Abstract
Plastics make considerable amount of solid waste in the worldwide corresponding to their use in many fields of our lives like construction, packaging, building, and so on. The manufacture of fuel-derived plastics is often dangerous to the environment; so, available waste administration systems including energy recovery operations and/or recycling are essential to solve this huge pollution. In this study, poly(ethylene terephthalate) (PET) bottle waste as considerable part of the total plastic waste bulk was recycled and white and fine-recycled PET powders (R-PET) were formed. The obtained R-PET showed semi-crystalline structure according to X-ray diffraction analysis. Then, the effect of functionalized multi-walled CNTs with D-glucose was investigated on R-PET properties. Transmission electron microscopy exhibited good dispersion of MWCNTs-Gl in the R-PET matrix. MWCNT-Gl as a nucleating agent increased the crystallization degree of R-PET.
1. Introduction
Plastics have been extensively used due to their benefits such as durability, cheapness, lightness, and also their usage in many areas like packaging, construction, electronic, and automotive applications. Since they have high degradation temperature, high resistance to UV radiation, and are commonly not biodegradable, they can stay on both sea and land for years generating significant amount of solid waste and environmental pollution in the world. [Citation1,2] At present, polymer recycling contributes in environmental protection as well as in petroleum resources preservation.[Citation3] Packaging is the main post-consumer waste for plastics and poly(ethylene terephthalate) (PET) bottles create considerable part of this region. PET is a low-cost, high-performance, semi-crystalline thermoplastic polymer that is used in the automobile and electronic industries and also has a variety of applications such as textiles, films, industrial fibers, bottle containers, and food packaging.[Citation4,5] However, inadequate thermal stability and mechanical properties of PET have hindered its valuable application in a wide range of industry.[Citation6]
Carbon nanotubes (CNTs) are ideal candidates as reinforcements for thermoplastic polymer composites that can improve the electrical, mechanical, and thermal properties of the polymers.[Citation7] One of the main subjects on polymer/CNT composites is how to improve the dispersion of CNTs in a polymer matrix. In fact, poor interfacial adhesion between the polymer and CNT restrict the improvement of properties in CNT-based composites.[Citation8] So as to disperse the CNTs in the polymer homogeneously, surface functionalization of CNTs is essential. Surface tension of the nanotubes decreases by the surfactant treatment, therefore, it improves the wettability of CNTs by polymer with the aid of functional groups.[Citation9–12]
The main purpose of this study is to examine the influence of CNT surface functionality on the morphology, crystallinity, and thermal properties of the nanocomposites (NCs) based on the recycled PET (R-PET) matrix using d-glucose-treated multi-walled CNTs (MWCNT-Gl). First, recyclable PET bottles were changed to white and fine powder through ultrasonic irradiation-assisted solution route. Then, MWCNT-Gl (1, 2, and 4 wt.% of polymer) was incorporated into the R-PET matrix under sonication conditions. All CNT samples were characterized by means of Fourier transform infrared (FT-IR), X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA), differential thermogravimetric (DTG), and differential thermal analysis (DTA).
2. Experimental details
2.1. Materials
The PET used in this study was obtained from Poroshat waste bottles (1500 mL) and it was used without any further processing. d-glucose, N,N-carbonyldiimidazole (CDI), N,N-dimethylacetamide (DMAc) as solvents were provided by Merck Chemical Co. (Darmstadt, Germany). Dimethylsulfoxide (DMSO) was obtained from DAEJUNG (Korea). The carboxyl-modified MWCNTs (MWCNT-COOH) with outer diameter of 8–15 nm, length of about 30 μm, carboxyl content 2.00 wt.%, and purity of >95 wt.% were purchased from Neutrino Co. (Tehran, Iran) and used without further purification.
