Abstract
In this article, polymerization of 1-hexene with FeCl3-doped Mg(OET)2/TiCl4/electron donor (ED) catalytic system is presented. For this purpose, first a number of TiCl4 catalysts supported on Mg(OEt)2 and Fe-doped Mg(OEt)2 supports were prepared with ethylbenzoate or dibutylphthalate as the internal EDs. After successive catalysts synthesis, they were employed in 1-hexene polymerization using cyclohexyl methyl dimethoxysilane as external ED as well as without it. The catalysts activity and molecular weight distribution (MWD) of poly 1-hexenes (PHs) were influenced strongly by both FeCl3 doping and donor presence so that a remarkable increase in the catalyst activity was seen in doped catalysts. Deconvolution of MWD curves revealed that increase in the type of active centers by introducing FeCl3 into the support should be responsible for the broadening of MWD of PHs. 13CNMR analysis indicated that while isotacticity does not change considerably by Fe doping, EDs increase its amount as high as 8–21%. Second, the stereoselective behavior of active Ti species in doped and undoped catalysts was fully explored by molecular modeling using density functional theory (DFT) method. Finally, with the aid of rheological measurements, the processability of polymers were evaluated and then the gel permeation chromatography (GPC) results were approved through the values obtained from model fitting as well as changes in moduli crossover modulus.
1. Introduction
Stereospecific heterogeneous α-olefin polymerization has been a major research field since the first discovery of Ziegler–Natta (ZN) olefin polymerization catalysts.[Citation1–4] Although most of the research efforts have been focused on the development of new catalytic systems for propylene polymerization,[Citation5–8] the design and synthesis of efficient catalysts for the stereoselective polymerization of longer chain α-olefins have also attracted extensive research interests since the polymeric materials based on α-olefins could find variety of applications. Low molecular weight (MW) poly(α-olefin)s are used as base materials for lubricating oil formulation, while high MW ones are used as pour point depressant and drag-reducers.[Citation9] Other applications include their usage in adhesives, foams, carriers in anti-perspirants and deodorants.[Citation10,11]
So far, many ZN as well as single-site metallocene and late transition metal catalysts have been reported that can polymerize higher α-olefins into their related (co)polymers.[Citation12–14] Based on these studies, it was evidenced that primary ZN catalysts based on MgX2 have less activity and stereospecificity in the polymerization of longer α-olefins and many efforts have been taken for solving these problems. In this respect, an electron donor (ED) was used to solve the stereospecifity problem, while some methods such as support modification was proposed to raise the activity. Selected modifiers were often chosen from organohalides,[Citation15–17] metal halides,[Citation18] and metalloide families.[Citation3] Recent investigations have shown that doping a suitable amount of Lewis acids in MgCl2 or Mg(OEt)2 supports would be an effective way to improve their catalytic performance in α-olefin polymerization.[Citation19] It is widely accepted that a high share of active sites are formed on the surface defects of MgX2 (magnesium alkoxide, and magnesium chloride) crystallites. Doping MgX2 with inorganic salts can change the distribution of these defects which lead to the better distribution of active center on the catalyst surface.[Citation18,20] Furthermore, there are some literature reporting control of molecular weight distribution (MWD) of polymer through modification of MgCl2-supported Ti-based ZN catalyst by doping inorganic compounds [Citation18,20–22] and Mg(OEt)2 support, by doping FeCl3/SiCl4.[Citation19] Based on these reports, changes in MW (both increase and decrease) of the resulted polymer and its distribution were seen in doped catalysts.
Following our previous study based on Mg(OEt)2 doping by FeCl3,[Citation19] which showed remarkable increase in activity and comonomer incorporation in linear low density polyethylene (LLDPE) synthesis, in this study, the system of Mg(OEt)2/FeCl3/SiCl4/ED/triethylaluminum (TEA)/TiCl4 is used for the stereospecific polymerization of 1-hexene in order to study the effect of several types of EDs on the stereospecifity of modified catalyst. While that this system has shown significant increase in the catalytic activity, enhanced stereospecificity (up to 87 mmmm%) was achieved as well. Obtained results indicated that our suggested system can be successfully used in large-scale production of higher α-olefin polymers.
2. Experimental section
2.1. Materials
Mg(OEt)2, tetrahydrofuran (THF), FeCl3, SiCl4, potassium hydroxide, and titanium tetrachloride (99%) were purchased from Merck (Darmstadt, Germany). n-hexane and toluene were supplied by Bandar Imam Petrochemical Co. (Mahshahr, Iran), distilled over calcium hydride, and stored over 13X and 4 Å type activated molecular sieves and sodium wire. TEA, ethylbenzoate (EB), di n-butylphthalate (DNBP), cyclohexyl methyl dimethoxysilane (CMMS), and 1-hexene of 93% purity were also purchased from Aldrich Chemical Co. (Munich, Germany). Nitrogen gas of 99.99% purity was supplied from Roham Co. (Tehran, Iran), then purified and dried by passing through KOH, activated silica gel, and 4 Å molecular sieve columns.
2.2. Preparation of the support
Preparation of the Mg(OEt)2 and Mg(OEt)2/FeCl3 (90/10 w/w) supports was accomplished according to a procedure in Ref. [Citation19].
2.3. Synthesis of catalysts
2.0 g Mg(OEt)2 or Mg(OEt)2/FeCl3 and 80 mL toluene were sequentially added in to a 500 mL two-necked reactor. After stirring for 30 min at 50 °C at which the mixture formed a homogeneous solution, the temperature was raised to 80 °C. Then, 4 mL TiCl4 was added to the solution at 80 °C. After 2 h, the liquid phase was removed, and the solid residue was washed twice with 100 mL of toluene. Then proper amount of internal ED (Mg/EB = 2–3 and Mg/DNBP = 8–10 mol/mol) has been added to the system followed by the addition of a mixture of toluene (100 mL) and TiCl4 (4 mL). The temperature was raised to 120 °C, and the mixture was stirred for another 2 h. The product was washed twice with 100 mL toluene and 4 times with n-hexane to remove unreacted TiCl4. The final catalyst was dried at 70 °C, under a flow of N2 atmosphere in 2 h.
