Abstract
A series of chains of triblock amphiphilic copolymers were synthesized by atom transfer radical polymerization (ATRP) techniques and post modified to polymeric dispersant for waterborne paint. Poly(butyl acrylate (BA))-b-poly(hydroxy ethyl methacrylate (HEMA))-b-poly(methyl methacrylate (MMA)) triblock copolymers having predetermined molecular weights were synthesized by ATRP using CuBr, 2-bromoisobutyrate, and pentamethyldiethylenetriamine as a catalytic system in dioxane at 80 °C. The copolymers were further reacted with cyclic chlorophosphate and triethyl amine to form dispersible modified poly(BA-HEMA-MMA). The synthesized copolymers were structurally evaluated by Fourier transform infrared, 1H NMR, 31P NMR, gel permeation chromatography (GPC), energy dispersive X-ray spectroscopy, and their hydroxyl equivalent, respectively. The surface activity of modified copolymers as dispersing additives was investigated by the surface tension analysis and wetting ability test. The ability of additives to function as wetting and dispersing agents was evaluated by analyzing their mechanical, optical, chemical, and rheological properties of water-based paints at different pigment volume concentrations. The effects of the chain length of copolymers on dispersibility and optical properties were studied. The optical properties of paints suggested that the dispersibility of modified poly (BA)-b-poly (HEMA)-b-poly (MMA) (MPBHM) increased with an increase in the molecular weight of the copolymer.
1. Introduction
Polymeric dispersing additives had established excellent performance for pigment stabilization mainly in water-based paint system.[Citation1] In waterborne system, dispersing agents must fulfill the some necessary requirements like it should not only allow fast wetting of pigment surface, but also assist in the stabilization of dispersed of pigment particles and for preventing the re-agglomeration of pigment particles for a long time during storage condition. However, the additional role of polymeric dispersant during the dispersion is to break down the agglomerates into fine particle size, which helps to provide high gloss, minute haze, and gives broad varieties of color shades when different pigment concentrates can be mixed.[Citation2–4] To achieve all these essential properties, the wetting and dispersing additives have to wet the pigment surface very fast and stabilized them for a longer duration of time which makes the coating esthetic and durable.
Polymeric pigment dispersants are the block copolymers which are characterized by their balanced chemical structure as they possess selective pigment affinic groups and soluble polymeric chains [Citation5] which not only assist in the effective dispersion of pigment particles in paint manufacturing, but also reduce the interfacial surface tension. Depending on the paint system (solvent and water-based), different mechanism for the stabilization of pigment exist.[Citation6] Solvent-based paint system, the stabilization of pigment particles was achieved by the steric stabilization mechanism, whereas in case of the aqueous system electrical stabilization is predominant. According to an adsorption–desorption phenomenon of polymer parts of the pigment surface, polymer with block copolymer are more beneficial due to the arrangement of the adsorbing parts of polymer to the pigment surface. Among different polymer architectures, block copolymers are well-matched to act as pigment dispersants.[Citation7] The basic invent is that they consist of both steric, stabilizer block, and a second block which carries pigment affinic groups. In this category, especially acrylic block copolymers have attracted much attention due their some inherent properties like transparency, UV resistance, water and alkali resistance, etc. However, the polymeric wetting and dispersing agents are much more effective in performance in comparison with the oligomer type of dispersant, because the number of anchoring groups and architecture of polymer can be designed by using suitable polymerization techniques.[Citation8]
In last decades, great advances have been made in the field of controlled free radical polymerizations by suppressing the contribution of termination and chain transfer reactions.[Citation9–14] In recent years, new methods for controlled free radical polymerization like nitroxide-mediated,[Citation15] reversible addition fragmentation chain transfer polymerization,[Citation16] ATRP have come up as an attractive and commercially viable alternatives. Among them ATRP was proven to be a very successful technique for the synthesis of triblock copolymers by the reason of macroinitiator can commence the polymerization of the second monomer to form an AB–diblock copolymer and further diblock macroinitiator can be polymerized with another monomer to form triblock copolymer.[Citation17–23]
ATRP technique plays vital role in the development of polymeric dispersant to provide an efficient path with precise control over molecular weight distribution.[Citation24] The synthesis of functional block copolymers and their modifications is one of the possible move toward the appropriate structural design of polymer chains with various functional groups leads to change in their physicochemical properties. Reuter et al. and Silber et al. studied the synthesis of block copolymers as dispersing agents and their effects on the pigment dispersion in water-based paint formulations.[Citation25,26] Jagtap et al. synthesized a block copolymer by reversible addition-fragmentation chain transfer polymerization; the copolymer was used as a wetting and dispersing agent and an emulsifier.[Citation27,28] Hence, controlled radical polymerization is the efficient tool for the development of polymeric pigment dispersants mainly for organic and inorganic pigments.
Modification of hydroxyl functional polymers with poly(2-(methacryloyloxy) ethyl phosphoryl choline) has prospective application in a series of fields like biomedical science, surface chemistry, and other biological fields, due to its distinct properties such as flexibility of chains and solubility in water.[Citation29,30] The block copolymer consists both hydrophilic and hydrophobic blocks are of significant importance for various applications and these materials could be used as surfactants, pigment dispersant.
In the present work, block copolymers poly(butyl acrylate (BA)-hydroxy ethyl methacrylate (HEMA)-methyl methacrylate (MMA)) having predetermined molecular weight, i.e. 10,000,15,000, and 20,000 with constant monomer composition were successfully designed by ATRP. Further, the hydrophilic block of copolymer. i.e. poly(HEMA) was modified to impart phosphate moiety which cannot only improve the water compatibility of copolymers, but also assists in the reduction of interfacial surface tension. These modified block copolymers with different molecular weight have been evaluated for their ability to disperse pigment particle in waterborne paint of different pigment volume concentration. From the results, it was concluded that the modified copolymers were effectively assisted in the dispersion and stabilization of pigment particles in during paint manufacturing.