2.2. Characterization
TGA, DTG, and DTA data of the composite materials were evaluated by STA503 TA instrument. All samples were dried at 120 °C for 12 h prior to the measurement. Weight loss was monitored from 25 to 800 °C at a heating rate of 20 °C/min in argon atmosphere. FT-IR spectra were recorded using a Jasco-680 (Tokyo, Japan) spectrometer at the wavenumber range of 400–4000 cm−1 [taken in potassium bromide (KBr)]. The morphology and state of MWCNTs dispersion within the polymer were observed by FE-SEM and TEM. FE-SEM and TEM micrographs were obtained using a HITACHI S-4160 instrument (Tokyo, Japan) and Philips CM 120 microscope (Netherlands) with an accelerating voltage of 150 kV. XRD analysis was performed on the PET and their NCs using Philips X’Pert MPD X-ray diffractometer with CuKα radiation of λ = 0.154 nm in the range of 10–80°. The composites were prepared using a mechanical probe sonicator (TOPSONICS, Tehran, Iran) operating at power 400 W.
2.3. Preparation of PET powder
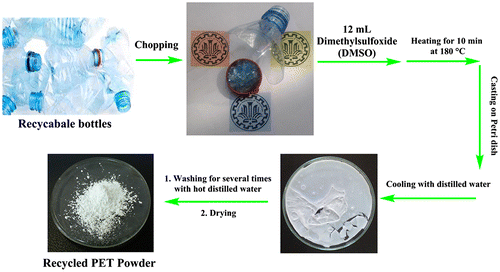
Recycled mineral water bottles were collected and chopped into small pieces of size 5 × 3 mm2. Uniformly chopped pieces of PET waste (3 g) were mixed with DMSO (12 mL). The mixture was heated at 180 °C with continuous stirring until the solution was completely clear and homogeneous (10–20 min). Then, the obtained solution was cast on a Petri dish and was rapidly cooled with distilled water. Finally, it was washed with hot distilled water several times to remove DMSO and was dried in oven at 100 °C for at least 24 h to minimize the effect of moisture and obtain white and fine R-PET powder. All stages for the preparation of PET powder have been represented in Scheme .
2.4. Surface functionalization of MWCNT with d-glucose
Surface functionalization of MWCNTs with glucose (MWCNT-Gl) was performed as previously reported.[Citation13] Schematic illustration of MWCNT functionalization is shown in Scheme .
2.5. Preparation of R-PET/MWCNT-Gl NCs
Composites containing 1, 2, and 4 wt.% of MWCNT-Gl were prepared through sonication in solution: at first, DMAc (16 mL) was added to a vessel containing 0.1 g of R-PET. It was sonicated for 15 min to be partially dissolved. Then, MWCNTs-Gl was added to the mixture and it was sonicated for 1 h. The resulting dispersion mixture was centrifuged for 10 min at 6000 rpm. Finally, it was further dried at 120 °C for 24 h to remove the residue solvent and a black solid was formed.
3. Results and discussion
3.1 Functionalization of MWCNTs
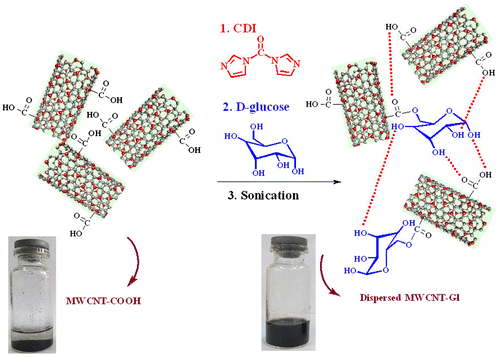
MWCNTs usually tend to bundle together due to high aspect ratio and large surface area in combination with the van der Waals attractions between the individual nanotubes. It causes difficult dispersion of MWCNTs in the polymer matrix.[Citation14] In this study, the chemical modification was accomplished to achieve uniform dispersion and good interfacial adhesion between MWCNTs and R-PET matrix. As it was shown in Scheme , MWCNT-COOH was functionalized with glucose as a biomolecule under an esterification reaction, catalyzed by CDI. Reaction was run in water solvent system as green media. Scheme also shows the stability of dispersions of MWCNTs-COOH and MWCNTs-Gl in DI water after 30 min of sonication. MWCNTsGl indicated highly dispersed and stable dark solution after 2 months of storage at room temperature, whereas the MWCNTs-COOH settled in water after three weeks.