2.4. 1-Hexene polymerization
A mixture of 0.08 mol of 1-hexene and 50 mL of n-hexane was taken in a three-necked round bottom flask and flushed with dry nitrogen. The amount of cocatalysts by a ratio of Al/Ti = 50 and CMMS (Al/Si = 40) were injected into the system by syringe. The polymerization started by adding 10 mg of catalyst to the system under nitrogen atmosphere. The mixture was stirred by a magnetic stirrer at 50 °C for 2 h. Then, the reaction was terminated by adding the acidified methanol. The obtained polymer was washed with methanol and dried under vacuum at 50 °C overnight.
2.5. Characterization techniques
Crystal structure of the supports and catalysts was determined by X-ray diffraction (XRD) on a Siemens D-5000 X-ray diffractometer (USA) operating at 40 kV and 25 mA with a copper target (λ = 1.54 Å) and at a scanning rate of 3° min−1. The morphologies of the catalysts and energy-dispersive X-ray spectroscopy (EDX) maps were observed by scanning electron microscopy (SEM model S-3000 N, Hitachi, Japan) after coating with a gold sputter coating machine (model E-1010, Hitachi, Japan). For determination of Ti content, after sample digestion in H2SO4, Ti was oxidized with H2O2 and analyzed by UV–visible spectrophotometer (λ = 410 nm) in a Shimadzu 100 spectrophotometer model 6800, USA. The molecular masses and their distributions were determined through gel permeation chromatography (Waters GPCV 150+, USA), using THF as the eluent at room temperature. The differential scanning calorimetry (DSC) tests were performed on a DSC Q 1000 of TA (USA), with samples of about 5 mg sealed in aluminum pans, under nitrogen atmosphere in a temperature range between −60 and 150 °C, at a heating rate of 10 °C min−1. Glass transition (Tg) and melting (Tm) temperatures were reported from the second heating scan. Dynamic mechanical thermal (DMTA) properties of the samples were carried out according to ASTM E1640-04 using a DMA, Tritec 2000, under the Tension mode at a frequency of 1 Hz. The temperature range was from −80 to 100 °C and the specimens were heated at a rate of 10 °C min−1. TGA curves were obtained with a Perkin Elmer Pyris Diamond, USA under nitrogen atmosphere with a heating rate of 10 °C min−1 and temperature range of 25–600 °C. The tacticity of polymers were obtained from the 13CNMR spectra recorded on a Bruker Avance-400 spectrometer in CDCl3 at frequency of 500 MHz. The melt rheological properties of the samples were determined using a stress-controlled MCR300 rheometer (Austria). The measurements were performed in the dynamic mode using 25 mm parallel plates geometry with gap settings according to the disk thickness under nitrogen atmosphere. The strain amplitude was set at 1% and checked to be within linear viscoelastic range by strain sweeps. The linear viscoelastic properties of samples were measured at 140 °C; the frequency was varied between 600 and 0.01 rad/s.
2.6. Computational details
DFT calculations were performed with the Gaussian 09 package [Citation23] using the BP86 level of theory.[Citation24] In all cases, the electronic configuration of the molecular systems was described with the standard split valence basis set with a polarization function of Ahlrichs and co-workers for H, C, Mg, and Cl (SVP keyword in Gaussian 09).[Citation25] For Ti and Fe, we used the small-core, quasi-relativistic Stuttgart/Dresden effective core potential, with an associated valence basis set (SDD keywords in Gaussian 09).[Citation26]
In case of undoped catalyst model, the doublet electronic state was used. Differently, for Fe-doped species, the quartet electronic state was favored, since Fe usually is in high spin mode.
Characterization of the located stationary points as transition state was performed by frequency calculations.
3. Results and discussion
3.1. Characterization of the supports and catalysts
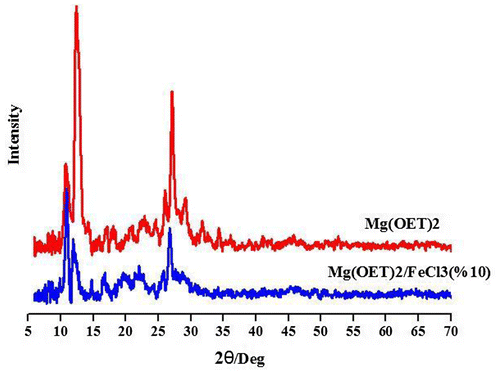
To obtain Mg(OEt)2/FeCl3 mixed support, Mg(OEt)2 together with 10 w/w% of FeCl3 were dispersed in THF and then the support extracted from the solution by settling and drying under N2 atmosphere. Figure shows the powder XRD patterns for pure Mg(OEt)2 and modified support (Mg(OEt)2/FeCl3). As can be seen, in the XRD pattern of Mg(OEt)2, three peaks appeared at 2θ mounts equal to 11°, 13°, and 27°. About pure FeCl3, it had already shown that five peaks appeared at 2θ = 19°, 39°, 42°, 55°, 68°.[Citation19] In the doped support, the introduction of FeCl3 caused a disorder in the crystalline structural of Mg(OEt)2, attributing to the reduction of the intensity of XRD patterns at 2θ ≈ 11°, 13° and 27°.[Citation18] It can be deduced from XRD patterns that FeCl3 was fully dissolved in Mg(OEt)2 structure. Such a behavior might be ascribed to the structures in which magnesium is partially replaced by Fe+2, since their ionic radii are very similar (Mg2+ = 0.88 Å, Fe+2 = 0.84 Å, and Fe+3 = 0.74 Å).
Four different catalysts were prepared by: (1) simple reaction of unmodified support with TiCl4 (Cat A), (2) reaction of Fe-doped Mg(OEt)2 with TiCl4 (Cat B), (3, 4) reaction of modified support with TiCl4 in the presence of EB and DNBP internal donors (Cat C and Cat D, respectively). The catalysts compositions were shown in Table .
Table 1. Composition, abbreviation, and Ti content of the prepared catalysts.
