Abstract
Silica nanoparticles (SiO2) were grafted with the precursor random copolymer of 1′-(2-acryloxyethyl)-3′,3′-dimethyl-6-nitrospiro-(2H-1-benzopyran-2,2′-indoline) (SPMA) and tert-butyl methacrylate (tBMA) by surface-initiated atom transfer radical polymerization (SI-ATRP), and SiO2-g-P(SPMA-co-methacrylic acid (MAA)) was obtained via chemical hydrolysis of the resulting precursor random copolymer in acidic conditions. From transmission electron microscopy, we observed the spherical morphology of monodispersed silica nanoparticles and core-shell structure of SiO2-g-P(SPMA-co-MAA). Energy dispersive spectroscopy, Fourier transform infrared spectra, X-ray photoelectron spectroscopy, and the thermogravimetric analysis indicated that the polymer had been successfully grafted onto the surface of silica nanoparticles. The dual-responsive properties were characterized by means of ultraviolet-visible spectrophotometer and dynamic light scattering. The average hydrodynamic diameter of SiO2-g-P(SPMA-co-MAA) increased from 185.7 to 212.7 nm under ultraviolet light irradiation for 5 min. Also, the particle size of SiO2-g-P(SPMA-co-MAA) increased with the rising pH value of surrounding condition.
1. Introduction
In recent years, silica nanoparticles (SNPs, SiO2) have received vast attention and become a research hotspot because there are a lot of hydroxyl groups on their surface which can be easily functionalized. They have been widely used in drug release control,[Citation1] bioimaging,[Citation2] biomedicine,[Citation3] catalyst,[Citation4] biosensor,[Citation5] gene delivery,[Citation6] diagnosis, and therapeutics,[Citation7] etc. Modified SNPs can keep all the properties of the inorganic nanoparticles and have special properties such as stimulus response and highly efficient adsorption of heavy metal ions.[Citation8,9]
The surface modification of silica nanoparticles is an important method for improving their properties and expanding their application, especially by preparing polymer brushes.[Citation10,11] Generally, polymer brushes can be obtained via covalent attachment approaches which usually contain ‘grafting to’ and ‘grafting from’ methods. Compared with ‘grafting to’, ‘grafting from’ method has no restrictions on terminal functional groups, and the grafting density is easy to control. Surface-initiated polymerization (SIP, ‘grafting from’) approach, based on initiators being immobilized with a surface to initiate polymerization, is a good choice to control the thickness, functionality, and density of polymer brushes with molecular precision.[Citation12–15] Therefore, surface-initiated atom transfer radical polymerization (SI-ATRP) is one kind of the effective method for preparing structure-controlled polymer brushes.[Citation16–23] With the development of the research on the smart material, stimuli-responsive polymers have attracted significant interest. When they are subjected to external environmental stimuli,[Citation24] such as the changes of solvent,[Citation25] temperature,[Citation26–28] ionic strength,[Citation29] pH,[Citation30,31] and light irradiation,[Citation32,33] the molecular chains of the corresponding stimuli-responsive polymer brushes will generate a conformational change, leading to the changes in the properties of polymer brushes.
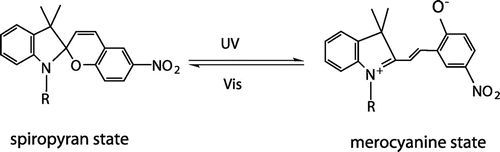
Spiropyran is a well-known photosensitive molecule which has a broad spectrum and near-quantitative property in both directions during the photoisomerization process.[Citation34–38] The combination of spiropyran units (SP) into polymer and then attaching them onto SNPs can sharply reduce the fatigue of light-responsive polymer brushes.[Citation39] As smart dynamic materials, the spiropyran-based copolymers have particular advantages for profound and lasting applications. Under the irradiation of ultraviolet (UV) light, a ring-closed and colorless spiropyran (SP) form switches into a ring-opened and strongly colored merocyanine (MC) form, and under visible (vis) light irradiation, the isomerization equilibrium will move to the reverse direction. Because the energy of the spiro C–O bond is relatively low, spiropyran will undergo a photocleavage of the spiro C–O bond under UV irradiation as shown in Scheme .