3.2 Composite preparation
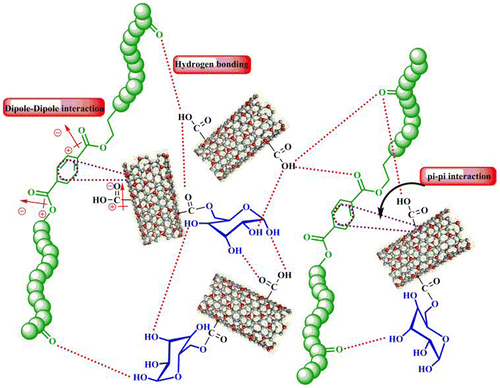
Recently, increased usage of synthetic polymers has caused the production of a significant amount of wastes based on polymer. They generally transfer hazard to the environment.[Citation15,16] One of the most successful examples of polymer recycling is PET recycling. This compound is fully recyclable and may be applied for manufacturing new produces in various industrial fields.[Citation17] Formation of an NC is an approach to further improve the properties of this polymer which can be reached by the addition of CNTs or other nanomaterials.[Citation18,19] In order to employ MWCNT-Gl as an effective reinforcement in PET composites, proper interfacial adhesion between the CNTs and polymer matrix is essential. In the first step, MWCNTs were functionalized with glucose to increase functional groups on the surface of nanotubes. Actually, MWCNTs-Gl stabilized their dispersion in the PET NCs through hydrogen bonding creation between hydroxyl groups on their surfaces and C=O groups in the R-PET. Also, there are aromatic rings in the R-PET backbone and these structures have strong interactions with CNT surfaces through π–π interactions. In addition to the mentioned interactions, there are dipole–dipole attractions too as illustrated in Scheme .
3.3. FT-IR spectroscopy
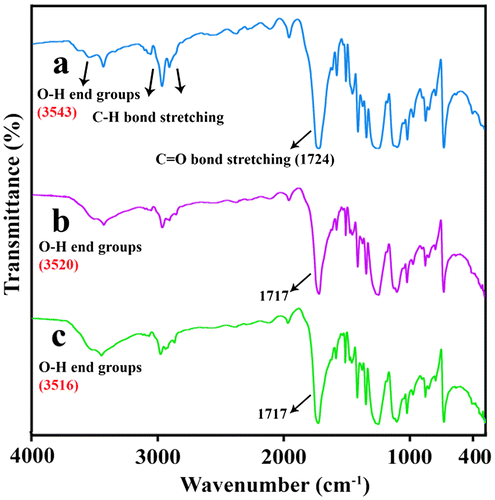
FT-IR spectrum of R-PET powder is presented in Figure (a). The presence of C = O bond stretching in conjugation with aromatic ring was appeared at 1724 cm−1. The absorption bands at 2906 and 2964 cm−1 were ascribed to C–H bond stretching (methylene groups). Hydroxyl end groups were observed at 3543 cm−1 and a peak at 3431 cm−1 was corresponding to the carbonyl overtone. The splitting of O–C–C asymmetric stretching was seen at 1119 and 1097 cm−1.[Citation20–23] Figures (b) and (c) show the spectra of R-PET/MWCNT-Gl NCs (2 and 4 wt.%). The observed shift in position of the hydroxyl stretching modes to a lower wavenumber, i.e. lower energy for all NCs, can be explained by the formation of hydrogen bonding between the MWCNT-Gl and R-PET medium.[Citation24]
3.4. Crystalline structure
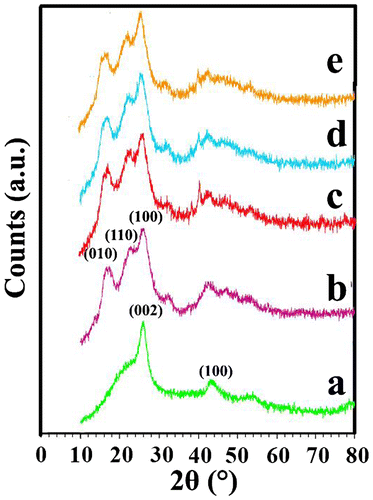
Figure shows the XRD patterns for the R-PET, MWCNT-Gl, and R-PET/MWCNT-Gl NCs of 1, 2, and 4 wt.%, respectively. R-PET displayed a semi-crystalline XRD pattern with well-defined reflection peaks at 2θ = 16.8°, 2θ = 22.9°, and 2θ = 26.3° corresponds to the (0 1 0), (1 1 0), and (1 0 0) planes which relates to a triclinic unit cell.[Citation25] The XRD patterns of the NC samples demonstrated narrow crystalline peaks, which are typical of R-PET.