UV–visible technique was used to achieve titanium content of the catalysts and the data were collected in Table . Results showed a decrease in the Ti content of the FeCl3-modified catalysts (Cat B, C, D) compared with it in unmodified one (Cat A). This can be due to the decrease in the binding energy of the Ti–MgCl2 support in the presence of Fe dopant which was obtained by DFT simulations.[Citation19] Furthermore, Ti content decreased by the addition of EB and DNBP donors, which shows the strong interaction between TiCl4 and the internal donors since TiCl4 can also behave as a Lewis acidic site to the donor molecules.[Citation19,27]
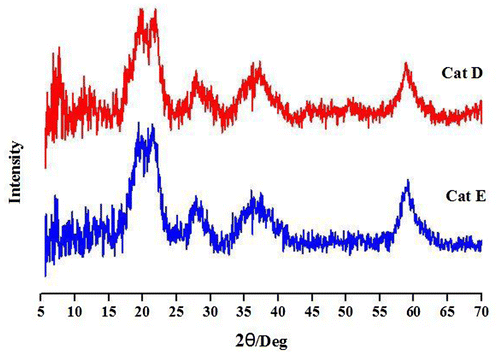
Then, to understand how the crystal structure of Mg(OEt)2 can be influenced by TiCl4 and/or ED, a structural investigation on Cat C and Cat D was carried out by X-ray technique. The obtained XRD patterns were shown in Figure . As can be seen, two peaks at 2θ of 11° and 13° in Mg(OEt)2 disappeared in Cat C and Cat D diffractograms which showed a XRD pattern similar to it in the activated MgCl2 with broad peaks at 2θ = 19°, 23°, 28°, 36° and 60° corresponded to the structurally disordered δ-MgCl2 (Figure ).[Citation28] These results confirmed the conversion of Mg(OEt)2 to MgCl2 by reacting with TiCl4, FeCl3, and even ED and SiCl4.[Citation27,29] According to the mentioned results, it could be deduced that TiC14, ED and SiCl4 in the Mg(OEt)2-supported catalyst play an important role in the distortion of crystal lattice of MgCl2 formed from the chlorination of Mg(OEt)2 by TiC14, FeCl3 and/or SiCl4.
Figure 3. Expanded side chain methylene (C3) resonance patterns of PHs synthesized using Cat B, Cat C, and Cat D.
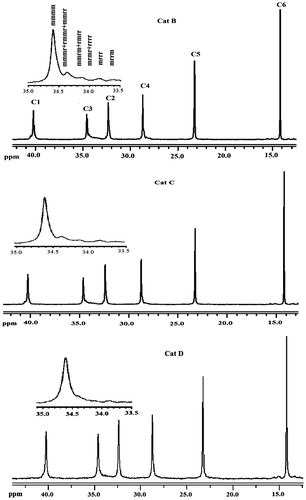
Figure 4. Expanded side chain methylene (C3) resonance patterns of poly(1-hexene) polymerized using Cat D in 25 °C.
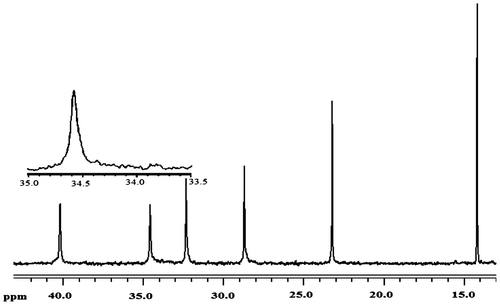
Figure 6. DSC curves of PHs obtained from (a) Cat C, Cat D, and Cat E in Tg region and (b) Cat C in Tm region.
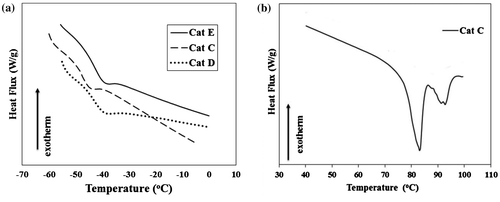
Figure 9. Employed models for the simulation of propene insertion into Ti-iBu active center. (a) MgCl-UD as undoped catalyst, (b) MgCl-D1 and (c) MgCl-D2 as doped catalyst models.
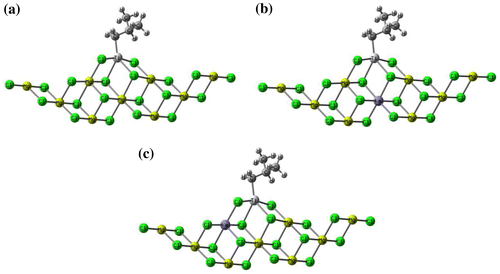
Figure 11. Storage and loss modulus versus frequency for poly(1-hexene) obtained with Cat-C, D and E at 140 °C.
The surface composition of the synthesized catalysts was obtained by EDX analysis and listed in Table .
Table 2. Elemental analysis of the prepared catalysts.
According to the information provided in Table , catalysts without internal donor, i.e. Cat A and Cat B, showed the highest amount of surface Cl atom. This amount decreased by using EB and DNBP donors in Cat C and Cat D while carbon content increased in the presence of internal donors. It was also observed that the Si % in catalysts surfaces varied, although its amount was invariant during all catalysts synthesis. Furthermore, there was a difference in the Fe content between added amount of 10%, which was used in support preparation step, and the amount in the final catalyst systems.