In addition to the photosensitive monomer, the pH-sensitive monomer such as methacrylic acid (MAA) is also a class of polymeric monomers that are extensively studied in the environmental response. MAA can be protonated at low pH values and deprotonated to negatively charged at relatively high pH values. The ionization/deionization process of PMAA generally occurs in the pH ranging from 4 to 8, bearing the carboxylic group with pKa around 5–6. PMAA is one of most typical pH-responsive polymers used as the carriers for drug delivery system which can achieve the precise targeted drug release. It has been widely applied in biomedical applications,[Citation40] coatings,[Citation41] and sensors.[Citation42]
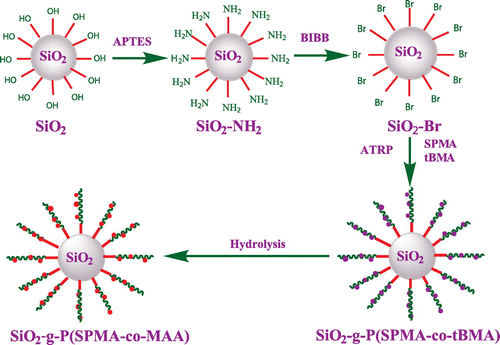
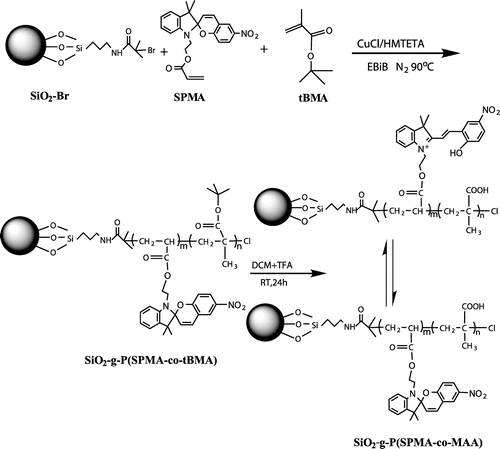
Inspired by the previous work, we designed and synthesized novel hybrid silica nanoparticles grafted with photo- and pH-sensitive random copolymer P(SPMA-co-MAA) by SI-ATRP. Carboxylic groups in MAA unit might poison the ATRP catalyst, so we chose tert-butyl methacrylate (tBMA) as the precursor of MAA unit. The synthetic strategy for the polymer-grafted SNPs is illustrated in Scheme . First, monodispersed SNPs were synthesized by the Stöber method,[Citation43] and then the amino-functionalized and 2-bromoisobutyrate-functionalized SNPs were prepared. Next, hybrid silica nanoparticles coated with P(SPMA-co-tBMA) were constructed by SI-ATRP. Finally, the resulting polymer brushes were hydrolyzed at acidic conditions, and ultimate P(SPMA-co-MAA) brushes were obtained.
2. Experimental
2.1. Materials
The precursor monomer tertiary-butyl methacrylate (tBMA, 99%, Aladdin) was passed through a column of basic alumina to remove inhibitors before use. Ethyl 2-bromoisobutyrate (EBiB, 98%, TCI) as the initiator, cuprous chloride (CuCl, ≥99.95%, Aladdin) as the catalyst, 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA, 97%,J&K) as the ligand, N,N-dimethylformamide (DMF, ≥99.5%), trifluoroacetic acid (TFA, 99%, Aladdin), ammonium hydroxide (25%), tetraethyl-orthosilicate (TEOS, 99.5%), ethanol (≥99.7%), tetrahydrofuran (THF, 99%), 2-bromoisobutyryl bromide (BiBB, 99%, J&K), dichloromethane (DCM, 99%, J&K), pyridine (99%, Aladdin), (3-aminopropyl)-triethoxysilane (APTES, 99.6%, J&K), toluene (≥99.5%) were used as received. The light-responsive monomer 1′-(2-acryloxyethyl)-3′,3′-dimethyl-6-nitrospiro-(2H-1-benzopyran-2,2′-indoline) (SPMA) was synthesized as described in literature.[Citation44]
2.2. Preparation of polymer brushes
2.2.1. Preparation of monodispersed SNPs(SiO2)by Stöber method
Under the condition of room temperature, ammonium hydroxide (25 mL) and ethanol (500 mL) were added into a two-neck round-bottom flask and stirred at a rotating speed of 300 rpm for 10 min. Next, the mixture of TEOS (12.5 mL) and ethanol (14 mL) was added into the flask at the rate of 1 mL/min by dropping funnel. The reaction was stopped after continuous stirring at room temperature for 20 h, and a white silica suspension was obtained. The SNPs were centrifugally separated from the suspension and washed with ethanol four times, and then the resulting SNPs were dispersed in ethanol (50 mL) with ultrasound.
2.2.2. Preparation of amino-functionalized SNPs (SiO2–NH2)
The SNPs suspension (45 mL) was placed in a 100-mL flask, and then the flask was sealed with rubber plug. Subsequently, the flask was immersed in an oil bath at 60 °C, and then ammonium hydroxide (50 μL) and APTES (0.4 mL) were added into reaction system dropwise. After 12 h, the reactant was cooled to room temperature and exposed to air. The obtaining mixture was washed and centrifugally separated with toluene for 4 times, and the resulting SiO2–NH2 was dispersed in toluene (50 mL).
2.2.3. Synthesis of 2-bromoisobutyrate-functionalized SNPs (SiO2–Br)
The amino-functionalized SNPs suspension (45 mL) and pyridine (0.3 mL) were placed in a 100-mL flask. Then, BiBB (0.25 mL) was added into the flask by syringe dropwise, and the mixture was stirred at 0 °C for 12 h. Then, the similar purification with SiO2–NH2 was carried out, and the final sediment (SiO2–Br) was dried under vacuum at 50 °C overnight.
2.2.4. Synthesis of hybrid silica nanoparticles coated with P(SPMA-co-tBMA) brushes (SiO2-g-P(SPMA-co-tBMA))
In a typical polymerization, SiO2–Br (100 mg), SPMA (73.6 mg), tBMA (582 μL), CuCl (2.7 mg), toluene (2 mL), DMF (0.1 mL), and EBiB (2 μL) were poured into a Schlenk tube equipped with a rubber septum, and the mixture was degassed three times using the freeze–pump–thaw procedure. Thereafter, HMTETA as the ligand (45.6 μL) was injected into the mixture and the tube was placed in an oil bath at a preset temperature (90 °C) for 24 h. The polymerization was manually stopped by exposing the reactant to air. The resultant was dispersed in THF, and centrifugally separated four times. On the one hand, the copper catalyst was removed by passing the centrifugal liquid through a neutral alumina column, and precipitation of the resulting transparent solution gave the free P(SPMA-co-tBMA) which was analyzed using GPC. On the other hand, the resulting solid from centrifugal separation was carefully washed with THF, and the nongrafted polymer was further removed by Soxhlet extraction for 24 h. SiO2-g-P(SPMA-co-tBMA) was obtained and dried under vacuum at 50 °C for 20 h.
