Abstract
The aim of study was to prepare novel polyamides with improved solubility and processability without sacrifice of their thermal and mechanical properties. Polyamides containing ether and sulfone units were obtained via condensation of a special diamine with various diacid chlorides. Poly(ether ether sulfone amide)s were obtained in good inherent viscosities ranging from 0.72 to 0.84 dL/g. All the polyamides were amorphous and readily soluble in polar solvents and swelled in CH2Cl2 and tetrahydrofuran. Flexible films of polymers were obtained by solution casting. Polyamide films exhibited good mechanical and thermal stability including the temperature for 10% weight loss of 449–476 °C.
1. Introduction
Notable performances particularly high thermal stability, barrier properties, strength, and modulus with flame retardancy, fatigue, and solvent resistance cause aromatic polyamides (PAs) among all polymers, are known as high-performance polymers. As a consequence, they are applied in various fields, including space and aviation, bullet-proof products, automotive applications, electronics, and so on.[Citation1–5]
Despite their excellent properties, it is known that aromatic polyamides have some deficiencies. The main problem is their poor fusibility and solubility that originates from rigid polymer backbones and formation of intermolecular hydrogen bonding.[Citation6–8] High moisture absorption also has negative effect on their mechanical properties, electrical insulating, and dielectric performance. Since their poor processability limits their applications, much effort for preparation of aromatic polyamides with high thermal stability, good solubility, low water absorption, superior mechanical properties, and low dielectric constant have been done.[Citation9–12]
One effective way for attaining mentioned desirable purposes is modification of polymer structure by incorporation of flexible linkages (e.g. –O–, –CH2–, and –SO2–) and side groups (e.g. –CBr3, –CF3, and –C6H5) to the backbone.[Citation13–19]
Introducing flexible units into polymer backbone leads to decreasing glass transition temperature and melting point due to lowering of interval rotational energy bonds and also increasing interaction of solvent molecules with the polymer backbone, which makes improved solubility and processability of related polymers.[Citation20–22]
The present work describes the synthesis of a diamine containing sulfone and ether flexible units. The monomer was characterized by spectral and elemental analyses, and utilized in preparation of poly(ether ether sulfone amide)s by indirect polycondensation using different diacid chlorides. Flexible films were obtained by solution casting. The properties of polyamides such as solubility, viscosity, thermal and mechanical properties, crystallinity, and moisture absorption were investigated to establish the structure–property relationship.
2. Experimental
2.1. Materials
All chemicals were purchased either from Merck or Aldrich Chemical Co. N-methyl-2-pyrrolidone (NMP), dimethylacetamide (DMAc), dimethylformamide (DMF), and toluene were distilled over calcium hydride under reduced pressure. Terephthaloyl chloride (TPC) and isophthaloyl chloride (IPC) were purified by sublimation. Adipoyl chloride was purified by vacuum distillation.
2.2. Instruments
Infrared measurements were performed on a Bruker IFS-48 FT-IR spectrometer (Ettlingen, Germany). The 1H-NMR spectra were recorded in dimethyl sulfoxide (DMSO-d6) solution using a Bruker Avance DPX 300 MHz instrument (GmbH, Germany). Elemental analyses were carried out on a CHN-O-Rapid Heraeus elemental analyzer (Wellesley, MA). Thermogravimetric analysis (TGA) was recorded on a Polymer Lab TGA-1500 (London, UK) in air at a heating rate of 10 °C/min. The dynamic mechanical measurements were performed on a Polymer Laboratories Dynamic Mechanical Thermal Analyzer (Model MK-II) over a temperature range of 25–300 °C at 1 Hz and a heating rate of 5 °C/min (Surrey, UK). The value of tan δ and the storage modulus vs. temperature were recorded for each sample. Inherent viscosities were measured by using an Ubbelohde viscometer in a concentration of 0.5 g/dL in DMAc at 30 °C. Wide angle X-ray diffraction was performed at room temperature on an X-ray Jeol Jdx-8030 diffractometer (London, UK) using Ni-filtered Cu Kα radiation (40 kV, 25 mA) with scanning rate of 3°/min. Tensile test was performed at room temperature for thin rectangular films with dimensions of 30 mm length, 10 mm width, and 0.05 mm thick, with a clamp distance of 20 mm using a mechanical (Universal) testing machine, SANTAM, STM-20, at a crosshead speed of 5 mm/min.