3.2. Catalysts activities
The performance of the synthesized catalysts was evaluated toward 1-hexene polymerization using TEA as cocatalyst. Activity results were collected in Table . As can be seen, Cat B showed nearly sevenfold increase in the activity (or 684%) compared with unmodified one (Cat A, 950 g polymer/g Ti h). This increase in activity was much higher than in HDPE (137%) and LLDPE (577%) synthesis.[Citation19] As reported in our recent manuscript, increase of Mulliken charge in active Ti center in the Fe-doped catalyst together with halogen-donating ability of SiCl4 could be responsible for the activity increase in partially doped catalyst.[Citation19] Furthermore, the activity increased as a result of the addition of internal ED and reached to 10,050 and 9900 g polymer/g Ti h in the case of EB and DNBP, respectively. On the other hand, the addition of CMMS as an external ED resulted in the considerable decrease in catalyst activity, probably due to some poisoning effect of external donor which was suggested by other authors.[Citation30,31]
Table 3. Activity of the synthesized catalysts toward 1-hexene polymerization.Table Footnotea
3.3. Characterization of poly1-hexenes
3.3.1 Stereo-regularity of polymers
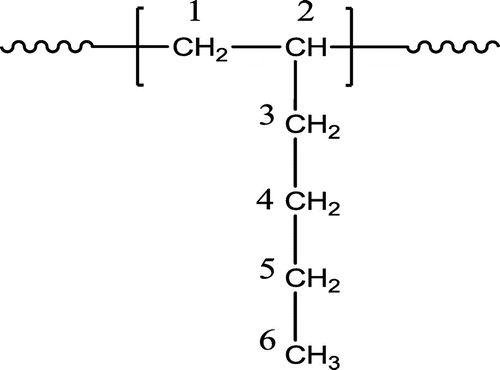
Carbon-13 nuclear magnetic resonance (13CNMR) spectroscopy was employed for micro-structural analysis of the polymers. Six types of C atoms with very similar chemical shifts existed in all of the spectra corresponded to the six C atoms in the poly 1-hexene (PH) structural unit (Scheme ).
In order to understand the configurational arrangement of 1-hexene monomer unit in the PHs obtained by using different catalytic systems, the side chain methylene carbon (C3 in Scheme ) was analyzed as the most sensitive carbon nuclei of pendant group toward stereo-regularity.[Citation30,32] Figure shows the expanded region of side chain methylene carbon (C3) of PHs obtained by different catalytic systems at 50 °C.
The character of the pentads was obvious. The isotactic pentad ‘mmmm,’ the ‘mmmr + rmmr + mmrr,’ the ‘mrrr,’ and the ‘mrrm’ usually show some peaks located at 34.7, 34.5, 34.0, and 33.9 ppm, respectively.[Citation33,34] The tacticity pentad content was calculated and shown as isotacticity (%) in Table . As it can be seen from Table , Fe-doping of the Mg(OEt)2 support decreased slightly isotactic content of the resulted polymer (from 66 to 65%), while that the isotactic pentad content increased with the addition of EB and DNBP internal EDs and reached to 74 and 77%, respectively. Indeed, the obtained PHs were largely stereoregular (Cat C: 83% and Cat D: 85%) so that the isotactic content was on higher side for DNBP compared to EB system, but syndiotactic content was on higher side for EB in compare with DNBP system.
Table 4. The tacticity pentad content in C3 methylene region.
It has already been shown that the extraction of internal donors from catalyst surface during polymerization decreases catalyst isospecificity.[Citation35] To prevent this deterioration, CMMS external donor was added during 1-hexene polymerization (Cat E) which led to the increase in catalyst isospecificity up to 87% with the total tacticity of 90%.
Concluding this section, our obtained results indicated that Fe doping, besides being helpful in catalyst activity increase toward higher α-olefin polymerization, does not change stereospecifity, considerably.
Generally, tacticity is considerably affected by polymerization temperature.[Citation13] To study such effect in Fe-doped catalyst, we have selected Cat D as highly active one and employed it in 1-hexene polymerization at lower temperature of 25 °C. The poly1-hexene produced by this system exhibited an increase in isotacticity up to 82%, while the syndiotacticy decreased to 9% by temperature decrease (Figure ). It was deduced from this section that the possibility of stereoerrors decreases at lower temperature due to the fact that α-olefins have enough energy to overcome higher energy barriers regarding stereoerrors at higher temperatures.[Citation36]
3.3.2 MW and MWD of PHs
The MW and MWD data for PHs prepared by different catalytic systems are listed in Table .
Table 5. Molecular weight and MWD of PHs by different catalysts.Table Footnotea
It is clear from the data in Table that the Mn and Mw of PHs decreased from 40,936 and 318,065 in undoped catalyst to 27,394 and 271,505 in Fe-doped catalyst (Cat B), respectively. Also, the MWD of the PH became wider in Cat B compare with in unmodified catalyst.
Furthermore, the MW and MWD were also influenced by the presence of EDs. The MW of the polymer increased in the presence of DNBP (Cat C) and reached to 48,802 and 483,425 for Mn and Mw, respectively. The use of CMMS as external donor in Cat D did not change the story and MW of the polymer increased as well. On the other hand, EB led to a decrease in the MW. Moreover, the polydispersity index of PH reduced slightly in Cat C and Cat E, probably due to poisoning of some non-specific sites by EDs which was suggested by Busico and coworkers.[Citation37]
3.3.3 The non-linear fitting of the MWD profiles of PH
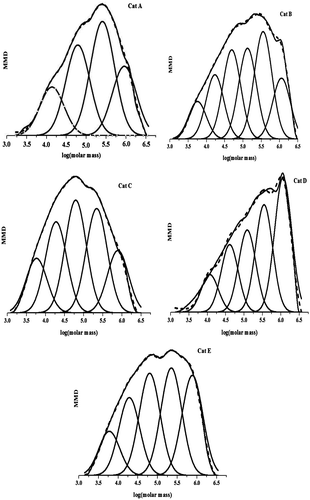
One way to find a better view of the distribution of the active center in heterogeneous catalysts is deconvolution of the MWD profile of polymer by the most probable distribution function (Flory function). In this function, Schulz-Flory distribution with distributing index equal to 2.0 is considered to evaluate polymers synthesized by one kind of active center in ZN catalysts. In this case, MWD is a superposition of the distribution index of each kind of active centers. Therefore, the distribution index of each component deconvoluted by the Flory function is 2. The species of active centers and the peak height proportion are denoted by the number of peaks and the relative weight of the polymer produced, respectively. Previous studies have shown that this method is efficiently useful for analyzing the active center distribution of heterogeneous catalysts.[Citation7]
Figure shows GPC curve of PH samples and their deconvolutions which were produced by different catalysts. For the synthesized samples, four to six Flory components can be deconvoluted to fit the MWD of PHs in a satisfactory manner. The deconvolution results showed that four types of active centers exist in undoped Mg(OEt)2-supported catalyst (Cat A), while six types of active centers exist in Fe-doped Mg(OEt)2-supported catalyst (Cat B). Accordingly, it was concluded that broadening of MWD of PHs is a result of increased derivatives of active centers formed by introducing FeCl3 into the support. Addition of EB, DNBP, and DNBP/CMMS decreased the number of active center types to five. With the addition of DNBP, the percentage of the Flory peaks of high MW was increased, while those of low MWs were decreased. This shows that DNBP can selectively poison the active centers which produce low-MW PHs.[Citation20]
3.3.4 Thermal properties of the PHs
The most common method used to determine glass transition is the DSC.[Citation38] In DSC, the glass transition temperature (Tg) of the polymers was obtained from the midpoint temperature of base line shift on the second heating traces. DSC thermograms of the PHs obtained by three catalysts Cat C, Cat D, and Cat E are shown in Figure , and their corresponding thermal transitions including the melting temperature (Tm) and the glass transition temperature are listed in Table . All analyzed PHs showed Tg at about −41 to −48 °C that was in good agreement with the data obtained by other researchers.[Citation30]
Table 6. The melting and glass transition temperatures by DSC trace of PHs obtained from different catalysts.