3.5. Dispersion of CNTs determined by FE-SEM and TEM
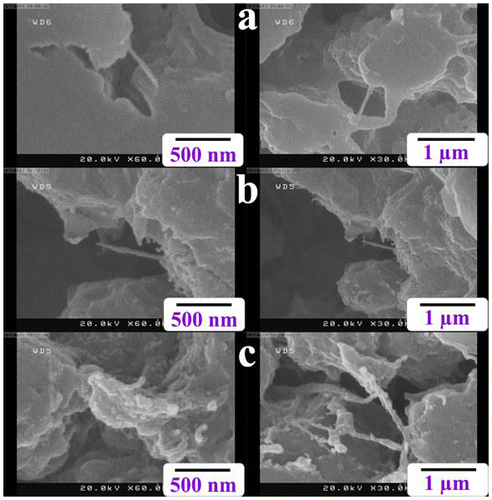
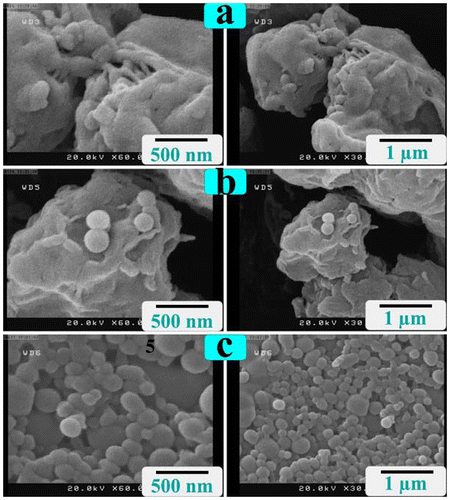
To disperse R-PET and to choice a proper solvent for the preparation of NCs, two solvents were employed, and different surface morphologies obtained, as they were shown in Figure . FE-SEM micrographs show that the original shape of R-PET was kept after undergoing ultrasonication in ethanol (Figure (b)). R-PET with spherical shapes was obtained when DMAc served as the dispersion medium (Figure (c)). Actually, the ultrasonication in DMAc caused the size of R-PET be closed to nanometer with a uniform morphology which sureties a strong interaction between nanofillers and the polymer. So, it was selected as a media for the NCs preparation.
Figure 3. FE-SEM micrographs of (a) R-PET before sonication, (b) R-PET after sonication in ethanol, and (c) R-PET after sonication in DMAc.
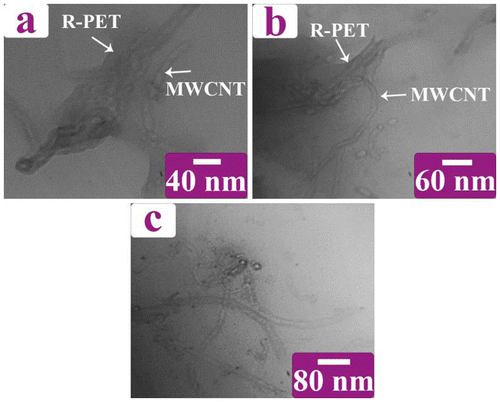
The dispersion of nanotubes into the R-PET matrix was assessed by FE-SEM and TEM micrographs too. Bright spots in the FE-SEM micrographs showed MWCNTs-Gl which have been surrounded by the polymer matrix revealing a good dispersion of nanotubes in the R-PET (Figure ). TEM images of the R-PET/MWCNT-Gl NC 4 wt.% at various magnifications are shown in Figure . This figure gives a view of the dispersed nanotubes. It also confirmed the presence of MWCNTs-Gl in the polymer matrix.
