Abstract
Development of water-soluble chemosensors that are selective and sensitive to Cu2+ ions is of tremendous importance owing to their potential applications in biological systems. In the present work, we report the synthesis of a new water-soluble polymer containing pendant rhodamine units that are capable of highly selective and sensitive detection of Cu2+ ions in aqueous medium. Poly(2-pyrrolidinemethyl acrylate) was prepared using RAFT polymerization technique. The pyrrolidine nitrogen group in the polymer was subjected to Aza-Michael type addition with ethyl acrylate that was followed by covalent linking of rhodamine units to the polymer. This polymer was completely water-soluble and found to be capable of sensing Cu2+ ions in aqueous medium. Cu2+-induced opening of the spirolactam ring of the rhodamine units resulted in rapid and easily noticeable colour change, thus enabling a highly selective detection of Cu2+ in μmol range. The ability of these polymeric systems to detect Cu2+ ions in complete aqueous media has more importance than use of organic solvents to solubilize the polymer as reported previously, and thus opened a new window for application of these systems in the detection of copper ions in biological systems.
Introduction
Presence of transition metals like copper, zinc and iron, even in trace amounts, are known to play critical roles inside living beings.[Citation1] The utility of metal ions cannot be ascertained unless we are able to quantify the metal content of cells and tissues. Previously, the quantification of the metal ions was done via Atom Absorption Spectroscopy [Citation2–4] and volumetric detection.[Citation5] With technological advancement, these studies subsequently gave way to Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) and preconcentration techniques involving solid-phase extraction of heavy metal ions as analytical methods owing to their superior sensitivity.[Citation6–9] The major drawbacks of these methods are time-consuming sample pre-treatment and separation, thus making them inconvenient for the fast detection of metal ions. In recent times, all these techniques have subdued by new colorimetric techniques. The techniques depend upon UV–vis absorption and/or fluorescence measurements of organic fluorophores,[Citation10–12] peptides [Citation13,14] and DNA,[Citation15–17] which can serve as high-performance chemosensors for metal ion detection in aqueous media. These have attracted much attention due to several advantages, such as high sensitivity, specificity and ease of operation in identifying the metal ions.
Fluorescent sensors have been of profound interest to the scientific community for years owing to their usefulness in the fields of clinical diagnostics, environmental monitoring, etc. for sensing and recognizing environmentally and biologically important ionic species, especially transition metal ions.[Citation1] Hence, researchers are constantly looking for new fluorescent sensors and tuning the known probes for better results. Rhodamine is a molecule which has been used extensively as a fluorescent labelling reagent and a dye laser source because of its excellent photophysical properties, such as long absorption and emission wavelengths elongated up to visible region, high fluorescence quantum yield and large absorption coefficient.[Citation18–21]
Recently in the field of metal sensing, chemosensors based on polymers have become more and more important due to some advantages like easy fabrication and signal amplification compared to a simple organic compound.[Citation22–24] Advances in synthetic chemistry and the advent of different polymerization techniques like reversible addition–fragmentation chain transfer (RAFT),[Citation25,26] atom transfer radical polymerization (ATRP),[Citation27] etc. have enabled the synthesis of polymers with well-controlled architectures e.g. block, star, graft and brush and various functional polymers with tuneable properties.[Citation28–31] These advancements in the field of polymer chemistry lead to design a fluorescent probe–polymer conjugate that would give faster response, high sensitivity and selectivity, which would not only allow the detection of different targets but also provide specific imaging of them within living cells.[Citation32–35]
Copper ions are present in various tissues, mainly in mitochondria, and are required for cellular respiration.[Citation1] Copper ions are also present throughout the brain and play an important role in the central nervous system.[Citation36] Copper ions act as a catalytic cofactor for numerous metal enzymes and also required for biosynthesis and pigment formation.[Citation2,37] However, at higher concentrations, this essential trace element becomes toxic and is responsible for oxidative stress, liver damage and various diseases, such as Menkes disease, Wilson’s disease and Alzheimer’s disease.[Citation38,39] The Cu consumption limit should not exceed 10–12 mg, as suggested by World Health Organization (WHO), whereas in drinking water the maximum allowable level (MAL) of Cu is ~20 μmol according to the US Environmental Protection Agency (EPA).[Citation40] For the last 10–20 years, Cu pollution has become a major problem all over the world because of frequent use of Cu2+-based compounds in industry and agriculture.[Citation41] Therefore, a selective detection of trace amounts of Cu2+ ion in aqueous medium is extremely important. As a result of its biological and environmental importance, a lot of effort has been devoted till date for the development of Cu2+-selective fluorescent chemosensors.[Citation42] The main problem with such fluorescent probes e.g. rhodamine and its derivatives, is their very low or zero sensitivity in aqueous medium. Most of the sensors based on these fluorophores are reported to work only in acetonitrile–water mixture or any other organic solvent. So, there is a great need for developing sensors that can selectively and sensitively detect Cu2+ in completely aqueous medium. Incorporation of such fluorescent probes into polymeric systems forming polymer–probe conjugates helps in designing sensors which are active in aqueous environment. In this work, we report a new water-soluble polymer containing rhodamine units that are capable of selective and sensitive detection of Cu2+ ions in completely aqueous environment.