Furthermore, during reheating of the polymer at the rate of 10 °C min−1, broad endothermic peaks which prolonged from 70 to 95 °C were observed as a result of melting for polymer from Cat C (see Figure (b)). It is widely accepted that tacticity has great influence on polymer crystallization.[Citation39] According to our obtained data, although Cat E showed higher tacticity, it seems that high MW of PH synthesized thereof prevented its crystallization and as a result no Tm was seen in DSC curve for this polymer. On the other hand, polymer C showed endothermic Tm peak in the second heating probably due to its lower MW which makes it prone for crystallization during heating–cooling cycle. Indeed, short polymer chains form crystals more readily than long chains, because the long chains tend to be more tangled. As a special remark, one can conclude from this section that although tacticity and MW act in different ways for polymer crystallization, effect of MW was much more significant in our obtained PHs than the stereoregularity.
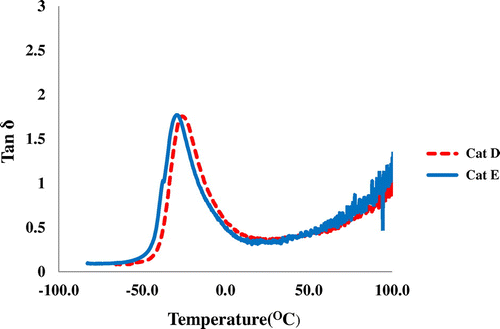
To confirm the Tg data obtained from DSC, DMTA analysis was also performed on PH samples from Cat C, Cat D, and Cat E. Although, DMTA is less commonly used, however, this method is more sensitive to transitions. In DMTA curves, Tg was obtained from the temperature at which tan δ went through a maximum (see Table ). The glass transition temperature of PHs was found in the range of −26.1 to −29.3 °C. Also, the representative DMTA curves are shown in Figure .
3.3.5 Thermogravimetric analysis (TGA)
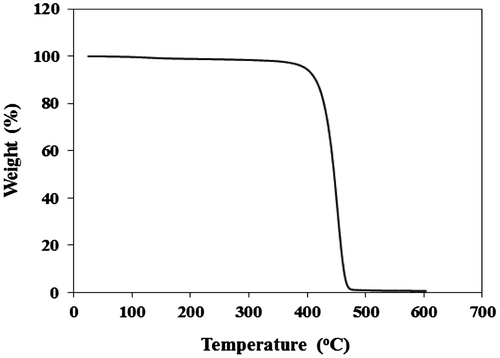
Thermogravimetric analysis (TGA) in air at a heating rate of 10 °C min−1 is usually applied for the evaluation of thermal stability.[Citation38,40] Thermal stability of the PH samples was investigated by TGA as TGA curve of sample from Cat D was shown in Figure as a representative curve.
All PHs displayed similar thermograms with single weight loss zone in which more than 99% of polymer was decomposed in the temperature range of 400–470 °C. This result was in good agreement with previous studies on thermal decomposition of polyolefins which showed one stage weight loss for this type of polymers.[Citation41]
3.3.6 DFT simulations
In the next part of our manuscript, we employed DFT simulations to assess the effect of Fe doping on the stereoselectivity of the ZN catalyst. We chose MgCl2, instead of Mg(OEt)2, since it was observed that during interaction with TiCl4 and SiCl4, Mg(OEt)2 is converted to MgCl2 due to Cl-donating ability of these compounds.[Citation16,42]
To this end, we considered three model systems: MgCl-UD, MgCl-D1 and MgCl-D2, see Figure for labels. They were considered as good representative model for ZN catalysts since there is growing evidence that the real active Ti species could have a similar environment.[Citation43–45] These systems mainly differ: (1) for the number of Fe atoms involved in the support (Figure (a) with (b) and (c)) and (2) the location of Fe atom toward Ti center (Figure (b) and (c)). These models were selected according to the data in Refs. [Citation18,Citation20] which suggested that in MCln (M is metal)-doped catalysts, a MCln/MgCl2 solution is formed. The theoretical stereoselectivity was computed, according to our previous work,[Citation46] as the energy difference between the two transition states related to the re- and si-enantiofaces of the propene coordination to the metal centre and named as ΔEre-si. It is worth mentioning that although, effect of different EDs (benzoate, phthalate, silanes, etc.) on the stereo regularity of the obtained polymer was studied by many authors,[Citation47–49] such effect in the doped catalysts was not considered yet. Indeed, in this manuscript, we are aimed to assess stereoselectivity of the doped ZN catalyst. Since, we did not find ΔEre-si data for the same model and computational method (as ones used in the present work) in the literature, we had to repeat these calculations for undoped catalyst too.