2. Experimental
2.1. Materials
BA, MMA was purchased from SD Fine Chem. Ltd, Mumbai (India). The inhibitor was separated by washing with 5% NaOH and passed through sodium sulfate. Copper (I) bromide (CuBr) pentamethyldiethylenetriamine (PMDETA), HEMA were purchased from Aldrich and used without purification. Ethyl 2-bromoisobutyrate was purchased from Spectrochem Ltd, Mumbai (India). Ethylene glycol, triethyl amine, dioxane, benzene, and POCl3 were purchased from SD Fine Chem. Ltd., Mumbai (India). Acrylic emulsion (AC-261Active content 50%) was procured from Indofil Chemical Co. Ltd and TiO2 (Rutile) was purchased from DuPont (India). Talc was purchased from Royalty minerals (Mesh size, 300), Mumbai, India. Cyclic ethylene chlorophosphate was synthesized in the lab using previously reported method.[Citation31] Water used for the experiments was purified by Millipore Qplus water purification system (resistance 18 MΩcm).
2.2. Preparation of polybutylacrylate Macroinitiator by ATRP (PBA)
In this experiment, BA (17.6 g, 0.1373 mol) and dioxane 15 g were introduced in three necked flask followed by CuBr (0.8423 g, 0.0058 mol), PMDETA (1.01 g, 0.0058 mol) and equipped with heating mantle, temperature controller, overhead stirrer, and condenser, which was then sealed with rubber septum. The flask contents were purged with nitrogen to remove any dissolved oxygen and followed by addition of ethyl 2-bromoisobutyrate 1.14 g (0.0058 mol). The polymerization was carried out at 80 °C. The reaction progress was determine by gravimetric analysis and the reaction mixture was viscous in 4 h. The resulting crude product was dissolved in tetrahydrofuran and the transition metal complex was removed by passing the solution through the column of neutral Al2O3. Then the solvent was removed by rotatory evaporator. The formulation for synthesis of polybutylacrylate macroinitiator (PBA) shown in Table .
Table 1. Data of molecular weight for PBA by ATRP at 80 °C in dioxane.
2.3. Chain extension to synthesis of PBA-b-Poly (Hydroxyethylmethacrylate) (PHEMA) by ATRP
Measured quantity of previously synthesized and purified PBA-Br macroinitiator 15.4 g (0.0052 mol) was dissolved in 10 g of dioxane and introduced into a three neck flask, which contain CuBr (0.75 g, 0.0052 mol), PMDETA (0.91 g, 0.0052 mol), and HEMA (26.46 g, 0.20 mol) under nitrogen purging. The polymerization was carried out at 80 °C till desired conversion was obtained. The progress of reaction was monitored by solid content determination. The crude product was dissolved in tetrahydrofuran and solution was passed through a column of Al2O3 for removal of transition metal complex. Then solvent was removed by rotatory evaporator. The data of block copolymerization from PBA by ATRP shown in Table .
Table 2. Data of block copolymerization from PBA by ATRP at 80 °C in dioxane.
2.4. Chain extension to synthesis of PBA-b-PHEMA-b-PMMA (PBHM) from PBA-b-HEMA-Br
In the usual experiment a dry three neck flask was charged with CuBr (0.54 g, 0.0038 mol), PMDETA (0.54 g, 0.0038 mol), and dioxane (20 ml) under the continuous purging of nitrogen to remove the oxygen in the flask followed by addition of PBA-b-PHEMA-Br macroinitiator (48 g, 0.0038 mol). Subsequently, copolymerization was carried out at 80 °C. The progress of the reaction was monitored by solid content determination and the resultant PBA-b-PHEMA-b-PMMA triblock co polymer was diluted in tetrahydrofuran and passed through a column of Al2O3 for removal of transition metal complex. Then solvent was removed by rotary evaporator. Data of triblock polymerization from PBA-b-PHEMA by ATRP shown in Table .
Table 3. Data of triblock polymerization from PBA-b-PHEMA by ATRP at 80 °C in dioxane.
2.5. Phosphorylation of PBA-b-PHEMA-b-PMMA (MPBHM)
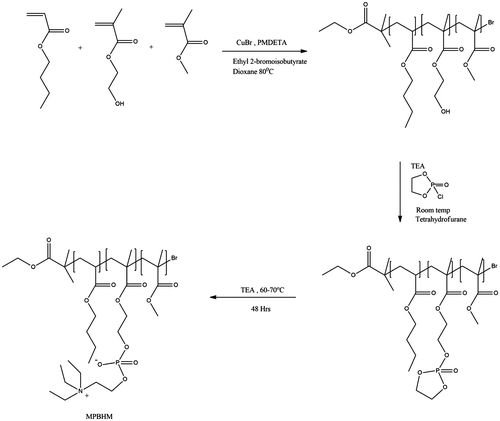
PBHM 10 g was dissolved in 20 g of anhydrous tetrahydrofuran in 100 ml two neck round bottom flask. Into the reaction mixture, triethyl amine (3.410 g, 0.033 mol) was charged and stirred for 2 h, followed by drop wise addition of cyclic ethylene chlorophosphate (4.8 g, 0.033 mol). Afterwards, the reaction was stirred for 4–5 h at room temperature and the solid by-product (salt of triethyl ammonium chloride) was removed by filtration and excess solvent was removed by the rotary evaporator which gives intermediate shown in Scheme . In the last step intermediate, 8 g were dissolved in 20 g tetrahydrofuran (THF) and charged in 100 ml reactor and triethyl amine (2.72 g, 0.026 mol) was added and heated to 65–70 °C with stirring for 2 days and the desired product MPBHM was obtained. A similar procedure was used to phosphorylation of other copolymers. The reaction scheme for the synthesis of triblock copolymer and post modification is shown in Scheme and recipe of modification given in Table .
Table 4. Recipe of post modification of PBHM.
3. Characterization and measurements
3.1. Physicochemical analysis of copolymers
The hydroxyl value of the PBHM’s synthesized was evaluated by acetic anhydride-pyridine method ISO 4629-1978 (E). The hydroxyl values were calculated by using an equation
where N = normality of alcoholic KOH solution; B = burette reading, for blank solution; S = burette reading for sample analyzed; W = weight of the sample analyzed.
3.2. Fourier transform infrared
Fourier transform infrared characterization was recorded on a Shimadzu 8400s spectrometer (Japan). All the spectra were measured at a 4 cm−1 resolution and scanned from 4000 to 400 cm−1; a total of 25 scans for each spectrum were performed.
3.3. 1H NMR spectroscopy (1H NMR and 31P NMR)
1H NMR and 31P NMR spectrum of sample were done using Bruker Biospin (Advance AV500WB, Germany) recorded at 400 MHz in CDCl3.