2.3. Monomer synthesis
Target diamine (DA) was prepared according to our reported procedure with minor modification in the final step.[Citation23]
2.4. Polymer synthesis
A 100 mL, two-necked, round-bottomed flask equipped with a nitrogen gas inlet tube, magnetic stirrer, and calcium chloride drying tube was charged with 5 mmol of the diamine and 35 mL of dry DMAc. The mixture was stirred at 0 °C for 0.5 h for dissolution. About 2.5 mL of propylene oxide was added into it and after a few minutes, 5 mmol of diacid chloride was added and the mixture was stirred at 0 °C for 0.5 h. The temperature was elevated to room temperature and the solution was stirred for 6 h. Polyamide was precipitated by pouring the flask content into water. It was filtered and purified using DMF-H2O solvent–nonsolvent system. After filtration, it was washed with methanol and hot water successively, and dried overnight under vacuum.
2.5. Preparation of polyamide films
The polyamide films were prepared by solution casting method. In this method, 15 wt% homogenous solution of the polymer in DMAc on the clean petridish was placed in vacuum oven at 110 °C overnight for evaporation of the solvent. Then film was slowly separated from the glass plate and was put in a vacuum oven at 150 °C for 6 h for drying more. The obtained films with 40–45 mm thickness were used for X-ray diffraction measurements, tensile tests, solubility tests, and thermal analyses.
3. Results and discussion
3.1. Monomer synthesis
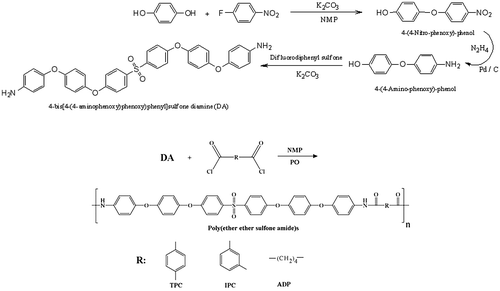
Diamines are important building blocks for preparation of variety of polymers including polyamides, polyimides, polyureas, and so on. Therefore, their structures can induce and dictate some specific properties to the final polymers. Hence, special attention should be paid to the design and preparation of a diamine. In this way, a diamine named as 4-bis[4-(4-aminophenoxy)phenoxy)phenyl]sulfone with four ether and one sulfone unit was synthesized through a three-step procedure as shown in Scheme . In the first step, hydroquinone was reacted with 1-fluoro-4-nitrobenzene via nucleophilic substitution reaction to make 4-(4-nitrophenoxy) phenol (NPP). Then NPP was reacted with N2H4 in the presence of Pd/C to produce 4-(4-aminophenoxy) phenol (APP) by reduction of NO2 to NH2 group. Finally, the APP was reacted with 4,4′-difluorodiphenyl sulfone via nucelophilic substitution reaction and the diamine was synthesized. The structure of all compounds was confirmed by 1H-NMR and FT-IR spectroscopic methods and elemental analysis with satisfied results.[Citation23]
3.2. Polymer synthesis
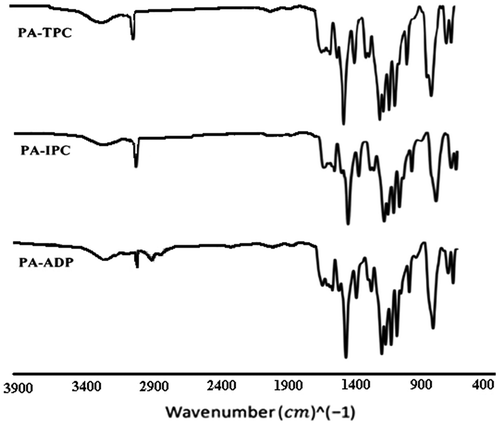
The polyamides were prepared in high yields by polycondensation reaction of the synthesized diamine with different aromatic diacid chlorides in DMAc and in the presence of a trace amount of propylene oxide as acid scavenger (Scheme ). The prepared polyamides showed yellow to brown color. FT-IR spectroscopy confirmed the structure of these polyamides by showing the absorptions at 3300–3400 cm−1 (N-H stretching), 1650–1670 cm−1 (C=O stretching) and 1500–1550 cm−1 (combined N–H bending and C–N stretching), with absorptions of aryl ether group. Figure exhibits the FT-IR spectrum of these polyamides. Also, suitable agreement between calculated and found amounts of C, H, and N in elemental analysis of poly(ether ether sulfone amide)s was observed (Table ).