The very low ΔEre-si of 0.23 kcal mol−1, calculated here for isolated Ti atom on the (1 1 0) lateral cut of undoped MgCl2 (MgCl-UD), describes well essentially nonstereoselective nature of this kind of active site in α-olefin polymerization in the absence of any external ED. This amount is in good agreement with the previous quantum mechanics calculations by Cavallo [Citation47], Busico [Citation48], and Ziegler [Citation49] on similar models. Fe doping did not change considerably the stereoselectivity of active species, since the ΔEre-si amount decreased slightly to 0.19 and 0.20 kcal mol−1 in doped catalyst models, i.e. MgCl-D1 and MgCl-D2, respectively. This observation confirms well similar isotacticity values for undoped (Cat A, 66%) and doped (Cat B, 65%) catalysts which were obtained in experimental section.
To investigate the influence of EB and DNBP donors on the stereoselective behavior of studied models, two molecules of donors were coordinated on both sides of the isolated Ti-active species, and the approximate transition states corresponding to re- and si-enantiofaces of the propene coordination to the metal center were calculated. Obtained results were depicted in Table . The numbers reported in Table clearly indicate that the donor strongly modified the stereoselectivity of the active species so that the si-enantioface of propene was clearly favored over the re-enantioface and the ΔEre-si raised to 2.69, 2.64, 2.50, and 2.48 kcal mol−1 for MgCl-D1-DNBP, MgCl-D2-DNBP, MgCl-D1-EB, and MgCl-D2-EB, respectively. As can be seen, although both donors modified the stereoselectivity of active center, DNBP showed more drastic effect in promoting the formation of active sites with more isospecificity. These ΔEre-si values validated qualitatively the isotacticity results that were obtained experimentally.
Table 7. ΔEre-si for the primary insertion of propene into the Ti-iBu bond of the models depicted in Figure in the presence and absence of DNBP and EB EDs.
3.3.7 Dynamic shear rheological measurements
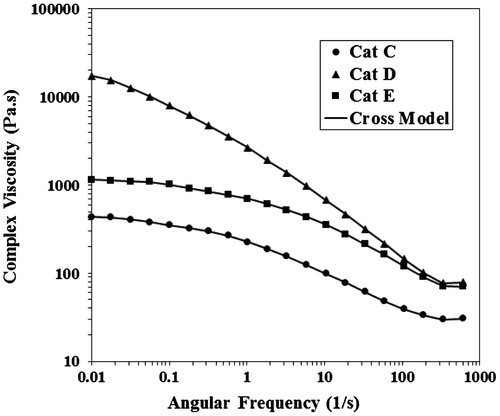
The momentous role of rheology in linear viscoelastic region to examine the molecular features such as MW as well as MWD is inevitable due to its outstanding reproducibility. So, shear rheological measurements were used as reliable endorsements for the results of GPC and as an unrivaled tool for the evaluation of melt processability. Figure shows the complex viscosity against angular frequency for the polymers from Cat C, Cat D, and Cat E. The shear viscosity at lower frequencies which is called zero-shear viscosity is related to MW regarding the Mark-Hawink equation as follows:(1)
where K and α are equation constants. It is obvious that higher zero-viscosity reveals the greater MW. No evident Newtonian plateau was observed for sample D and the onset of shear thinning shifted to lower frequencies which resulted in broader power-law region and was attributed to wider MWD. Having η* vs. ω, the power-law shear thinning index (n), zero-shear viscosity (ηo), and relaxation time of the polymer melt were obtained by fitting the Cross model to the data using the following equation:(2)
The acquired fitted parameters were shown in Table . Despite having higher viscosity, Cat D showed lower power-law index that accounts for its better processability. To bear this in mind, at sufficiently high shear rates, the melt viscosity appeared to depend primarily on number-average MW () rather than weight-average MW; and these observations from the curves were in excellent agreement with the GPC results.
Table 8. The cross-fitting parameters and crossover point characteristics.
The curves of storage modulus and loss modulus were plotted against frequency within linear viscoelastic region and shown in Figure . In their molten state when subjected to applied deformation, polymers behave as a solid-like material (elastic) or liquid-like material (viscous) and the two storage modulus (G′) and loss modulus (G″) are characteristics of these two responses, respectively. Thus, the variation of G′ and G″ over wide range of frequencies is a measure of relative motion of all molecules in the bulk of polymer. During frequency sweep experiment, the storage modulus intercepts loss modulus which indicates a transition from viscous dominant to elastic dominant behavior and the modulus at this point is called crossover modulus (GC). In the point, the crossover frequency (ωC) is defined as the frequency at which . The crossover frequency and modulus are of special interest since they are considered as a measure of MW as well as MWD.[Citation50] The measured GC and ωC were reported in Table . It is shown that the crossover frequency is inversely dependent to the longest relaxation time which is used to make the MW comparison of various samples. It’s worth mentioning that samples with lower GC cast light on their broader MWD through which the results from GPC measurements were qualitatively validated.
4. Conclusions
In this study, synthesis of PH using Mg(OEt)2/TiCl4 and Mg(OEt)2/FeCl3/SiCl4/TiCl4/ED ZN catalytic systems has been considered. The results showed that catalyst activity increases significantly by the addition of FeCl3/SiCl4 and internal EDs. The 13CNMR results showed that the catalyst prepared from different supports (doped and undoped) without internal donor possesses nearly similar isotacticity of 65–66%, while that the isotactic content increased with the addition of EB and DNBP internal EDs and reached to 74 and 77%, respectively. It was found that increasing the type of active centers through introducing FeCl3 into the support should be responsible for the broadening of MWD of PHs. The results of DSC indicated Tgs in the range of −48–(−41) °C for all PHs and Tm of 83 °C for solely PH sample with the lowest MW (i.e. polymer from Cat C). TGA analysis revealed a single weight loss zone in which more than 99% of polymer decomposed in the temperature range of 400–470 °C. DFT simulation confirmed slightly less isotactic behavior of doped catalyst, obtained in experimental section, since ΔEre-si decreased in doped system compared to unmodified one. Finally, conclusions regarding GPC results were endorsed through peerless technique of rheology revealing that samples with higher zero viscosity and lower crossover modulus had greater MW and broader MWD, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors thank MOLNAC (www.molnac.unisa.it) for its computer facilities.
