature of the POSS than the neat polyimide. The decrease in dielectric constant (from 3.5 to 2.9) by the incorporation of POSS (1 wt%) can be due to the internal free volume of POSS in the polymer and reduced rotational freedom of the polymeric chains due to the presence of rigid and cage POSS.[Citation30] This fact is further confirmed by the dielectric constant of the PI/POSS 3% and PI/POSS 5% nanocomposites being 2.5 and 2.1, respectively.
Conclusions
A new optically active PI and its nanocomposites with POSS have been developed with well-defined architecture through the synthesis of the diamine (MDAP) from L-tryptophan. The SEM, AFM and TEM outcomes indicated that the nanoparticles were dispersed rather homogeneously in the PI matrix on nanoscales. The PI–POSS hybrid films possess higher thermal stability than the pure PI film (T10% 402.4 for neat PI and 470.5 for PI/POSS 5%). The reduced dielectric constant (from 3.5 for neat PI to 2.1 for PI/POSS 5%) of these PI–POSS hybrids can be explained in terms of creating porous silsesquioxane nanocores of the POSS and the increase in free volume by the presence of the rigid and large POSS structure resulting in a loose PI network. Therefore, the new chiral PI and its nanocomposites with POSS are novel materials (with high thermal stability and excellent electrical properties) suitable for electronic industries, non-linear optical devices, optical switches and so on. In addition because of the existence of amino acids in the polymer pendent group, these polymers are expected to be biodegradable and are therefore classified under environmentally friendly polymers.[Citation31,32]
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors acknowledge the University Grand Commission, New Delhi, for funding this project. The authors also acknowledge DST (FIST) and UGC (SAP) for the financial support extended to procure instrumental facilities.
References
- Yang CP, Su YY. Properties of organosoluble aromatic polyimides from 3’- trifluoromethyl-3,4’-oxydianiline. Polymer. 2003;44:6311–6322.10.1016/S0032-3861(03)00684-0
- Hajipour AR, Zahmatkesh S, Ruoho AE. An investigation into the synthesis and characterization of new optically active poly(ester-imide) thermoplastic elastomers, derived from N,N’-(pyromellitoyl)-bis-l-leucine, synthetic diols and polyethyleneglycol-diol (PEG-200). React. Funct. Polym. 2007;67:1040–1051.10.1016/j.reactfunctpolym.2007.06.012
- Ataei SM, Sarrafi Y. Novel thermally stable polyimides based on flexible diamine: synthesis, characterization and properties. Eur. Polymer J. 2004;40:2009–2015.10.1016/j.eurpolymj.2004.05.027
- Zhuo L, Haiwang S, Minhui H, et al. Atomic oxygen-resistant and transparent polyimide coatings from [3,5-bis(3-aminophenoxy)phenyl]phosphine oxide and aromatic dianhydrides: Preparation and characterization. Prog. Org. Coat. 2012;75:49–58.
- Reddy DS, Chou CH, Shu CF, et al. Synthesis and characterization of soluble poly(ether imide)s based on 2,2’-bis(4-aminophenoxy)-9,9’-spirobifluorene. Polymer. 2003;44:557–563.10.1016/S0032-3861(02)00828-5
- Yagci H, Mathias LJ. Synthesis and characterization of aromatic polyamides and polyimides from trimethyl- and di-t-butylhydroquinone-based ether-linked diamines. Polymer. 1998;39:3779–3786.10.1016/S0032-3861(97)10378-0
- Ulbricht M. Advanced functional polymer membranes. Polymer. 2006;47:2217–2262.10.1016/j.polymer.2006.01.084
- Nakano T. Optically active synthetic polymers as chiral stationary phases in HPLC. J. Chromatogr. A. 2001;906:205–225.10.1016/S0021-9673(00)00944-4
- Qiding M, Yu M, Lianxun G, et al. Synthesis and characterization of optically active aromatic polyimides derived from 2,2’-bis(2-trifluoro-4-aminophenoxy)-1,1’- binaphthyl and aromatic tetracarboxylic dianhydrides. J. Polym. Sci., Part A: Polym. Chem. 1999;37:4536–4540.
- Tagle LH, Terraza CA, Ortiz P, et al. Synthesis of oligomeric silicon-containing poly(imide-amide)s derived from trimellitic anhydride and amino acids. Vibration spectral, optical thermal and morphological characterization. J. Macromol. Sci., Part A: Pure Appl. Chem. 2012;49:562–570.