3.6. Thermal stability
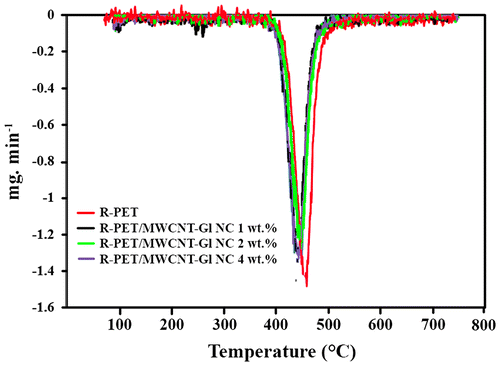
Figure shows TGA thermograms of the R-PET and R-PET/MWCNT-Gl NCs of 1, 2, and 4 wt.%. The char residue of the samples at 700 °C were 18, 18, 22, and 21 wt.% for R-PET and NCs of 1, 2, and 4 wt.%. The R-PET/MWCNT-Gl NC of 2 and 4 wt.% exhibited higher char yield than R-PET. A well-defined DTG peak (Tmax) centered at 460 °C is seen for R-PET which values for R-PET/MWCNT-Gl NCs of 1, 2, and 4 wt.% were 427, 434, and 433 °C, respectively (Figure ). This main weight loss could be attributed to the partial degradation of the polymer. The reduction in initial decomposition temperatures of the obtained NCs could be ascribed to the removal of d-glucose and other functional groups of MWCNTs bonded to the polymer chains which do not exist in the pure PET.[Citation26]
Figure 6. TGA thermograms of (a) R-PET, (b) R-PET/MWCNT-Gl NC 1 wt.%, (c) R-PET/MWCNT-Gl NC 2 wt.%, and (d) R-PET/MWCNT-Gl NC 4 wt.%.
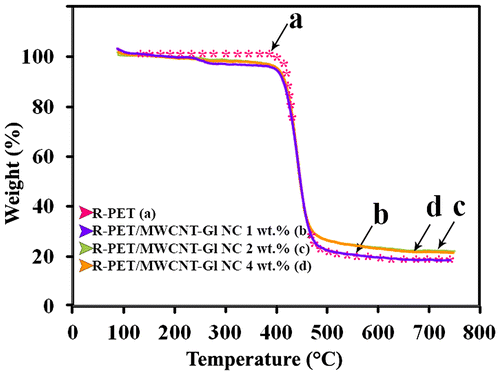
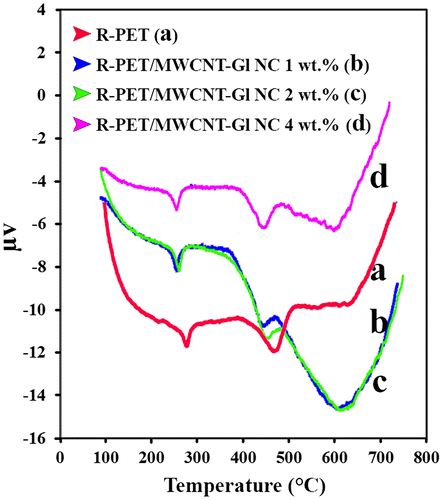
Figure demonstrates DTA curves for R-PET and NCs. The endothermic peak at ~220 °C is ascribed to the melting–transition temperature (Tm).[Citation27,28] The endothermic peak of Tm for R-PET is recorded at 270 °C and its value is directly related to the nanofiller loading.[Citation27,28] Tm values for NCs of 1, 2, and 4 wt.% were 253, 250, and 243 °C, respectively. DTA data show that the melting points are substantially shifted. The introduction of MWCNTs decreased the melting point of the composites. It seems that the interaction of nanotubes with this polar polymer matrix could weaken intermolecular interactions between the polymer chains.