References
- Ma Z, Wang L, Wang W, et al. Study of propylene polymerization catalyzed by spherical MgCl2-supported Ziegler-Natta catalyst system: preparation of spherical support. Polym-Plast. Technol. Eng. 2005;44:1475–1483.10.1081/200065168
- Wang J, Wang L, Feng L, et al. Studies on synthesis of low isotactic polypropylene by using a novel MgCl2-supported and low Ti-loading catalyst. Polym-Plast. Technol. Eng. 2005;44:501–510.10.1081/PTE-200048308
- Parada A, Rajmankina T, Chirinos J, et al. Catalytic systems based on TiCl4/MgCl2/SiCl4-n(OR)n for olefin polymerization. Des. Monomers Polym. 2003;6:1–10.10.1163/156855503321127484
- Sun T, Wang L, Dong X, et al. An insight into the chain-propagation mechanism of propylene polymerization catalyzed by traditional Ti-based Ziegler-Natta catalysts in view of recently developed catalysts. Des. Monomers Polym. 2006;9:117–127.10.1163/156855506776382691
- Hadian N, Hakim S, Nekoomanesh-Haghighi M, Bahri-Laleh N. Storage time effect on dynamic structure of MgCl2.nEtOH adducts in heterogeneous Ziegler-Natta catalysts. Polyolefins J. 2014;1:33–41.
- Rahbar A, Nekoomanesh-Haghighi M, Bahri-Laleh N, et al. Effect of water on the supported Ziegler-Natta catalysts: optimization of the operating conditions by response surface methodology. Catal. Lett. 2015;18:1–10.
- Yu H, Wang L, Ye Z, et al. Study on morphology and particle size distribution of polypropene catalyzed by novel spherical Ziegler-Natta catalyst. Polym-Plast. Technol. Eng. 2004;43:1115–1128.10.1081/PPT-200030050
- Zhao Z, Wang L, Wang J, et al. Study on the microstructures of polypropylene synthesized by a novel MgCl2-supported and low Ti-loading Ziegler-Natta catalyst. Des. Monomers Polym. 2007;10:477–486.10.1163/156855507782401204
- Kim I, Zhou J-M, Chung H. Higher α-olefin polymerization catalyzed by rac-Me2Si(1-C5H2-2-CH3-4-tBu)2Zr(NMe2)2/Al(iBu)3/[Ph3C][B(C6F5)4]. J. Polym. Sci., Part A: Polym. Chem. 2000;38:1687–1697.
- Kaur SND, Naik DG, Singh G, et al. Poly(1-octene) synthesis using high performance supported tiranium catalysts. J. Appl. Polym. Sci. 2010;115:229–236.10.1002/app.v115:1
- Kothari AV, Makwana UC, Desai BK, et al. Stabilized polypropylene synthesis using supported titanium catalyst system in presence of phenolic compounds. Des. Monomers Polym. 2014;17:582–589.10.1080/15685551.2014.907611
- Tao X, Gao W, Huo H, et al. Achiral Cs-symmetric half-sandwich Scandium(III) complexes with imine−cyclopentadienyl ligands catalyze isotactic polymerization of 1-Hexene. Organometallics. 2013;32:1287–1294.
- Liu F, Gao H, Hu Z, et al. Poly(1-hexene) with long methylene sequences and controlled branches obtained by a thermostable α-diimine nickel catalyst with bulky camphyl backbone. J. Polym. Sci., Part A: Polym. Chem. 2012;50:3859–3866.10.1002/pola.v50.18
- Bisz MBE. A comparative study on the polymerization of 1-octene promoted by vanadium and titanium complexes supported by phenoxyimine and salen type ligands. J. Polym. Res. 2013;20:164.
- Bahri-Laleh N, SeifaliAbbas-Abadi M, Nekoomanesh-Haghighi M, et al. Effect of halocarbon promoters on polyethylene properties using MgCl2 (ethoxide type)/TiCl4/AlEt3/H2 catalyst system. J. Appl. Polym. Sci. 2010;117:1780–1786.
- Bahri-Laleh N, Arabi H, Mehdipor-Ataei S, et al. Activation of Ziegler-Natta catalysts by organohalide promoters: A combined experimental and density functional theory study. J. Appl. Polym. Sci. 2012;123:2526–2533.10.1002/app.34589
- Xiao A, Wang L, Liu Q, et al. Synthesis of low isotactic polypropylene using MgCl2/AlCl3-supported Ziegler-Natta catalysts prepared using the one-pot milling method. Des. Monomers Polym. 2008;11:139–145.10.1163/156855508X298044
- Fregonese D, Bresadola S. Catalytic systems supported on MgCl2 doped with ZnCl2 for olefin polymerization. J. Mol. Catal. A: Chem. 1999;145:265–271.
- Bazvand R, Bahri-Laleh N, Nekoomanesh-Haghighi M, et al. Highly efficient FeCl3 doped Mg(OEt)2/TiCl4-based Ziegler-Natta catalysts for ethylene polymerization. Des. Monomers Polym. 2015;18:599–610.10.1080/15685551.2015.1041089
- Chen Y-P, Fan Z-Q, Liao J-H, et al. Molecular weight distribution of polyethylene catalyzed by Ziegler-Natta catalyst supported on MgCl2 doped with AlCl3. J. Appl. Polym. Sci. 2006;102:1768–1772.10.1002/(ISSN)1097-4628
- Jiang X, Tian X, Fan Z, et al. Control of the molecular weight distribution and tacticity in 1-Hexylene polymerization catalyzed by TiCl4/MgCl2-NaCl/TEA catalysis system. J. Mol. Catal. A: Chem. 2007;275:72–76.
- Garoff T, Leinonen T. Mn doping of the Ziegler-Natta PP catalyst support material. J. Mol. Catal. A: Chem. 1996;104:205–212.10.1016/1381-1169(95)00193-X
- Gaussian 09, Revision E.01, Frisch, MJ, Trucks, GW, Schlegel, HB, et al. Gaussian, Inc., Wallingford CT, 2009.