Table 1. Polymer characterization data.
3.3. Viscosity
The prepared PAs showed inherent viscosities in the range of 0.72–0.84 dL/g in DMAc at 30 °C with an Ubbelohde suspended level viscometer, inferring that synthesized polymers had high molecular weights (Table ).[Citation10,19,24–26]
3.4. Solubility behavior
The solubility behavior of these poly(ether ether sulfone amide)s was tested qualitatively by dissolving 0.3 g of polymers in 10 mL of various organic solvents at room temperature and the results were summarized in Table . As it was seen, all the poly(ether ether sulfone amide)s had very good solubility in aprotic polar solvents such as NMP, DMAC, DMF, and DMSO. In addition, they had also good solubility in moderate polar solvents such as m-cresol and THF at room temperature or upon heating. In comparing to the other polyamides, better solubility of the prepared polymers could be related to presence of the four flexible ethers and one sulfone units in the polymer backbone. Also, ADP-derived polyamides showed better solubility than other polymers due to the aliphatic nature of ADP moiety. The excellent solubility makes these polyamides to be potential nominee for useful applications example casting processes.
Table 2. Solubility of poly(ether ether sulfone amide)s.
3.5. Thermal properties
The thermal behavior of PAs was evaluated by DMTA and TGA techniques. A summary of thermal behavior and stability data of PAs were given in Table . The glass transition temperature (Tg) values of the polyamides were in the range of 190–218 °C measured by DMTA. The ADP–DA polymer exhibited the lowest Tg value (190 °C) due to the presence of the aliphatic sections in the backbone chain of the polymer and its more flexibility in comparison to aromatic sections, whereas the highest Tg (218 °C) was observed for the TPC–DA polymer because of the effect of the high symmetry and rigidity of the backbone.
Table 3. Thermal properties of polyamides.
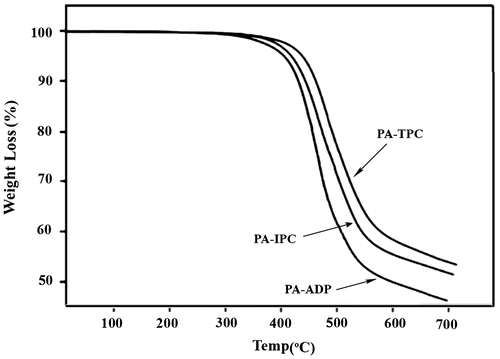
The thermal stability of these polymers was studied by TGA in air at a heating rate of 10 °C/min. TGA curves for the polyamides are shown in Figure . All the polymers exhibited good heat resistance with negligible weight loss up to 400 °C. The temperatures for 10% weight loss, which are consequential for evaluating the thermal stability of the polymers, were in the range of 449–476 °C implying their high thermal stability. The char yield for these polymers were in the range of 48–55% at 700 °C.