2.2.5. Preparation of SiO2-g-P(SPMA-co-MAA) by hydrolysis
SiO2-g-P(SPMA-co-tBMA) particles (60 mg), dichloromethane (30 mL), and trifluoroacetic acid (1 mL) were stirred at room temperature for 24 h. Then, the mixture was washed and centrifugally separated with dichloromethane and ethanol alternatively for four times, and ultimate P(SPMA-co-MAA) brushes were obtained and dried under vacuum at 50 °C overnight.
2.3. Characterization
The size and morphology of bare SiO2 and SiO2-g-P(SPMA-co-MAA) were determined by transmission electron microscopy (TEM) using a Titan G2 60–300 on a copper grid with a carbon membrane.
The dispersion stability of the nanoparticles was evaluated by the n-value as reported in the previous literatures.[Citation45] The n-value was calculated from the absorbance of the nanoparticle suspension at different wavelengths. The concentration of nanoparticles in ethanol is 3 mg/4 mL.
The elemental composition of polymer brushes was estimated using energy dispersive spectroscopy (EDS).
Fourier transform infrared spectra (FT-IR) were taken with a Nicolet model 6700 ranging from 450 to 4000 cm−1.
X-ray photoelectron spectroscopy (XPS) measurements were implemented on an ESCALAB MKII multitechnique spectrometer with a standard Mg Kα excitation source.
The thermogravimetric analysis (TGA) was performed on NETZSCH STA 449C instrument under an air atmosphere with a heating rate of 10 °C/min in the range from room temperature to 800 °C.
The specific surface area of the nanoparticles was characterized by nitrogen adsorption/desorption curves obtained from a Micromeritics ASAP2010 apparatus, and it was calculated with the corrected BET equation.
The molecular weight of the free precursor copolymer was determined on Agilent Technologies Series 1200 GPC system. The eluent (THF) was pumped through the system at a fixed flow rate of 1 mL/min. Commercially available polystyrene sample with narrow molecular weight distribution was used as the standard to generate a calibration curve.
The structure was determined by 1H NMR spectroscopy using a 500 MHz Bruker AVANCE III NMR spectrometer in CDCl3.
UV–vis spectra were taken with a Shimadzu UV-2450 Spectrometer ranging from 200 nm to 800 nm. The concentration of all the samples was 6.8 × 10−2 wt%.
Dynamic light scattering (DLS) was carried out on a Zetasizer Nano instrument Zen 3600 from Malvern Instruments. The concentration of all the samples was 3.3 × 10−2 wt%.
3. Results and discussion
3.1. Synthesis and characterization of SiO2-g-P(SPMA-co-MAA)
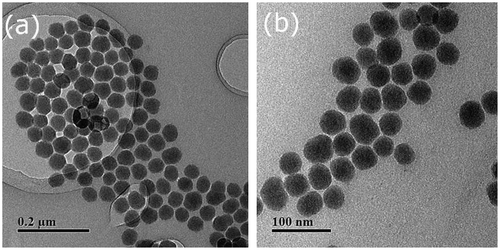
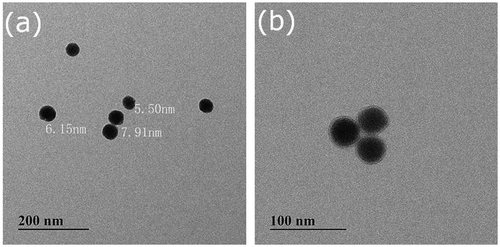
The preparation route of hybrid silica nanoparticles coated with double responsive random copolymer P(SPMA-co-MAA) is shown in Scheme . TEM images of bare silica nanoparticles and hybrid nanoparticles SiO2-g-P(SPMA-co-MAA) dispersed in anhydrous ethanol are shown in Figures and , respectively. Monodispersed and ordered silica nanoparticles are obviously observed in Figure (a) and (b), and their morphology is almost spherical with the diameter of 40–50 nm.
As can be seen from Figure (a) and (b), the thickness of the grafting polymer layer is about 5–8 nm. In the previous report, n-value is utilized to assess the dispersion stability of the nanoparticles suspension. The higher the n-value is, the better the dispersion stability is. In the present work, it is determined by the absorbance at different wavelengths according to the following equation:
where τ is the absorbance of the suspension; λ is the wavelength used in the experiment; α is a constant.
Then, n-value can be calculated from the plotting between ln τ and ln λ as shown in Figure S1 (see the Supporting Information). For the nanoparticles in our system, the relatively high n-value (n = 2.3) indicates that the resulting nanoparticles have good dispersion stability. This is because the whole reaction is carried out in the solvent which avoids particle aggregation caused by repeated drying.