- Perdew JP. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B. 1986;33:8822–8824.10.1103/PhysRevB.33.8822
- Schäfer A, Horn H, Ahlrichs R. SV SVP TVP Ahlrichs basis set. J. Chem. Phys. 1992;97:2571.10.1063/1.463096
- Küchle W, Dolg M, Stoll H, et al. J. Chem. Phys. 1994;100:7535.10.1063/1.466847
- Pokasermsong P, Praserthdam P. Comparsion of activity of Ziegler-Natta catalysts prepared by recrystallization and chemical reaction methods towards polymerization of ethylene. Eng. J. 2009;13:57.10.4186/ej
- Thushara KS, Gnanakumar ES, Mathew R, et al. Toward an understanding of the molecular level properties of Ziegler-Natta catalyst support with and without the internal electron donor. J. Phys. Chem. C. 2011;115:1952–1960.10.1021/jp1078289
- Lee D-H, Jeong Y-T, Soga K, et al. Propylene polymerization with Mg(OEt)2/benzoyl chloride/TiCl4–triethyl aluminum/external donor catalyst systems. J. Appl. Polym. Sci. 1993;47:1449–1461.10.1002/app.1993.070470816
- Vasilenko IV, Kostjuk SV. The influence of cocatalysts on 1-hexene polymerization with various supported magnesium-titanium catalysts. Polym. Bull. 2006;57:129–138.10.1007/s00289-006-0543-1
- Lim S-Y, Choung S-J, Song K-H. Studies on the effects of external electron donor on propylene polymerization. Kor. J. Chem. Eng. 1996;13:21–29.
- Kaur S, Naik DG, Singh G, et al. Poly(1-octene) synthesis using high performance supported titanium catalysts. J. Appl. Polym. Sci. 2010;115:229–236.10.1002/app.v115:1
- Jiang X, Tian X, Fan Z, et al. Control of the molecular weight distribution (MWD) and tacticity of 1-hexene polymerization catalyzed by TiCl4/MgCl2-NaCl/TEA catalysis system. J. Mol. Catal. 2007;275:72–76.
- Asakura T, Demura M, Nishiyama Y. Carbon-13 NMR spectral assignment of five polyolefins determined from the chemical shift calculation and the polymerization mechanism. Macromolecules. 1991;24:2334.10.1021/ma00009a033
- Soga K, Shiono T, Doi Y. Influence of internal and external donors on activity and stereospecificity of ziegler-natta catalysts. Makromol. Chem. 1988;189:1531–1541.
- Galland GB, Da Silva LF, Nicolini A. Tacticity of poly-α-olefins from poly-1-hexene to poly-1-octadecene. J. Polym. Sci., Part A: Polym. Chem. 2005;43:4744–4753.10.1002/(ISSN)1099-0518
- Busico V, Corradini P, De Martino L, et al. Polymerization of propene in the presence of MgCl2-supported Ziegler-Natta catalysts, 1. The role of ethyl benzoate as ‘internal’ and ‘external’ base. Die Makromol. Chem. 1985;186:1279–1288.
- Mehdipour-Ataei S, Amirshaghaghi A, Bahri N. Structure–property relations in heat resistant polyesters with built-in ether and imide units. Eur. Polymer J. 2006;42:2646–2654.10.1016/j.eurpolymj.2006.06.029
- Khan A. Influence of tacticity on structural ordering of isotactic polypropylene under annealing. Canadian Chem. Trans. 2014;2:46–56.
- Mehdipour-Ataei S, Bahri-Laleh N, Amirshaghaghi A. Comparison of one-step and two-step methods of polyimidization and substitution effect in the synthesis of new poly(ester-imide)s with bulky pendent group. Polym. Degrad. Stab. 2006;91:2622–2631.10.1016/j.polymdegradstab.2006.05.004
- da Silva GM, de Mello Ferreira IL, da Costa MPM, et al. Copolymerization of 1-hexene and 1-dodecene with 1,3-butadiene by a versatate/diisobutylaluminum hydride/t-butyl chloride catalyst system. Polímeros. 2014;24:153–161.
- Hamedani GN, Moghaddam MBF, Marandi R, et al. Ziegler catalyst and method of synthesizing the same. United States patent 2013/0030134 A1.
- Bahri-Laleh N, Correa A, Mehdipour-Ataei S, et al. Moving up and down the Ti-oxidation state in Ziegler Natta catalysis. Macromolecules. 2011;44:778–783.10.1021/ma1023582
- Correa A, Bahri-Laleh N, Cavallo L. How well can DFT reproduce key interactions in Ziegler-Natta systems? Macromol. Chem. Phys. 2013;214:1980–1989.10.1002/macp.v214.17
- Bahri-Laleh N, Nekoomanesh-Haghighi M, Mirmohammadi SA. A DFT study on the effect of hydrogen in ethylene and propylene polymerization using a Ti-based heterogeneous Ziegler-Natta catalyst. J. Organomet. Chem. 2012;719:74–79.10.1016/j.jorganchem.2012.08.017
- Bahri-Laleh N, Falivene L, Cavallo L. Testing DFT ability to predict the stereoselectivity of group 4 metallocenes in propylene polymerization. Polyolefins J. 2014;1:139–146.
- Correa A, Credendino R, Pater JTM, et al. Theoretical investigation of active sites at the corners of MgCl2 crystallites in supported Ziegler-Natta catalysts. Macromolecules. 2012;45:3695–3701.10.1021/ma3001862
- Busico V, Cipullo R, Polzone C, et al. Propene/ethene-[1-13C] copolymerization as a tool for investigating catalyst regioselectivity, 2 – The MgCl2/TiCl4-AlR3 system. Macromolecules. 2003;36:2616–2622.10.1021/ma034138o
- Flisak Z, Ziegler T. DFT study of ethylene and propylene copolymerization over a heterogeneous catalyst with a coordinating lewis base. Macromolecules. 2005;38:9865–9872.10.1021/ma0516844
- Ansari M. Rheology and processing of high density polyethylenes (HDPEs): effect of molecular characteristics [ PhD Dissertation]; 2012.