4. Conclusions
PET bottle waste was recycled and changed to white and fine-recycled PET powder. Then, novel R-PET/MWCNT NCs with 1, 2, and 4 wt.% MWCNT content were prepared through ultrasonic irradiation-assisted solution method. To improve the compatibility between the R-PET polymer and nanotubes, MWCNT-COOH was functionalized with d-glucose carbohydrate as biomolecule. When, DMAc served as the dispersion medium, spherical shapes of R-PET with smaller size were gained. FT-IR spectra showed shift in the position of hydroxyl-stretching modes to a lower wavenumber due to the creation of intermolecular hydrogen bonding between the nanotubes and the polymer. TEM micrographs represented well-dispersed nanotubes in the polymer matrix. The addition of MWCNT-Gl could weaken intermolecular interactions between the R-PET chains and decreased the melting points of the composites.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
Supports from the Research Affairs Division of Isfahan University of Technology (IUT), National Elite Foundation (NEF), and Center of Excellency in Sensors and Green Chemistry Research (IUT) are gratefully acknowledged.
References
- Guru M, Cubuk MK, Arslan D, et al. An approach to the usage of poly)ethylene terephthalate (PET waste as roadway pavement material. J. Hazard. Mater. 2014;279:302–310.
- Wang CQ, Wang H, Liu YN. Separation of polyethylene terephthalate from municipal waste plastics by froth flotation for recycling industry. Waste Manage. 2015;35:42–47.10.1016/j.wasman.2014.09.025
- Yang P, Zhou Q, Li XY, et al. Chemical recycling of fiber-reinforced epoxy resin using a polyethylene glycol/NaOH system. J. Reinf. Plast. Compos. 2014;33:2106–2114.10.1177/0731684414555745
- Bizarria MTM, Giraldi ALF de M, de Carvalho CM, et al. Morphology and thermomechanical properties of recycled PET–organoclay nanocomposites. J. Appl. Polym. Sci. 2007;104:1839–1844.10.1002/(ISSN)1097-4628
- Rodríguez-Uicab O, May-Pat A, Avilés F, et al. Influence of processing method on the mechanical and electrical properties of MWCNT/PET composites. J. Mater. 2013;2013:1–10.10.1155/2013/656372
- Kim JY, Kim SH. High performance PET/carbon nanotube nanocomposites: preparation, characterization, properties and applications. InTech. 2012;97–121. doi:10.5772/50413.
- Yesil S, Bayram G. Poly(ethylene terephthalate)/carbon nanotube composites prepared with chemically treated carbon nanotubes. Polym. Eng. Sci. 2011;51:1286–1300.10.1002/pen.v51.7
- Zaman HU, Hun PD, Khan RA, et al. Effect of multi-walled carbon nanotubes on morphology, mechanical and thermal properties of poly(ethylene terephthalate) nanocomposites. Fuller. Nanotubes Carbon Nanotubes. 2013;21:701–711.10.1080/1536383X.2012.654540
- Mallakpour S, Abdolmaleki A, Borandeh S. l-Phenylalanine amino acid functionalized multi walled carbon nanotube (MWCNT) as a reinforced filler for improving mechanical and morphological properties of poly(vinyl alcohol)/MWCNTcomposite. Prog. Org. Coat. 2014;77:1966–1971.10.1016/j.porgcoat.2014.07.005
- Mallakpour S, Zadehnazari A. The effect of carboxylated multi-walled carbon nanotubes on reinforcement efficiency of thiazole bearing poly(amide-imide) composites. Des. Monomers Polym. 2014;17:275–285.10.1080/15685551.2013.840502
- Song YS, Youn JR. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon. 2005;43:1378–1385.10.1016/j.carbon.2005.01.007
- Aroon MA, Matsuura T, Ismail AF. A comparison between β-cyclodextrin and chitosan as soft organic materials for surface modifcation of MWCNTs. Int. J. Nanosci. Nanotechnol. 2012;8:71–78.