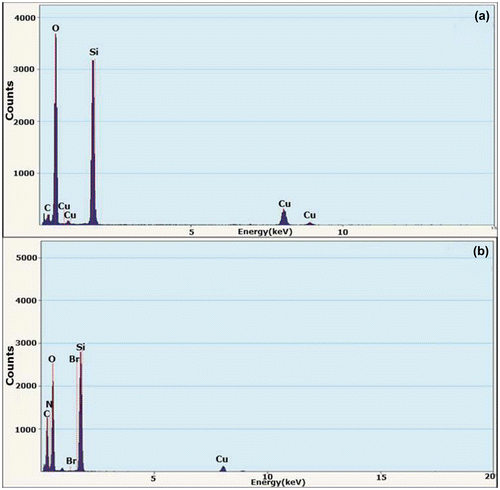
In order to further confirm the structure of the polymer brush, EDS is employed to identify the element compositions of SiO2 and SiO2-g-P(SPMA-co-MAA), and the results are shown in Figure . Compared with bare silica nanoparticles, carbon element content in hybrid nanoparticles increases significantly while silicon element as well as oxygen element content decreases obviously. At the same time, the peaks of bromine and nitrogen element are observed in the curve of hybrid nanoparticles. In addition, the appearance of copper element is caused by copper grids in the EDS experiments. These phenomena prove that the silica surface has been grafted with a layer of polymer, which is consistent with the TEM results.
Figure 3. EDS spectrograms of (a) bare silica nanoparticles and (b) hybrid silica nanoparticles coated with P(SPMA-co-MAA).
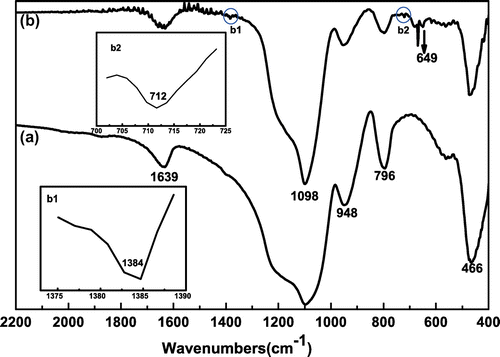
Successful preparation of SiO2-g-P(SPMA-co-MAA) is also affirmed by FT-IR spectroscopy in Figure . It is evident that characteristic absorption peaks of major functional groups appear in the curve of bare silica as demonstrated in Figure (a). The strong and wide absorption peak at 1098 cm−1 is ascribed to antisymmetric stretching vibration of Si–O–Si bond. The absorption peaks at 796 and 466 cm−1 can be assigned to symmetric stretching vibration and bending vibration of Si–O bond. The absorption peak at 1639 cm−1 appears from the bending vibration of H–O–H bond in water. At the same time, the absorption peak at 958 cm−1 is assigned to bending vibration of Si–OH bond. Compared with bare silica in Figure (a), there are a few new characteristic peaks for SiO2-g-P(SPMA-co-MAA) in Figure (b). The out-of-plane bending vibrations of C–H bond which is connected with carbon–carbon double bond and benzene ring appear at 649 and 712 cm−1, respectively. The weak absorption peak at 1384 cm−1 is ascribed to deformation vibration of C–H which is derived from the methyl. These results indicate that the polymer molecules have been grafted on the surface of silica nanoparticles.
Figure 4. FT-IR spectra of (a) bare silica nanoparticles and (b) hybrid silica nanoparticles coated with P(SPMA-co-MAA).
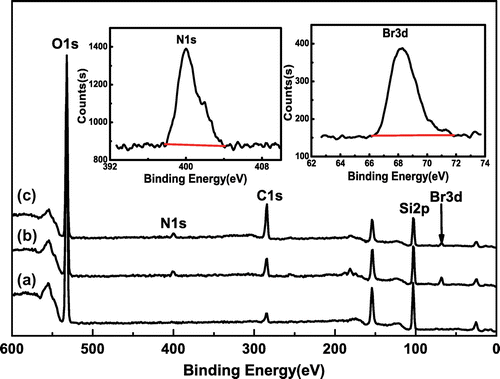
As we know, XPS is usually used to analyze the status of surface chemical bond, and the result is demonstrated in Figure . The spectrum of SiO2 in Figure (a) has the strong peaks at 284.87, 532.92, and 103.49 eV, attributing to the binding energy of C1s, O1s, and Si2p, respectively. Meanwhile, the new peaks at 401.52 and 68.15 eV are clearly observed from the XPS spectrum of SiO2–Br in Figure (b), assigning to the binding energy of N1s and Br3d, respectively. The XPS spectrum of SiO2-g-P(SPMA-co-MAA) is shown in Figure (c), and the peak of C1s at 284.77 eV has been strengthened, while the peak of Br3d at 68.25 eV has been weakened.
The composition of the surface elements of SiO2, SiO2–Br, and SiO2-P(SPMA-co-MAA) is demonstrated in Table . Compared with SiO2, both silicon and oxygen element contents in SiO2–Br and SiO2-P(SPMA-co-MAA) decline, while carbon content rises. The emergence of bromine and nitrogen element is obviously found in Table .
Table 1. Surface elements composition of SiO2, SiO2–Br, and SiO2-P(SPMA-co-MAA).
For the purpose of analyzing the surface elements’ chemical status more clearly, O1s high-resolution spectra of SiO2 and SiO2-P(SPMA-co-MAA) are scanned, and the results are fitted with the ‘XPSPEAKFIT’ software shown in Figure . The binding energy of relative bonds and attribution are listed in Table . After P(SPMA-co-MAA) is grafted on the surface of SiO2, the relative content of Si–O bond decreases, meanwhile the relative content of C–O bond increases, and an obvious C=O bond (531.44 eV) fitting peak appears. All these evidences indicate that the random copolymer brush has been successfully prepared by SI-ATRP and chemical hydrolysis.