3.4. GPC
Molecular weight and polydispersity index (PDI) of the copolymers were determined by GPC (Waters Co. USA) equipped with a refractive index detector (Waters 4714), HPLC pump (Waters 501), and a series of Styragel HT 2 columns (pore size: 10 μm, 7.8 mm × 300 mm). Tetrahydrofuran was used as an eluant at a flow rate of 1 ml/min and a pump pressure of 3 mPa at 40 °C. Polystyrene was used as an internal standard.
3.5. Energy-dispersive X-ray spectroscopy (EDXS)
Energy-dispersive X-ray spectroscopy (EDXS) analysis of MPBHM was carried out using Quanta 200 SEM instrument (FEI Company, USA). The EDX were recorded for measurement of percentage of elemental content.
3.6. Measurement of surface activity
Surface tension and interfacial tension was measured against n-heptane was determined at 25 °C, using Kruss tensiometer (k 100), using standard platinum rod (PL03) as probe for measurement.
3.7. Measurements of wetting time
The canvas was cut into 1-inch diameter discs having an average weight of 0.55 g. The disc was floated on the surface of copolymer solution 1% w/v and the stopwatch started. The time required for the disc to begin to sink was measured accurately and noted as the wetting time.
3.8. Qualitative analysis of functional block copolymers
All three synthesized functional block copolymers were analyzed qualitatively for the confirmation of quaternary nitrogen using CTAB as standard.
3.9. Evaluation of pigment dispersant and paint properties
3.9.1. Degree of dispersion
The degree of fineness of the grinded pigment was measured by using Hegman gage according to ASTM D1210-05.
3.9.2. Determination of paint viscosity
The viscosities of paints were determined using a Rheometer (MCR 101, Anton Paar, Germany) by cone and plate geometry with spindle CP-35-2, at room temperature. The viscosities were analyzed at constant shear rates, according to ASTM D4287-00.
3.10. Optical properties
3.10.1. Whiteness index and yellowness index
Color values such as the whiteness index, yellowness index, spectral reflectance values, and chromatic strength of the samples were measured using a reflectance spectrophotometer (Color Eye 7000 A, GretabMacbeth, USA). The whiteness index was determined using the CIE Granz 82 method, and the yellowness index was determined using the ASTM-E313-73 (D1925) method.
3.10.2. Gloss of paint
The gloss of dry paint was measured on a digital mini gloss meter (Rhopoint Instruments, ASTM D 523-99).
4. Result and discussion
4.1. Physicochemical analysis
The hydroxyl value of triblock copolymer was determined as per ISO 4629-1978 (E). Physicochemical analysis of PBHM are shown in Table . Hydroxyl value of copolymers is important for further modification of poly(HEMA) block.
Table 5. Hydroxy value of PBHM.
4.2. FT-IR analysis of copolymers
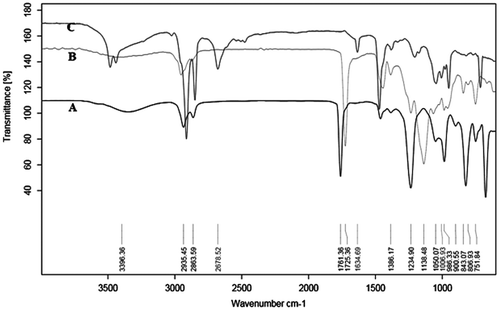
The FT-IR spectra in Figure evaluate the copolymer and modified copolymer before and after reaction with cyclic ethylene chlorophosphate and triethyl amine respectively. Spectrum A) Shows the sharp characteristic bands of the copolymer at 1750–1720 cm−1 due to C=O stretching vibration of ester linkage and symmetric methylene modes at 2853 cm−1. The characteristic peak for hydroxyl group was observed at 3396 cm−1. However, in a spectrum (B) the disappearance of peak at 3396 cm−1 confirms the reaction of hydroxyl groups with cyclic ethylene chlorophosphate and new peak appears due to asymmetric phosphate group vibration at 1232 cm−1 observed to be useful vibrational modes at 1050 cm−1 due to (–P–O–CH2–) and P=O stretching frequency at 1391 cm−1, respectively. Furthermore, in a spectrum (C) the characteristic peak in 986 cm−1 due to –(N + (CH2–CH3)3) and these confirms the quaternization of triblock copolymer.
4.3. 1H NMR of PBHM and MPBHM
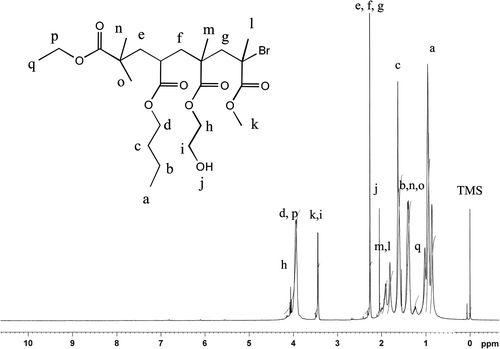
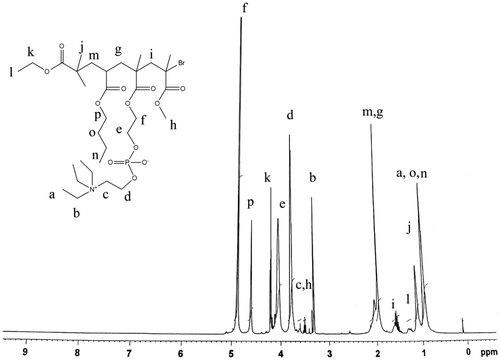
The synthesized copolymers were also characterized by NMR spectroscopy to confirm the modification of copolymers with phosphates ester and with quaternized amine. The nuclear magnetic resonance (NMR) spectrum of PBHM is shown in Figure . The terminal methyl protons in long chains of BA were confirmed by a peak triplet at 0.96 ppm (a, 3H) and peak triplet due to side chain group of ethyl bromoisobutyrate was observed at 1.10 ppm (q, 3H). The signals obtained as multiplet at 1.33 and 1.58 ppm (b, 2H and c, 2H, respectively) could be attributed to the methylene protons of aliphatic chains. The proton associating with a carbon atom of the ether group was observed with peak triplets at 4.01 ppm (d, 2H) and 4.21 ppm (h, 2H). The signal obtained as a doublet at 2.0 ppm (j, 1H) and a peak triplet (i, 2H) because of adjacent methylene protons. The characteristic sharp signal obtained as a singlet at 3.41 ppm (k, 3H) corresponded to the adjacent C–O linkage. The peak singlet at 1.98 ppm (l, m 2H) were observed because of adjacent methylene protons. The signal obtained as a singlet at 2.21 ppm (f, g 2H) corresponded to the respective adjacent quaternary carbon. The characteristic sharp peak singlet at 2.0 ppm (n, 3H and o 3H) corresponded to the methyl proton of side chain polymer.