In comparison to common aromatic polyamides with no ether and sulfone linkages such as poly(p-phenyleneterephthalamide) and poly(m-phenyleneisophthalamide) with Tg in the range of 250–400 °C, it should be mentioned that presence of four ether and one sulfone flexible linkages in the poly(ether ether sulfone amide)s decreased their Tg to 190–218 °C and consequently better processability of these polymers was resulted. Instead, due to their higher rigidity the initial decomposition temperature (TIDT) and temperature for 10% weight loss (T10) for poly(p-phenyleneterephthalamide) and poly(m-phenyleneisophthalamide) begin from 425 to 580 °C respectively, that is higher than the poly(ether ether sulfone amide)s.[Citation27–29]
3.6. Mechanical properties
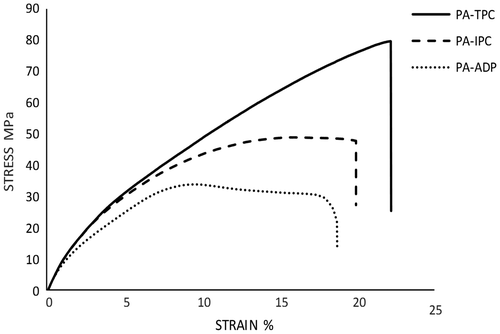
The tensile properties of polyamides measured for the flexible films using standard procedure at 25 °C were presented in Figure and the related data were tabulated in Table . As shown, tensile strengths and elongation at break of these polyamide films were in the range of 79–32 MPa, and 19–22%, respectively. ADP–DA polyamide exhibited lowest elongation at break about 19%, due to the presence of weak bonds of aliphatic groups in the polymer backbone. PA–TPC showed the best mechanical properties, which could be attributed to the presence of rigid phenyl groups in the main chain and strong aromatic bonds as well as more symmetric structure. It is worth mentioning that polyamides with no ether and sulfone linkages such as poly(p-phenyleneterephthalamide) and poly(m-phenyleneisophthalamide) show higher strength (0.6–3 GPa) and modulus (50–150 GPa) in respect to the prepared poly(ether ether sulfone)s that could be mainly related to their highly rigid and oriented structures and also crystallinity.[Citation27,30–32]
Table 4. Mechanical properties of polyamides.
3.7. X-Ray diffraction of the polyamides
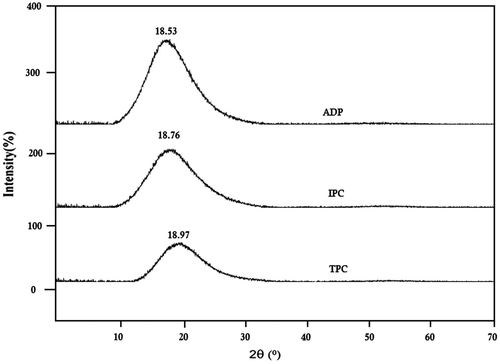
To investigate crystallinity of the polymers, wide angle X-ray powder diffractometry (WAXD) of the polymers was done in the region of 2θ = 5–70° at room temperature. The WAXD Diagram of polyamides was shown in Figure . These patterns indicated that all the PAs were amorphous. It could be attributed to the presence of five flexible linkages (–O– and –SO2–) in the main chain of the polymer which led to a decrease in chain to chain interactions and poor chain packing of polymers thus, presenting amorphous poly(ether ether sulfone)s with better solubility and processability.
3.8. Water absorption
Generally, as a result of the interaction between polar amid groups and water, polyamides are considered as hydrophilic polymers, which is a great issue due to the absorption of water that can gradually lead to a decrease in mechanical properties. Thus, the stability of polymer in the environment decreases by water absorption. In comparison to the reported polyamides,[Citation33,34] the amount of water absorbed by the prepared polymer films at room temperature was moderate (in the range of 1.56–5.30%).
4. Conclusions
Briefly, a sulfone ether diamine monomer was synthesized and then was used to prepare a series of novel poly(ether ether sulfone amide)s via polycondensation reactions with various aromatic and aliphatic diacid chlorides. These new polyamides exhibited good mechanical properties, thermal stability, suitable water absorptions, and acceptable progress in the solubility. These characteristics indicated that the presence of flexible (four ether and one sulfone) linkages in the polymer backbone have played an important role in the improvement of solubility and processability of polymer without sacrifice of thermal and mechanical stability. Therefore, in this research, the main problem of rigid aromatic polyamides was overcome by preparation of such monomer and related polyamides.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kiani H, Nasef M, Javadi A, et al. Highly refractive, transparent, and solution processable polyamides based on a noncoplanar ortho-substituted sulfonyl-bridged diacid monomer containing chlorine side groups. J. Polym. Res. 2013;20:1–12.