Figure 6. High-resolution XPS spectra and curve fitting of O1s of (a) SiO2 and (b) SiO2-P(SPMA-co-MAA).
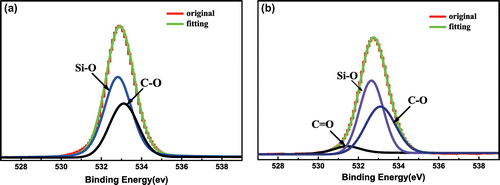
Table 2. O1s high-resolution XPS analysis of SiO2 and SiO2-P(SPMA-co-MAA).
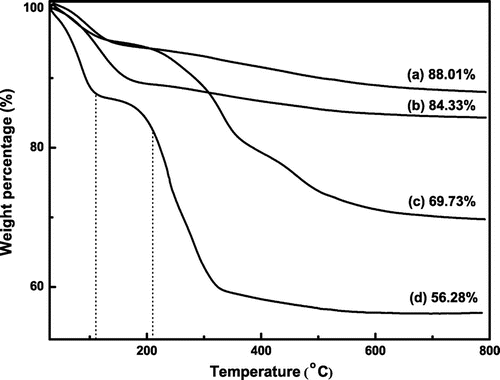
Figure illustrates the TGA results of SiO2, SiO2–NH2, SiO2–Br, and SiO2-g-P(SPMA-co-MAA). As can be seen from Figure (a), the bare silica particles only have a slight weight loss, and weight retention maintains 88.01% at 800 °C, which is possibly caused by the loss of the associated water. TGA curve of the amino-functionalized silica nanoparticles in Figure (b) shows the loss of amine group, and the final weight retention stabilizes at about 84.33%. The curve of the SiO2–Br is illustrated in Figure (c). In addition to the weight loss of physically absorbed water, the second weight loss at 210 °C is due to the decomposition of ATRP initiator grafted on the particles, and finally weight retention stabilizes at about 69.73%. TGA curve of the hybrid silica nanoparticles coated with P(SPMA-co-MAA) in Figure (d) can be divided into three stages. The initial weight loss stage ranging from room temperature to 110 °C is caused by desorption of physically absorbed water and dehydroxylation. The second weight loss stage ranging from 110 to 210 °C shows a moderate decrease which results from the loss of the initiator decomposition. The last sharp weight loss stage ranging from 210 to 800 °C is mainly attributed to the P(SPMA-co-MAA) which are grafted on the surface of silica nanoparticles, and the final remaining weight percentage is 56.28%.
Figure 7. The thermogravimetric analysis of (a) SiO2, (b) SiO2–NH2, (c) SiO2–Br, and (d) SiO2-P(SPMA-co-MAA).
The weight loss measured by TGA enables us to calculate the initiator grafting density (GI) as described in the literature.[Citation46]
where W% is the weight loss of various nanoparticles from TGA analysis, NA is the Avogadro’s number, Minitiator is the molar mass of the grafted initiator, and Ssp is the specific surface area. According the BET result, we can know Ssp = 135 m2/g (Figure S2 in the Supporting Information), so the initiator grafting density is about 4.65 molecules/nm2.
In addition, the polymer grafting density (GP) can be also determined from the TGA results as presented below:
where Mn, grafted polymer is the number average molecular weight of the grafted polymer. We get it from GPC result of the free polymer, since the molar weight of the free chains by the sacrificial initiator usually matches those of the grafted chains as reported elsewhere. In the present work, the number average molecular weight of the free P(SPMA-co-tBMA) is about 47,700 (Figure S3 in the Supporting Information), so we can calculate the number average molecular weight of P(SPMA-co-MAA) as 30,300 according to 1H NMR spectrum as shown in Figure S4 (see the Supporting Information). Then, polymer grafting density can be approximately determined as 0.053 chains/nm2, which is consistent with Save and Charleux’s report.
In addition, we can give a rough estimate on the length of the grafted chains (L) from polymer grafting density according to the following relationship[Citation47]:
where ρ is the density of grafted polymer chains, and it can be approximately thought as 1. In a typical experiment, the length of the grafted chains is about 3 nm, which is in good agreement with the thickness of the grafting polymer layer from TEM analysis.
3.2. Stimuli-responsive behaviors of hybrid nanoparticles SiO2-g-P(SPMA-co-MAA)
To explore the light/pH double responsive behaviors of polymer brushes, UV–vis absorption spectrometer is used to record the changes of absorbance upon different light conditions, and DLS is utilized to get the changes of particle sizes on different pH values.
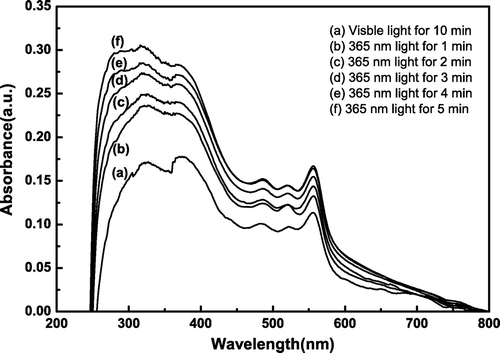
Under the visible light irradiation for 10 min, it is interesting that there is an appearance of an absorption peak at 556 nm in Figure . This is due to the influence of carboxylic groups in MAA unit, and it leads to the ring-opening of spiropyran unit. After the polymer is grafted onto the silica surface, the free movement of the molecules is restricted to some extent. Full ring-closure of the spiropyran unit is difficult to be achieved even if it is under the relatively long irradiation of visible light. When it is irradiated with UV light for 1 min, the absorbance at 556 nm goes up from 0.11349 to 0.13254. Expectedly, with the extension of irradiation time, the absorbance increases gradually, meaning that more spiropyrans transform to MC isomers. These results verify that the spiropyran-based random copolymer brush SiO2-g-P(SPMA-co-MAA) has a good photo-responsive behavior.