Figure revels the NMR spectrum of MBBHM. The characteristic sharp signal obtained as triplet at 0.98 ppm, 3.30 ppm (a, b 2H) of quaternary triethyl amine group, respectively. However, the important peak obtained as triplet at 3.68 and 3.81 ppm (c, d 2H) of ethylene linkage of (N + CH2–CH2–O). The characteristic signal obtained as triplet at 4.05 and 4.85 ppm (e, f 2H) of (PO–CH2–CH2–O) linkage, respectively. The characteristic peak doublet at 2.01 ppm (g, m 1H) due to methylene linkage of (C–H). The peak obtained triplet at 1.34 and 4.21 ppm (l, k 2 H) corresponds to side chain of ethyl group. The sharp signal obtained as singlet at 1.10 and 3.45 ppm (j, h 3H) due to C–CH3 and O–CH3 groups, respectively.
The terminal methyl protons in long chains of BA were confirmed by a peak triplet at 0.96 ppm (n, o 3H). The characteristics peak at 4.80 ppm (p, 2H) due to O–CH2 linkage. Thus, from the NMR spectrum the PBHM was successfully modified to MPBHM with quaternized phosphate ester moiety.
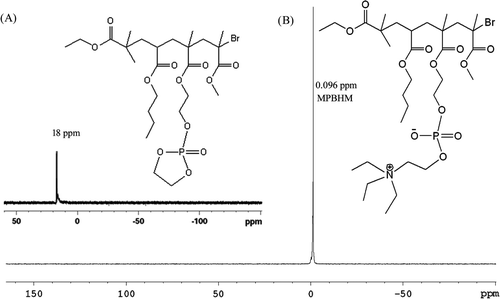
4.4. 31P NMR of MPBHM
Figure (A) shows the 31P NMR of MPBHM copolymer after reaction with cyclic ethylene chlorophosphate to generate ester. The absorption at 18 ppm corresponds to the presence of cyclic ethylene phosphate ester (–P–O–) linkages. Mostly, the chemical shift of the acyclic or cyclic phosphate is found at 18 ppm range. This peak corresponds to the (–P–O–CH2) absorption which is also occurred in the up field chemical shift region. From the 31P spectra it concluded that the PBHM was completely reacted with hydroxyl group present in copolymer. However, Figure (B) shows the 31P NMR spectra showed distinct signal for the MPBHM at 0.096 ppm corresponds to the presence of ethylene phosphate ester (–P–O–) linkages with quaternary amine. From the 31P spectra it was concluded that the MPBHM was successfully modified.
4.5. GPC
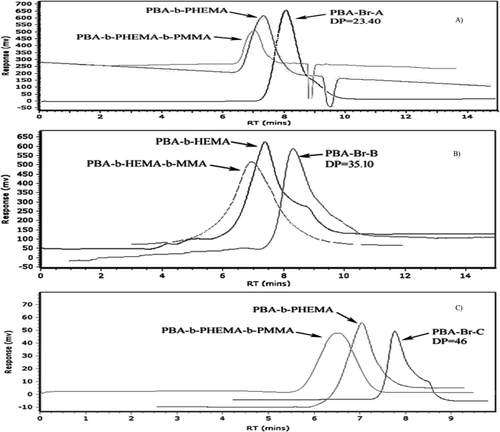
The GPC analysis showed that the number average molecular weight is well matched with experimental values (MnTheo) and PDI values show good control over the molecular distribution. This indicates that block copolymers synthesized by ATRP are in well controlled with constant number of growing chains throughout the reaction. The GPC analysis confirmed that number average molecular weights are in good agreement with the theoretical molecular weight and polydispersity index values obtained less than 1.3 shows good control over polymerization.
The GPC traces of copolymers shown in Figure . The synthesis of PBA macroinitiator and PBA-PHEMA diblock copolymers by ATRP resulted in well-controlled polymers with narrow molecular weight distribution. In Figure (A)–(C) shows the GPC traces for PBA-Br-A macroinitiator (DP = 23.40), PBA-Br-B macroinitiator (DP = 35) PBA-Br C macroinitiator (DP = 46), respectively. All GPC traces are narrow monomodal, and GPC traces of the diblock macroinitiator is shifted to lower elution volume from that of the macroinitiator, indicating an increase in molecular weight was taken to higher conversion.
4.6. Kinetics of polymerization
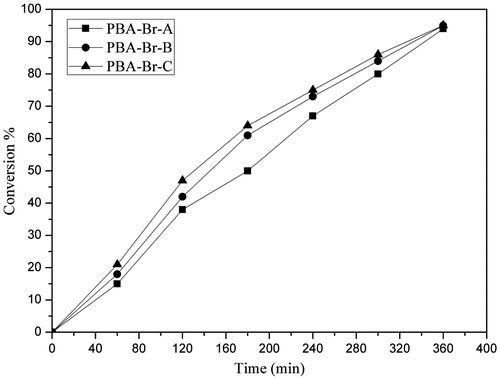
The kinetic plot of monomer conversion with time shown in Figure depicts that monomer conversion increases with the reaction time, which is a characteristic feature of control radical polymerization. Polymerization proceeds steadily with a constant concentration of growing radical species during conversion. The molecular weights obtained in PBHM-10, PBHM-15, and PBHM-20 agreed practically well with the theoretical molecular weight in case of homopolymerization of PBA but whenever chain extension block polymerization took place its molecular weight increases and its result shown in Tables and .