- Baldwin AF, Ma R, Huan TD, et al. Effect of incorporating aromatic and chiral groups on the dielectric properties of poly(dimethyltin esters). Macromol. Rapid Commun. 2014;35:2082–2088.10.1002/marc.v35.24
- Okamura A, Hirai T, Tanihara M, et al. Synthesis and properties of novel biodegradable polyamides containing α-amino acids. Polymer. 2002;43:3549–3554.10.1016/S0032-3861(02)00111-8
- Mallakpour S, Hatami M. Bionanocomposites preparation and characterization: dispersion of surface-modified ZnO nanoparticles in optically active poly(amide-imide) derived from 3,5-diamino-N-(4-hydroxyphenyl)benzamide and amino acid. Des. Monomers.Polym. 2011;14:461–473.10.1163/138577211X587654
- Qin L, He XW, Li WY, et al. Molecularly imprinted polymer prepared with bonded β-cyclodextrin and acrylamide on functionalized silica gel for selective recognition of tryptophan in aqueous media. J. Chromatogr. A. 2008;1187:94–102.10.1016/j.chroma.2008.02.004
- Kusakabe K, Ichiki K, Hayashi J, et al. Preparation and characterization of silica polyimide composite membranes coated on porous tubes for CO2 separation. J. Membr. Sci. 1996;115:65–75.10.1016/0376-7388(95)00290-1
- Leu CM, Chang YT, Wei KH. Polyimide-side-chain tethered polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric film applications. Chem. Mater. 2003;15:3721–3727.10.1021/cm030393b
- Ayandele E, Sarkar B, Alexandridis P. Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials. 2012;2:445–475.10.3390/nano2040445
- Cordes DB, Lickiss PD, Rataboul F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010;110:2081–2173.10.1021/cr900201r
- Kuo SW, Chang FC. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011;36:1649–1696.10.1016/j.progpolymsci.2011.05.002
- Wang F, Lu X, He C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011;21:2775–2782.10.1039/C0JM02785E
- Gnanasekaran D, Madhavan K, Reddy BSR. POSS related polymer nanocomposites. J. Sci. Ind. Res. 2009;68:437–464.
- Govindaraj B, Sundararajan P, Sarojadevi M. Synthesis and characterization of polyimide/polyhedral oligomeric silsesquioxane nanocomposites containing quinolyl moiety. Polym. Int. 2012;61:1344–1352.10.1002/pi.v61.8
- Leu CM, Reddy GM, Wei KH, et al. Synthesis and dielectric properties of polyimide-chain-end tethered polyhedral oligomeric silsesquioxane nanocomposites. Chem. Mater. 2003;15:2261–2265.10.1021/cm0208408
- Balaji S, Sarojadevi M. Synthesis and characterization of optically active polyimides and their octa(aminophenyl)silsesquioxan nanocomposites. High Perform. Polym. 2015:1–15. doi:10.1177/0954008315591021.
- Tamaki R, Tanaka Y, Asuncion M, et al. Octa(aminophenyl) silsesquioxane as a nanoconstruction site. J. Am. Chem. Soc. 2001;123:12416–12417.10.1021/ja011781m
- Chen BK, Chiu TM, Tsay SY. Synthesis and characterization of polyimide/silica hybrid nanocomposites. J. Appl. Polym. Sci. 2004;94:382–393.10.1002/(ISSN)1097-4628
- Li G, Wang L, Ni H, et al. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: a review. J. Inorg. Organomet. Polym. 2001;11:124–154.
- Bourbigot S, Turf T, Bellayer S, et al. Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym. Degrad. Stab. 2009;94:1230–1237.10.1016/j.polymdegradstab.2009.04.016
- Parveen A, Thirukumaran P, Sarojadevi M. Low dielectric materials from fluorinated polybenzoxazines. Polym. Adv. Technol. 2014;25:1538–1545.10.1002/pat.v25.12
- Lee YJ, Huang JM, Kuo SW, et al. Polyimide and polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric applications. Polymer. 2005;46:173–181.10.1016/j.polymer.2004.10.003
- Mallakpour S, Kolahdoozan M. Synthesis and properties of thermally stable and optically active novel wholly aromatic polyesters containing a chiral pendent group. Eur. Polym. J. 2007;43:3344–3354.10.1016/j.eurpolymj.2007.06.006
- Pan H, Qiu Z. Biodegradable poly( l -lactide)/polyhedral oligomeric silsesquioxanes nanocomposites: enhanced crystallization, mechanical properties, and hydrolytic degradation. Macromolecules. 2010;43:1499–1506.10.1021/ma9023685