- Knijnenberg A, Bos J, Dingemans TJ. The synthesis and characterisation of reactive poly(p-phenylene terephthalamide)s: a route towards compression stable aramid fibres. Polymer. 2010;51:1887–1897.10.1016/j.polymer.2010.03.015
- Shockravi A, Javadi A, Abouzari-Lotf E. Fluorinated ortho-linked polyamides derived from non-coplanar 1,1′-thiobis(2-naphthol): synthesis and characterization. Polym. J. 2011;43:816–825.10.1038/pj.2011.74
- Javadi A, Abouzari-Lotf E, Mehdipour-Ataei S, et al. High refractive index materials: a structural property comparison of sulfide- and sulfoxide-containing polyamides. J. Polym. Sci., Part A: Polym. Chem. 2015;53:2867–2877.10.1002/pola.v53.24
- Hsiao SH, Lin KH. Soluble aromatic polyamides bearing asymmetrical diaryl ether groups. Polymer. 2004;45:7877–7885.10.1016/j.polymer.2004.09.030
- Shockravi A, Mehdipour-Ataei S, Abouzari-Lotf E, et al. Sulfide and sulfoxide based poly(ether-amide)s: Synthesis and characterization. Eur. Polym. J. 2006;42:133–139.10.1016/j.eurpolymj.2005.06.031
- Lee HS, Kim SY. Synthesis of poly(arylene ether amide)s containing CF3 groups by nitro displacement reaction of AB-type monomers. Macromol. Rapid Commun. 2002;23:665–671.10.1002/1521-3927(20020801)23:12<665::AID-MARC665>3.0.CO;2-A
- Cheng L, Jian XG. Synthesis of new soluble aromatic poly(amide imide)s from unsymmetrical extended diamine containing phthalazinone moiety. J. Appl. Polym. Sci. 2004;92:1516–1520.10.1002/(ISSN)1097-4628
- Jalalian E, Mehdipour-Ataei S, Babanzadeh S, et al. Silicon-containing poly(amide-imide)s: preparation, characterization, and properties. Des. Monomers Polym. 2015;18:714–722.10.1080/15685551.2015.1070502
- Mehdipour-Ataei S, Heidari H. Synthesis and characterization of novel soluble and thermally stable polyamides based on pyridine monomer. Macromol. Symp. 2003;193:159–168.10.1002/masy.200390049
- Rostami E. Synthesis and characterization of new polyamides containing pyridine thioether units in the main chain under microwave irradiation (MW) and their nanostructures. Int. J. Polym. Mater. 2013;62:175–180.10.1080/00914037.2011.641632
- Lee HS, Kim SY. Synthesis of poly(arylene ether amide)s containing CF3 groups by nitro displacement reaction of AB-type monomers. Macromol. Rapid Commun. 2002;23:665–671.10.1002/1521-3927(20020801)23:12<665::AID-MARC665>3.0.CO;2-A
- Zulfiqar S, Ishaq M, Ahmad Z, et al. Synthesis, static and dynamic light scattering studies of soluble aromatic polyamide. Polym. Adv. Technol. 2008;19:1250–1255.10.1002/pat.v19:9
- Calderón V, Schwarz G, García F, et al. Synthesis and characterization of new aromatic polyamides bearing crown ethers and acyclic ethylene oxide units in the pendant structure. III. benzo-18-crown-6 systems and their open-chain counterparts. J. Polym. Sci., Part A: Polym. Chem. 2006;44:6252–6269.10.1002/(ISSN)1099-0518
- Mehdipour-Ataei S, Babanzadeh S, Abouzari-Lotf E. Nicotinic-based poly(amide-ether-imide)s: a new category of soluble, heat-resistant, and flame-retardant polyimides. Des. Monomers Polym. 2015;18:451–459.10.1080/15685551.2015.1041076
- Kausar S. Fabrication and characteristics of poly(benzimidazole/fluoro/ether/siloxane/amide)/sulfonated polystyrene/silica nanoparticle-based proton exchange membranes doped with phosphoric acid. Int. J. Polym. Mater. Polym. Biomater. 2015;64:184–191.10.1080/00914037.2014.936589