The kinetics graph of the macroinitiator plotted against Ln [M]0/[M] versus time is shown in Figure . A straight line through the origin indicates that monomer consumption follows the first-order kinetics. Furthermore, the concentration of propagating radicals remains constant during polymerization, where [M]0 represents the initial concentration of the monomer. The molecular weights increased linearly with conversion and polydispersity remained relatively low, indicating a well-controlled radical polymerization and a fast exchange between active and dormant species.
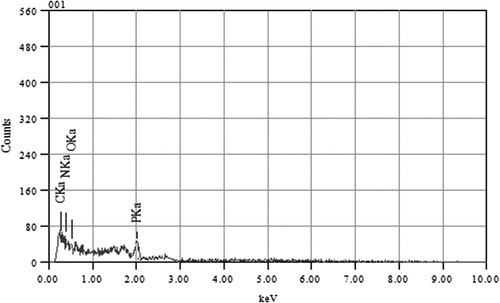
4.7. Energy dispersive X-ray spectroscopy (EDXS)
EDXS data for the modified MPBHM were obtained on a JEOL JSM 6390LA instrument SEM shown in Figure . EDXS analysis confirms that Phosphate moiety was successfully incorporated in hydrophilic part of triblock copolymer. The elemental analysis data are shown in Table .
Table 6. Elemental analysis of MPBHM.
4.8. Qualitative determination of MPBHM
The samples of MPBHM were analyzed by a qualitative method for confirmation of quaternization, in which 1% aqueous solution of MPBHM was taken into the test tube. Then NaOH was added until the solution becomes basic, 5 ml of CHCl3, and 0.5% solution of bromophenol blue indicator was added to the copolymer solution, respectively. On shaking this solution results in the two distinct layers, the organic layer becomes blue, which concludes that presence of the quaternary group in MPBHM.[Citation32]
4.9. Evaluation of MPBHM as wetting and dispersing agent for emulsion paint
4.9.1. Wetting time
The canvas was cut into 1-inch diameter discs having an average weight of 0.55 g. The disc was floated on the surface of copolymer solution 1% w/v and the stopwatch started. The time required for the disc to begin to sink was measured accurately and noted as the wetting time.[Citation33] The result of wetting time shown in Table .
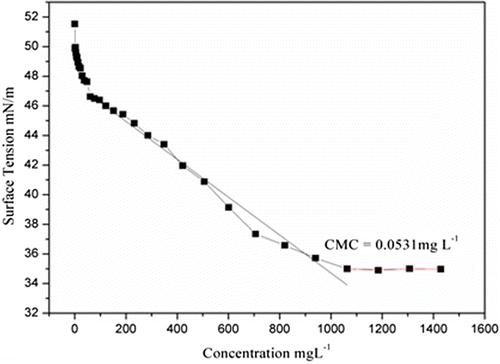
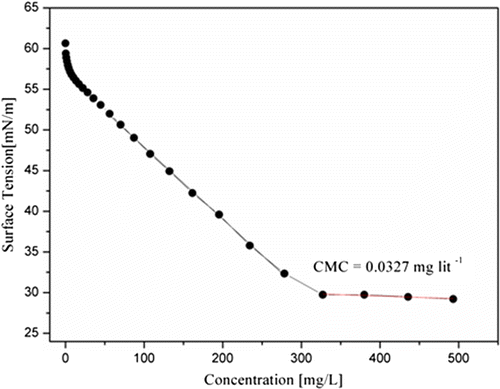
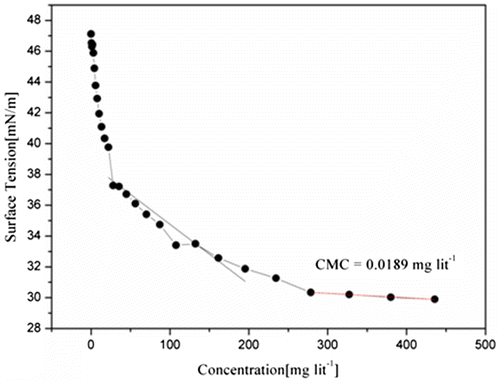
Table 7. Data of surface tension and Critical Micelle Concentration of MPBHM.
4.9.2. Hydrophilic–lipophilic balance determination
The hydrophilic-lipophilic balance (HLB) of MPBHM was evaluated by using Griffin’s method, adopted from the literature, approximate HLB values can be estimated from the composition of polymeric surfactant. According to this method, HLB values of the synthesized triblock copolymers as reported in Table results shows the HLB values of triblock copolymers was determined by theoretical methods based on calculating the number of Hydrophilic and lipophilic chains in polymers this method applied to the amphiphilic copolymers, allows the estimation of an approximate HLB value for the composition of the dispersant or macrosurfactant, according to the equation.[Citation34]
where WH and WL being the weight fraction of the hydrophilic and hydrophobic segments. The HLB values of all MPBHM was shown in table 10. According to this method surfactant with HLB values in the range 3–6 stabilize W/O emulsion, whereas those with HLB values 6–9 are good wetting agent. However, HLB values in the range 7–15 stabilized O/W emulsion.
4.9.3. Surface activity and Critical Micelle Concentration
The surface tension of aqueous solutions of amphiphilic triblock copolymers and was measured, using by Kruss Tensiometer at 25 °C using Millipore water and the readings were recorded in the range of 56–29 dyne/cm. Futhermore, if any liquids, oligomers, or polymers which reduces the surface tension of medium shows better wetting as compared to other having higher surface tension. It was observed that the MPBHM-20 shows good surface activity with low surface tension as compared to MPBHM-15 and MPBHM-10. This is due to increasing hydrophobic chains in polymers. Dynes/cm is the surface tension of all three modified triblock copolymers. However, Experiments show the amphiphilic triblock copolymers MPBHM-20 exhibits lowest critical micelle concentration (CMC) 0.0189 g/l with lower surface tension, this could be due to the increase in hydrophobic chains of polymers. However, in case of MPBHM-10 and MPBHM-15, it shows higher surface tension and CMC 0.0536 g/l, 0.0327 g/l because of number of hydrophobic blocks.[Citation35] Data of Surface tension Critical Micelle Concentration of MPBHM are shown in Table
Figures – reveal that the surface tension of MPBHM decreases along with the increase in concentration up to the CMC levels it again constant with surface tension.