- Mallakpour S, Zadehnazari A. One-pot synthesis of glucose functionalized multi-walled carbon nanotubes: dispersion in hydroxylated poly(amide-imide) composites and their thermo-mechanical properties. Polymer. 2013;54:6329–6338.10.1016/j.polymer.2013.09.048
- Mallakpour S, Soltanian S. Studies on preparation and microstructure characterization of novel composites based on functionalized multiwalled carbon nanotubes and chiral poly(ester-imide) containing S-valine linkages. Polym. Plast. Technol. Eng. 2014;53:1583–1589.10.1080/03602559.2014.919640
- Strain IN, Wu Q, Pourrahimi AM, et al. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A. 2015;3:1632–1640.10.1039/C4TA06191H
- Webb HK, Arnott J, Crawford RJ, et al. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers. 2013;5:1–18.
- Ptièek Siroèiæ A, Fijaèko A, Hrnjak-Murgiæ Z. Chemical recycling of postconsumer poly(ethylene-terephthalate) bottles – depolymerization study. Chem. Biochem. Eng. 2013;27:65–71.
- May-Pat A, Avilés F, Toro P, et al. Mechanical properties of PET composites using multiwalled carbon nanotubes functionalized by inorganic and itaconic acids. eXPRESS Polym. Lett. 2012;6:96–106.
- Mallakpour S, Zadehnazari A. Chiral poly(amide-imide)/carbon nanotube bionanocomposites containing hydroxyl pendant groups and L-phenylalanine amino acid: synthesis, preparation of thin films, and thermomechanical behavior. Soft Mater. 2012;11:494–502.
- Mamoor GM, Shahid W, Mushtaq A, et al. Recycling of mixed plastics waste containing polyethylene, polyvinylchloride and polyethylene terephthalate. Chem. Eng. Res. Bull. 2013;16:25–32.
- Vijayakumar S, Rajakumar PR. Infrared spectral analysis of waste pet samples. Int. Lett. Chem. Phys. Astron. 2012;4:58–65.
- Umamaheswari S, Murali M. FTIR spectroscopic study of fungal degradation of poly(ethylene terephthalate) and polystyrene foam. Chem. Eng. 2013;64:19159–19164.
- Oromiehieh A, Meldrum IG. Characterization of polyethylene terephthalate and functionalized polypropylene blends by different methods. Iranian Polym. J. 1999;8:193–204.
- Fortunati E, Puglia D, Monti M, et al. Cellulose nanocrystals extracted from okra fibers in PVA nanocomposites. J. Appl. Polym. Sci. 2013;128:3220–3230.10.1002/app.v128.5
- Cruz-Delgado VJ, Ávila-Orta CA, Espinoza-Martínez AB, et al. Carbon nanotube surface-induced crystallization of polyethylene terephthalate (PET). Polymer. 2014;55:642–650.10.1016/j.polymer.2013.12.029
- Mallakpour S, Abdolmaleki A, Rostami M. Hybrid S-valine functionalized multi-walled carbon nanotubes/poly(amid-imide) nanocomposites containing trimellitimidobenzene and 4-hydroxyphenyl benzamide moieties: preparation, processing, and thermal properties. J. Mater. Sci. 2014;49:7445–7453.10.1007/s10853-014-8449-z
- Herrero M, Martínez-Gallegos S, Labajos FM, et al. Layered double hydroxide/polyethylene terephthalate nanocomposites. Influence of the intercalated LDH anion and the type of polymerization heating method. J. Solid State Chem. 2011;184:2862–2869.10.1016/j.jssc.2011.08.017
- Martínez-Gallegos S, Herrero M, Rives V. In situ microwave-assisted polymerization of polyethylene terephtalate in layered double hydroxides. J. Appl. Polym. Sci. 2008;109:1388–1394.10.1002/(ISSN)1097-4628