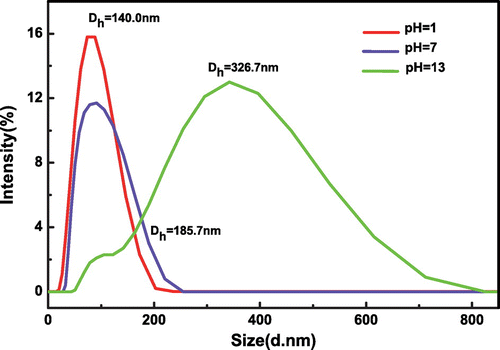
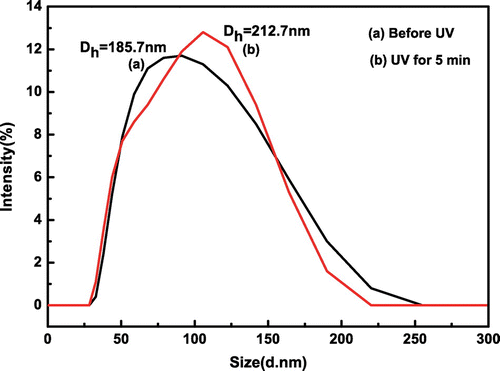
With the purpose of investigating the photosensitive behaviors of hybrid nanoparticles, DLS spectra are presented in Figure . As can be seen, the average hydrodynamic diameter increases from 185.7 to 212.7 nm under UV irradiation for 5 min. This reason is that SPMA is activated by ultraviolet light, and then the hydrophobic structure has become the hydrophilic structure. The particle sizes increase with the decrease of hydrophobic interaction and the enhancement of hydrophilic interaction.
Figure 9. The hydrodynamic diameters distributions of SiO2-g-P(SPMA-co-MAA) under different light conditions.
The DLS characterization is employed to study the effect of pH on particle sizes and distribution of SiO2-g-P(SPMA-co-MAA), and the results are shown in Figure . As can be seen, the hydrodynamic diameter of hybrid silica nanoparticles SiO2-g-P(SPMA-co-MAA) rises from 140.0 to 185.7 nm when pH value changes from 1 to 7. The hydrodynamic diameter increases from 185.7 to 326.7 nm when pH value changes from 7 to 13. The schematic illustration of pH-responsive behavior is shown in Scheme . It is noted that the wide particles size distribution at pH 13 is caused by the deprotonation of carboxyl (–COOH) in the MAA unit. Under the condition of alkali, the MAA unit in the random copolymer brushes is hydrophilic, and the electrostatic repulsion between the carboxyl anions (–COO−) makes the copolymer brushes more fluffy and swelling which leads to the increase in particle size. Moreover, because the copolymer on the surface is random, the strength of the electrostatic repulsion is unequal in different chain units, and this contributes to the appearance of a broad peak. The other reason is that the hydrophobic isomer of SPMA is transformed into the hydrophilic MCH isomer under acidic condition, and the resulting MCH isomer usually switches into hydrophilic MC isomer under alkaline condition.
4. Conclusion
In this study, we report the successful synthesis of organic/inorganic hybrid nanoparticles SiO2-g-P(SPMA-co-MAA) by SI-ATRP and chemical hydrolysis, and the double stimuli-sensitive behaviors of the hybrid nanoparticles have been investigated. TEM is used to show core/shell structure of the hybrid nanoparticles, and EDS demonstrates the change of element content before and after SI-ATRP. Both FT-IR and XPS verify the existence of chemical bonding on the interface between inorganic silica and polymeric layer. Also, the three-stage TGA curve of hybrid silica nanoparticles coated with P(SPMA-co-MAA) three stages is observed. The dual-responsive properties are characterized by means of UV–vis spectrophotometer and DLS. The average hydrodynamic diameter of SiO2-g-P(SPMA-co-MAA) increases from 185.7 to 212.7 nm under ultraviolet light irradiation for 5 min. Also, the particle size of SiO2-g-P(SPMA-co-MAA) increases with the rising pH value of surrounding condition.
All the results in the present work indicate that the hybrid particles are dual-sensitive, which may provide a diverse range of potential applications such as drug delivery, diagnostics, tissue engineering, biosensors, coatings, and textiles. As one of ‘grafting from’ methods, SI-ATRP can offer dense and strong ‘living’ centers for the polymeric molecular chains. In addition, the grafting density and molecular weight of the resulting polymers can be better controlled by SI-ATRP than by traditional polymerization process.
Supporting information available
The plotting between ln τ and ln λ in the absorbance experiment to assess dispersion stability, nitrogen adsorption/desorption isothermal curve of the silica nanoparticles, GPC result of the free copolymer P(SPMA-co-tBMA) obtained from the sacrificial initiator, and 1H NMR spectrum of the precursor copolymer P(SPMA-co-tBMA) in CDCl3.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/15685551.2015.1136536.