4.10. Paint formulation for MPBHM
MPBHMs were incorporated in waterborne paint system formulation as wetting and dispersing additive. Paints with various pigment volume concentration, i.e. 35, 45, and 55 was formulated with 2.5% concentration of additive using high speed disperser. Paints were formulated by using TiO2, talc, and acrylic emulsion as a binder. The pigment dispersion was checked on Hegman gauze. This paint was applied on asbestos panels and allows to dry and then the performance was evaluated. Table shows the paint formulation recipe at different pigment volume concentration (PVC).
Table 8. Paint formulation.
4.10.1. Ease of pigment dispersion
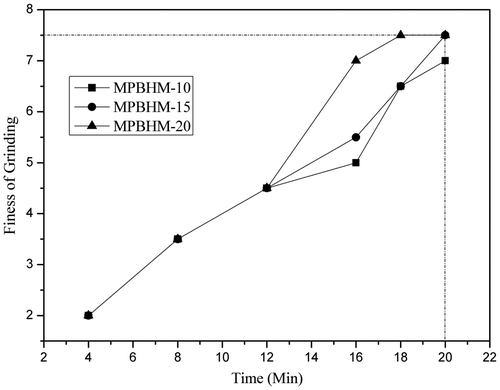
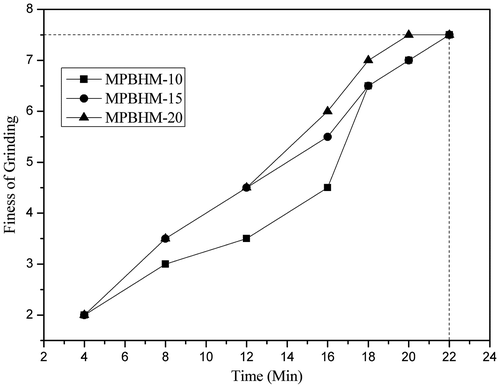
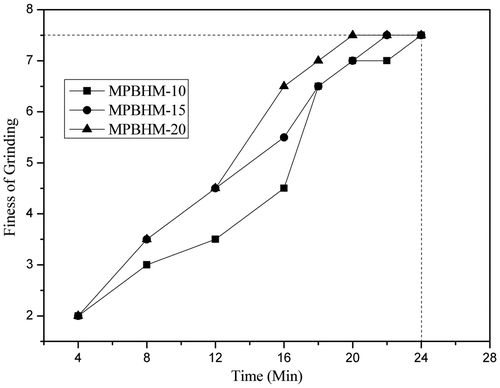
The ease of pigment dispersion was determined according to ASTM D1210. Ease of dispersion was checked at regular interval of time during the pigment grinding process till 7+ dispersion was observed. From that it was concluded that fineness of grinding of pigment was more than 7 of MPBHM-10 in 20 min of dispersion and in case of MPBHM-20 it was on 18 min, This is because if the polymeric dispersant chains are too short, then they will not give a suitably thick wall to prevent flocculation. It means that too low a molecular weight will cause dispersion instability and will lead to loss of properties, but the chains are too long, they have a tendency to fold back on themselves. Too high a molecular weight will also give reduced performance for better dispersion of pigment particles preferably the chains should be free to move in the dispersing medium.[Citation36] Polymeric dispersants provide make them very efficient to anchor on pigments for effective in providing electrical stabilization. The ease of dispersion of 35,45, and 55 PVC paint was studied; it is shown in Figures –.
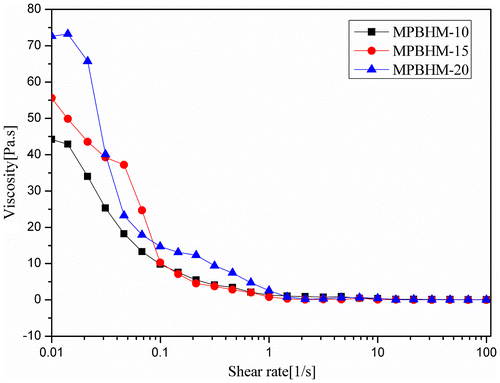
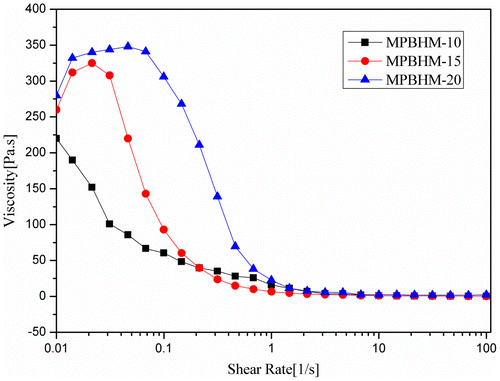
4.10.2. Determination of paint viscosity using cone and plate geometry
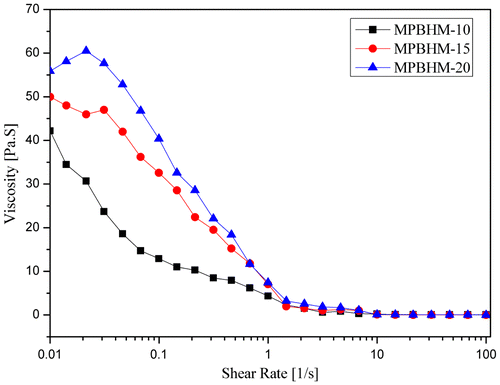
The graphical data shown in Figures – indicate that because the PVC increases from 35 to 55%, paint viscosity also increases. All additive formulations (MPBHM-10, MPBHM-15, and MPBHM-20) with 2.5% at constant shear rates showed shear thinning effects. Shear thinning behavior was observed more at 45 and 55 PVCs than that at 35 PVC. Paint viscosity decreased considerably at a low PVC.