- Shockravi A, Mehdipour-Ataei S, Abouzari-Lotf E, et al. Soluble and thermally stable polyamides bearing 1,1′-thiobis(2-naphthoxy) groups. Eur. Polym. J. 2007;43:620–627.10.1016/j.eurpolymj.2006.10.029
- Oroujzadeh M, Mehdipour-Ataei S, Esfandeh M. New proton exchange membranes based on sulfonated poly(arylene ether sulfone) copolymers: effect of chain structure on methanol crossover. Int. J. Polym. Mater. Polym. Biomater. 2015;64:279–286.10.1080/00914037.2014.936600
- Hsiao SH, Chang YM, Chen HW, et al. Novel aromatic polyamides and polyimides functionalized with 4-tert-butylphenylamine groups. J. Polym. Sci., Part A: Polym. Chem. 2006;44:4579–4592.10.1002/(ISSN)1099-0518
- Mehdipour-Ataei S, Zigheimat F. Soluble poly(amide-imide)s containing oligoether spacers. Eur. Polym. J. 2007;43:1020–1026.10.1016/j.eurpolymj.2006.11.034
- Abouzari-Lotf E, Shockravi A, Javadi A. Heat-resistant and soluble fluorinated poly(amide imide)s based on non-coplanar ortho-linked diimide-dicarboxylic acid. Polym. Degrad. Stab. 2011;96:1022–1028.10.1016/j.polymdegradstab.2011.01.004
- Akbarian-Feizi L, Mehdipour-Ataei S, Yeganeh H. Investigation on the preparation of new sulfonated polyimide fuel cell membranes in organic and ionic liquid media. Int. J. Polym. Mater. Polym. Biomater. 2014;63:149–160.10.1080/00914037.2013.812086
- Abbasi F, Mehdipour-Ataei S, Khademinejad S. Novel type of highly soluble and thermally stable poly(sulfone ether imide)s. Des. Monomers Polym. 2015;18:789–798.10.1080/15685551.2015.1078112
- Yamazaki N, Matsumoto M, Higashi F. Studies on reactions of the N-phosphonium salts of pyridines. XIV. Wholly aromatic polyamides by the direct polycondensation reaction by using phosphites in the presence of metal salts. J. Polym. Sci., Part A. Polym. Chem. 1975;13:1373–1380.10.1002/pol.1975.170130609
- Morgan PW. Synthesis and properties of aromatic and extended chain polyamides. Macromolecules. 1977;10:1381–1390.10.1021/ma60060a040
- Brignac EP. Process for increasing polyamide viscosity and polyamide composition prepared thereby. United States Patent 3509107 A. 1970.
- Yang HH. Kevlar aramide fiber. New York (NY): Wiley; 1993.
- Chen WC, Sauer JA, Hara M. Ionic poly(p-phenylene terephthalamide)s: solubility and thermal stability. J. Polym. Sci., Part B: Polym. Phys. 2001;39:2888–2897.10.1002/(ISSN)1099-0488
- Horta A, Coca J, Diez FV. Degradation mechanism and kinetics of a high thermally stable aromatic polyamide. Adv. Polym. Technol. 2000;19:120–131.10.1002/(ISSN)1098-2329
- Graf R, Lohaus G, Börner K, et al. β-lactams, their polymerization and use as raw materials for fibers. Angew. Chem., Int. Ed. Engl. 1962;1:481–488.10.1002/anie.v1:9
- Foo CC, Chai GB, Seah LK. Mechanical properties of nomex material and nomex honeycomb structure. Compos. Struct. 2007;80:588–594.10.1016/j.compstruct.2006.07.010
- Su FH, Zhang ZZ, Liu WM. Tribological and mechanical properties of Nomex fabric composites filled with polyfluo 150 wax and nano-SiO2. Compos. Sci. Technol. 2007;67:102–110.10.1016/j.compscitech.2006.03.029
- Batzer H, Kreibich UT. Influence of water on thermal transitions in natural polymers and synthetic polyamides. Polym. Bull. 1981;5:585–590.
- Jördens C, Wietzke S, Scheller M, et al. Investigation of the water absorption in polyamide and wood plastic composite by terahertz time-domain spectroscopy. Polym. Test. 2010;29:209–215.10.1016/j.polymertesting.2009.11.003