Supporting_Information.zip
Download Zip (728.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was financially supported by Natural Science Foundation of China [grant number 21376271], Natural Science Foundation of Hunan Province [grant number 2015JJ2174], Scientific Research Foundation for the Returned Overseas Chinese Scholars, Undergraduates innovation training project, and Excellent seedling funding of Central South University.
References
- Li ZZ, Wen LX, Shao L, et al. Fabrication of porous hollow silica nanoparticles and their applications in drug release control. J. Controlled Release. 2004;98:245–254.10.1016/j.jconrel.2004.04.019
- Santra S, Yang H, Dutta D, et al. TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem. Commun. 2004;24:2810–2811.10.1039/b411916a
- Xu H, Yan F, Monson EE, et al. Room-temperature preparation and characterization of poly(ethylene glycol)-coated silica nanoparticles for biomedical applications. J. Biomed. Mater. Res. 2003;66A:870–879.10.1002/(ISSN)1097-4636
- Radu A, Byrne R, Alhashimy N, et al. Spiropyran-based reversible, light-modulated sensing with reduced photofatigue. J. Photochem. Photobiol., A. 2009;206:109–115.10.1016/j.jphotochem.2009.05.022
- Biggs BW, Hunt HK, Armani AM. Selective patterning of Si-based biosensor surfaces using isotropic silicon etchants. J. Colloid Interface Sci. 2011;369:477–481.
- Kim MH, Na HK, Kim YK, et al. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano. 2011;5:3568–3576.10.1021/nn103130q
- Wang L, Zhao W, Tan W. Bioconjugated silica nanoparticles: development and applications. Nano Res. 2008;1:99–115.10.1007/s12274-008-8018-3
- Cui H, Liu H, Chen S, et al. Synthesis of amphiphilic spiropyran-based random copolymer by atom transfer radical polymerization for Co2+ recognition. Dyes Pigm. 2015;115:50–57. 10.1016/j.dyepig.2014.12.008
- Chen S, Liu H, Cui H, et al. Synthesis of spiropyran-containing random copolymer by atom transfer radical polymerization and its complexation with metal ions. Des. Monomers Polym. 2015;18:574–582.10.1080/15685551.2015.1045226
- Jalalian E, Mehdipour-Ataei S, Babanzadeh S, et al. Silicon-containing poly(amide-imide)s: preparation, characterization, and properties. Des. Monomers Polym. 2015;18:714–722.10.1080/15685551.2015.1070502
- Achilleos DS, Vamvakaki M. End-grafted polymer chains onto inorganic nano-objects. Materials. 2010;3:1981–2026.10.3390/ma3031981
- Jones DM, Brown AA, Huck WTS. Surface-initiated polymerizations in aqueous media: effect of initiator density. Langmuir. 2002;18:1265–1269.10.1021/la011365f
- Jones DM, Smith JR, Huck WTS, et al. Variable adhesion of micropatterned thermoresponsive polymer brushes: AFM investigations of poly(n-isopropylacrylamide) brushes prepared by surface-initiated polymerizations. Adv. Mater. 2002;14:1130–1134.10.1002/1521-4095(20020816)14:16<1130::AID-ADMA1130>3.0.CO;2-7
- Paniagua SA, Kim Y, Henry K, et al. Surface-initiated polymerization from barium titanate nanoparticles for hybrid dielectric capacitors. ACS Appl. Mater. Interfaces. 2014;6:3477–3482.10.1021/am4056276
- Klok HA, Genzer J. Expanding the polymer mechanochemistry toolbox through surface-initiated polymerization. ACS Macro Lett. 2015;4:636–639.10.1021/acsmacrolett.5b00295
- Lee SH, Dreyer DR, An J, et al. Polymer brushes via controlled, surface-initiated atom transfer radical polymerization (ATRP) from graphene oxide. Macromol. Rapid Commun. 2010;31:281–288.10.1002/marc.v31:3
- Ding S, Floyd JA, Walters KB. Comparison of surface confined ATRP and SET-LRP syntheses for a series of amino (meth)acrylate polymer brushes on silicon substrates. J. Polym. Sci., Part A: Polym. Chem. 2009;47:6552–6560.10.1002/pola.v47:23
- Nagase K, Watanabe M, Kikuchi A, et al. Thermo-responsive polymer brushes as intelligent biointerfaces: preparation via ATRP and characterization. Macromol. Biosci. 2011;11:400–409.10.1002/mabi.v11.3
- Saigal T, Xu J, Matyjaszewski K, et al. Emulsification synergism in mixtures of polyelectrolyte brush-grafted nanoparticles and surfactants. J. Colloid Interface Sci. 2014;449:152–159.