The modified copolymer MPBHM-20 incorporated in a waterborne paint formulation showed highest viscosity than MPBHM-10 and MPBHM-15. However, in all formulations, the dispersed paint showed high viscosity in a low shear region, which avoids settling of paints during transportation and storage.[Citation37] During dispersion, a polymeric dispersant reduces the interactions between pigment particles and lowers viscosity. However, decreased viscosity is always beneficial because paint viscosity is an important characteristic during application. Low paint viscosity increases fluidity and leveling of paints and enhances the ease of brushing. In the shear region 0.01 s−1, a leveling zone, all the additives containing formulations exhibited a low viscosity, indicating very good flow and leveling.[Citation38]
4.11. Optical properties of paint
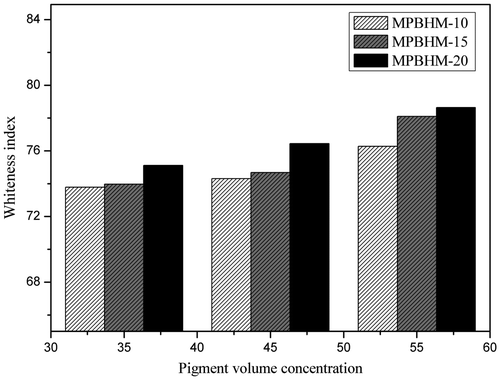
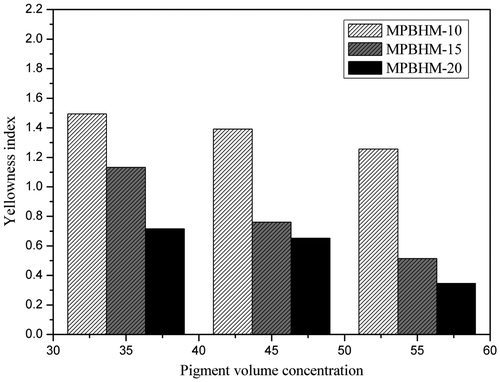
4.11.1. Whiteness and yellowness index
In waterborne paint system, optical properties i.e. color, gloss, and opacity is an important phenomenon which affects the properties of paint. The whiteness index is one of the properties of paint as it deals with ease of dispersion of pigment. The whiteness index of paint is directly proportional to the extent of pigment dispersion. The whiteness index of paint with varying concentration of MPBHM was measured shown in Figures and . It was observed that the whiteness index of the paint increases with wetting and dispersing concentration. However, due to introduction of MPBHM dispersant in paint formulation, it helps in pigment dispersion by electrostatic stabilization mechanism and also in avoiding agglomerization and improves whiteness index of paint.
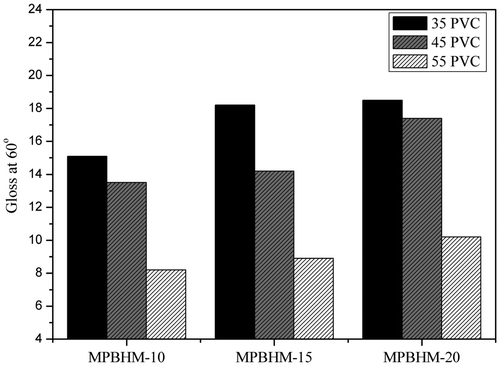
4.11.2. Determination of gloss of paint
Figure reveals the gloss of emulsion paint. High gloss is obtained pigment particles or flocculates which extend beyond from the surface interfere with the direct reflection. Since wetting and dispersing additives inhibit flocculation, they increase the gloss of a coating. From these results, it was observed that the molecular weight of additives in paint increases its gloss because it reduces the flocculation of pigment and stabilization of pigment particle.[Citation39,40] However, as the molecular weight of the copolymer increases its gloss, as the PVC increases, gloss decreases. At lower PVC, i.e. 35% MPBHM-10 exhibited better gloss; however, at higher PVC, i.e. 55% MPBHM-20 is better among others in gloss improvement.
5. Conclusion
Poly(BA-b-HEMA-b-MMA) triblock copolymers were successfully synthesized by ATRP using CuBr/PMDETA as catalyst system. These hydrophilic blocks of copolymers were modified by incorporating phosphate and quaternary nitrogen moieties. The modification of copolymers were confirmed by FT-IR, 1H, and 31P NMR, EDXS and from physicochemical analysis. These modified copolymers were used as wetting and dispersing additive in waterborne paint system. From the performance properties of paints, it was concluded that modified triblock copolymers act as an efficient wetting and dispersing additive which assist to improve the optical properties of paint. The HLB balance of modified copolymers also helps in reduction of interfacial surface tension which not only facilitate the wetting and dispersion of pigment particles, but also avoid the re-agglomeration of pigment particle during storage.
Acknowledgments
The author immensely likes to thank to UGC Green tech Grant for providing financial support and Institute of Chemical technology for conducting research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Reuter E, Silber S, Psiorz C. The use of new blockcopolymeric dispersing agents for waterborne paints – theoretical and practical aspects. Prog. Org. Coat. 1999;37:161–167.10.1016/S0300-9440(99)00072-7
- Bieleman J. Additives for coatings. New York: Wiley; 2000. p. 67–95.
- Malshe VC. Sikchi MA. Basic of paint technology: part 1. Mumbai: Antar Prakash Center for Yoga; 2004. p. 8–22.
- Florio JJ, Daniel JM. Handbook of coating additives. 2nd ed. New York: Marcel Dekker; 2004.
- Schofield JD. Handbook of coating additives. New York: Marcel Dekker; 1992. p. 71–104.
- Patton TC. Paint flow and pigment dispersion. New York: Willey Interscience; 1979.
- Jakubauskas HL. Use of A-B Block polymers as dispersants for non-aqueous coating systems. J. Coat. Technol. 1986;58:71–82.
- Krishnan R, Srinivasan KSV. Synthesis and characterization of amphiphilic block copolymers of methyl methacrylate with poly (ethylene oxide) macroinitiators formed by atom transfer radical polymerization. J. Appl. Polym. Sci. 2004;97:989–1000.
- Mu A, Gaynor SG, Matyjaszewski K. Synthesis of amphiphilic block copolymers by atom transfer radical polymerization (ATRP). Macromolecules. 1998;31:6046–6052.
- Greszta D, Mardare D. Living radical polymerization. 1. Possibilities. Macromolecules. 1994;27:638–644.
- Gr P, Kazmaier PM, Gordon K. Narrow molecular weight resins by free-radical polymerization process. Macromolecules. 1993;26:2987–2988.
- Wayland BB, Basickes L, Mukerjee S, Wei M, Fryd M. Living radical polymerization of acrylates initiated and controlled by organocobalt porphyrin complexes. Macromolecules. 1997;30:8109–8112.10.1021/ma9707493
- Kato M, Kamigaito M, Sawamoto M, Higashimuras T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphoshine)ruthenium(II)methylaluminium bis (2,6 di-ter-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules. 1995;28:1721–1723.10.1021/ma00109a056
- Wang J, Matyjaszewski K. Control1ed ‘living’ radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995;117:5614–5615.
- Nowakowska M, Zapotoczny S, Karewicz A. Synthesis of Poly (sodium styrenesulfonate-block-vinylnaphthalene) by nitroxide-mediated free radical polymerization. Macromolecules. 2000;33:7345–7348.10.1021/ma991817j
- Chiefari J, Chong YKB, Ercole F, Krstina J, Jeffery J, Le TPT. Living free-radical polymerization by reversible addition – fragmentation chain transfer: the RAFT process. Macromolecules. 1998;31:5559–5562.10.1021/ma9804951
- Davis KA, Matyjaszewski K. ABC triblock copolymers prepared using atom transfer radical polymerization techniques. Macromolecules. 2001;34:2101–2107.10.1021/ma002050u
- Matyjaszewski K. Controlled/‘living’ radical polymerization. Halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(11) redox process. Macromolecules. 1995;28:7901–7910.
- Matyjaszewski K, Patten TE, Xia J. Controlled/‘living’ radical polymerization. Kinetics of the homogeneous atom transfer radical polymerization of styrene. J. Am. Chem. Soc. 1997;119:674–680.10.1021/ja963361g
- Grimaud T, Matyjaszewski K. Controlled/‘living’ radical polymerization of methyl methacrylate by atom transfer radical polymerization. Macromolecules. 1997;30:2216–2218.10.1021/ma961796i
- Matyjaszewski K, Gaynor SG, Mu AHE. Preparation of hyperbranched polyacrylates by atom transfer radical polymerization. 2. Kinetics and mechanism of chain growth for the self-condensing vinyl polymerization of 2- ((2-bromopropionyl) oxy) ethyl acrylate. Macromolecules. 1997;30:7034–7041.10.1021/ma970634z
- Scroll P, For D. Atom transfer radical polymerization using poly (vinylidene fluoride) as macroinitiator. Des. Monomers Polym. 2004;7:181–189.
- Taylor P, Amin A, Sarkar R, Moorefield CN, Newkome GR. Synthesis of polymer – clay nanocomposites of some vinyl monomers by surface-initiated atom transfer radical polymerization. Des. Monomers Polym. Monomer Polym. 2013;16:528–536.
- Matyjaszewski K. Atom transfer radical polymerization: from mechanisms to applications. Isr. J. Chem. 2012;52:206–220.
- Xia J, Matyjaszewski K. Controlled/‘living’ radical polymerization. homogeneous reverse atom transfer radical polymerization using AIBN as the initiator. Macromolecules. 1997;30:7692–7696.10.1021/ma9710085
- Wang W, Dong Z, Xia P, Yun D, Zhung Q. Reverse atom transfer radical polymerization of methyl acrylate using AIBN as the initiator under heterogeneous conditions. Macromol. Rapid Commun.. 1998;19:647–649.10.1002/(ISSN)1521-3927
- Saindane P, Jagtap RN. Progress in Organic Coatings RAFT copolymerization of amphiphilic poly (ethyl acrylate-b-acrylic acid) as wetting and dispersing agents for water borne coating. Prog. Org. Coatings [Internet]. 2015;79:106–114. doi:10.1016/j.porgcoat.2014.07.016
- Sontakke TK, Jagtap RN. Synthesis of AA-MMA block copolymer by RAFT polymerization and used as emulsifier cum macroinitiator and its influence on the film properties. J. Dispers. Sci. Technol. [Internet]. 2013 [ cited 2015 Jan 1];34:1575–1584. Available from: http://www.tandfonline.com/doi/abs/10.1080/01932691.2012.756377
- Chen H, Liang Y, Wang M, Lv P, Xuan Y. Reverse ATRP of ethyl acrylate with ionic liquids as reaction medium. Chem. Eng. J. 2009;147:297–301.10.1016/j.cej.2008.11.007
- Min KE, Li MEI, Matyjaszewski K. Preparation of gradient copolymers via ATRP using a simultaneous reverse and normal initiation process. I. Spontaneous gradient. J. Polym. Sci., Part A: Polym. Chem. 2005;3616–3622.10.1002/(ISSN)1099-0518
- Ansari BWH, Noori S, Naqvi AZ. Interaction between Zwitterionic surfactants and amphiphilic drug: a tensiometric study. Z. Phys. Chem. 2013;227:441–458.
- Zhinong GAO, Shuxin TAI, Qi Z. Synthesis and surface activity of biquaternary ammonium salt gemini surfactants with ester bond. J. Nat. Sci. 2008;13:227–231.
- Saad AH, Kadhim RB. Formulation and evaluation of herbal shampoo from ziziphus spina leaves extract. Int. J. Res. Ayurveda Pharm. 2011;2:1802–1806.
- Tan B, Grijpma DW, Nabuurs T, et al. Crosslinkable surfactants based on linoleic acid-functionalized block copolymers of ethylene oxide and 3-caprolactone for the preparation of stable PMMA latices. Polymer. 2005;46:1347–1357.10.1016/j.polymer.2004.11.070
- El-Dougdoug WIA. Synthesis and evaluation of anionic copolymeric surfactant as dispersing agent for some heterocyclic azo-dyes synthesis and evaluation of anionic copolymeric surfactant as dispersing agent for some. J. Dispers. Sci. Technol. 2010;31:1298–1306.
- Patton TC. Paint flow and pigment dispersion. 2nd ed. New York: Willey Interscience; 1979.
- Malshe VC. Basic of paint technology. 1st ed. Mumbai; 2002. p. 20–22.
- Deka A, Dey N. Rheological studies of two component high build epoxy and polyurethane based high performance coatings. J. Coat. Technol. Res. 2013;10:305–315.10.1007/s11998-012-9445-3
- Malshe VC. Basics of paint technology. 1st ed. Mumbai; 2002. p. 128–129.
- Malshe VC. Basics of paint technology. 1st ed. Mumbai; 2002. p. 76–78.

![Figure 7. Plot of ln ([M]0/[M]) versus time for ATRP of PBA-Br.](/cms/asset/3a46b6bf-6b6b-43dd-96e4-a7dbfd611e8f/tdmp_a_1136534_f0007_b.gif)