- Liu H, Chen S, Cui H, et al. Fabrication of triple responsive polymer brushes and their catalytic performance after loading palladium. RSC Adv. 2015;5:72444–72452.10.1039/C5RA13245B
- McCaig HC, Myers E, Lewis NS, et al. Vapor sensing characteristics of nanoelectromechanical chemical sensors functionalized using surface-initiated polymerization. Nano Lett. 2014;14:3728–3732.10.1021/nl500475b
- Chen JK, Bai BJ. pH-switchable optical properties of the one-dimensional periodic grating of tethered poly(2-dimethylaminoethyl methacrylate) brushes on a silicon surface. J. Phys. Chem. C. 2011;115:21341–21350.10.1021/jp207728f
- Wu T, Zou G, Hu JM, et al. Fabrication of photoswitchable and thermotunable multicolor fluorescent hybrid silica nanoparticles coated with dye-labeled poly(N-isopropylacrylamide) brushes. Chem. Mater. 2009;21:3788–3798.10.1021/cm901072g
- Blum AP, Kammeyer JK, Rush AM, et al. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015;137:2140–2154.10.1021/ja510147n
- Meng H, Hu J. A brief review of stimulus-active polymers responsive to thermal, light, magnetic, electric, and water/solvent stimuli. J. Intell. Mater. Syst. Struct. 2010;21:859–885.10.1177/1045389X10369718
- Shanmuganathan K, Capadona JR, Rowan SJ, et al. Stimuli-responsive, mechanically-adaptive polymer nanocomposites. ACS Appl. Mater. Interfaces. 2011;21:2812–2822.
- Sudhakar K, Madhusudana Rao K, Subha MCS, et al. Temperature-responsive poly(N-vinylcaprolactam-co-hydroxyethyl methacrylate) nanogels for controlled release studies of curcumin. Des. Monomers Polym. 2015;18:705–713.10.1080/15685551.2015.1070497
- Lu C, Urban M. Rationally designed gibbous stimuli-responsive colloidal nanoparticles. ACS Nano. 2015;9:3119–3124.10.1021/nn507489p
- Tan BH, Tam KC, Lam YC, et al. Microstructure and rheology of stimuli-responsive nanocolloidal systems effect of ionic strength. Langmuir. 2004;20:11380–11386.10.1021/la0481290
- Gao W. pH-responsive nanoparticles for drug delivery. Mol. Pharm. 2010;7:1913–1920.10.1021/mp100253e
- Mane SR, Dinda H, Sathyan A, et al. Increased bioavailability of rifampicin from stimuli-responsive smart nano carrier. ACS Appl. Mater. Interfaces. 2014;6:16895–16902.10.1021/am504402b
- Lu Y, Zhou T, Fan Q, et al. Light-responsive viscoelastic fluids based on anionic wormlike micelles. J. Colloid Interface Sci. 2013;412:107–111.10.1016/j.jcis.2013.09.014
- Jin C, Sun X, Wu L. Synthesis and characterization of N,N-bis(2-hydroxyethyl) cinnamamide as a photo-responsive monomer. Des. Monomers Polym. 2011;14:47–55.10.1163/138577210X541196
- Jang AR, Jeon EK, Kang D, et al. Reversibly light-modulated dirac point of graphene functionalized with spiropyran. ACS Nano. 2012;6:9207–9213.10.1021/nn303539y
- Shao N, Jin JY, Cheung SM, et al. A spiropyran-based ensemble for visual recognition and quantification of cysteine and homocysteine at physiological levels. Angew. Chem. 2006;118:5066–5070.10.1002/(ISSN)1521-3757
- Andersson J, Li S, Lincoln P, et al. Photoswitched DNA-binding of a photochromic spiropyran. J. Am. Chem. Soc. 2008;130:11836–11837. 10.1021/ja801968f
- Rosario R, Gust D, Hayes M, et al. Photon-modulated wettability changes on spiropyran-coated surfaces. Langmuir. 2002;18:8062–8069.10.1021/la025963l
- Renkecz T, Mistlberger G, Pawlak M, et al. Molecularly imprinted polymer microspheres containing photoswitchable spiropyran-based binding sites. ACS Appl. Mater. Interfaces. 2013;5:8537–8545.10.1021/am401958e
- Radu A, Byrne R, Alhashimy N, et al. Spiropyran-based reversible, light-modulated sensing with reduced photofatigue. J. Photochem. Photobiol., A. 2009;206:109–115.10.1016/j.jphotochem.2009.05.022
- Liu DE, Kotsmar C, Nguyen F, et al. Macromolecule sorption and diffusion in HEMA/MAA hydrogels. Ind. Eng. Chem. Res. 2013;52:18109–18120.10.1021/ie402148u
- Chevalier Y, Hidalgo M, Cavaillé JY, et al. Structure of waterborne organic composite coatings. Macromolecules. 1999;32:7887–7896.10.1021/ma990561e
- Peng S, Zhu P, Mhaisalkar SG, et al. Self-supporting three-dimensional ZnIn2S4/PVDF–Poly(MMA-co-MAA) composite mats with hierarchical nanostructures for high photocatalytic activity. J. Phys. Chem. C. 2012;116:13849–13857.10.1021/jp302741c
- Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968;26:62–69.10.1016/0021-9797(68)90272-5
- Huang CQ, Wang Y, Hong CY, et al. Spiropyran-based polymeric vesicles: preparation and photochromic properties. Macromol. Rapid Commun. 2011;32:1174–1179.10.1002/marc.v32.15
- Mitani Y, Teshima T, Yoshida Y, et al. Dispersibility of fumed silica in simple media. J. Ceram. Soc. Jpn. 1993;101:707–712.10.2109/jcersj.101.707
- Pasetto P, Blas H, Audouin F, et al. Mechanistic insight into surface-initiated polymerization of methyl methacrylate and styrene via ATRP from ordered mesoporous silica particles. Macromolecules. 2009;42:5983–5995.10.1021/ma9003506
- Zhao B. Synthesis of binary mixed homopolymer brushes by combining atom transfer radical polymerization and nitroxide-mediated radical polymerization. Polymer. 2003;44:4079–4083.10.1016/S0032-3861(03)00322-7