Experimental section
Materials and methods
Rhodamine B, ethylenediamine, ethyl acrylate and L-proline were purchased from Sigma–Aldrich (St. Loius, MO, USA) and used as received. 2,2′-Azobisisobutyronitrile (AIBN, Sigma-Aldrich) was recrystallized twice from methanol prior to use. Other chemicals and solvents were purchased from Merck (Darmstadt, Germany) and Sisco Research Laboratories Pvt. Ltd. (Mumbai, India) and purified by standard procedures whenever required. S-1- Dodecyl-S′-(α,α′-dimethyl-α″-acetic acid) trithiocarbonate (DDMAT) was synthesized according to the previously reported literature.[Citation33,43] Ethylenediamine derivative of rhodamine B (1, Scheme (A)) was synthesized following reported procedures with some modifications.[Citation44] Exact details for the synthetic procedure of ethylenediamine derivative of rhodamine B (1, Scheme(A)) along with 1H NMR spectra (Figure S1) are provided in the Electronic Supplementary Information. 1H NMR spectra of all the compounds were recorded on a Bruker DPX-200/400 MHz NMR spectrometer, using residual solvent signal as the internal standard. The UV–vis absorption and fluorescence spectra were collected using a Shimadzu (model No. UV-2450) spectrophotometer and a Hitachi (model No. F-7000) spectrofluorimeter, respectively. The steps followed for the synthesis of polymer (P3, Scheme (B)) used for sensing experiments are provided below.
Synthesis of (S)-1-Boc-2-pyrrolidinemethanol (2, Scheme (B))
About 2.5 g (0.02 mol, 1 eq) of L-Proline was taken in a dry double-necked round-bottomed flask in which 40 mL of dry DCM was added and stirred under inert nitrogen atmosphere. To the slurry, 3.5 g (0.035 mol, 1.65 eq) of dry triethylamine was added. Then, 7.8 g (0.035 mol, 1.65 eq) of Boc-anhydride was added dropwise for 15 min and the reaction mixture stirred for overnight giving a clear solution. To this clear solution, 2.4 g (0.06 mol, 3 eq) of lithium aluminium hydride was added slowly in ice cold condition and the mixture was left to stir for 5 h in room temperature. After that, the reaction vessel was cooled to 0 °C and the excess lithium aluminium hydride was quenched with ice-cold water. The compound was filtered through Celite bed in a G2 crucible and the eluent was extracted with ethyl acetate thrice. The ethyl acetate part was then collected and solvent was evaporated in a rotary evaporator followed by purification of the product using column chromatography which finally yielded yellow coloured liquid compound. 1HNMR (CDCl3): δ 1.45 (s, 9H); δ 1.86 (br, 4H); δ 3.35 (br, 2H); δ 4.00 and δ 4.11 (br, 2H); δ 4.23 (d, 1H).
Synthesis of (S)-1-Boc-2-pyrrolidinemethyl acrylate (3, Scheme (B))
Two grams (0.01 mol, 1 eq) of (S)-1-Boc-2-pyrrolidinemethanol were taken in a dry double-necked round-bottomed (rb) flask and 40 mL of dry dichloromethane (DCM) was added to it and the reaction mixture was stirred under nitrogen atmosphere in ice cold condition for 15 min. One gram (0.01 mol, 1 eq) of triethylamine was added dropwise to it and left to stir for another 10 min. About 1.1 g (0.012 mol, 1.2 eq) of acryloyl chloride was then added to the mixture dropwise and the reaction mixture was stirred at room temperature for 24 h. The DCM part was washed with water until all the unreacted acryl chloride was removed. Then the DCM part was washed with ice-cold water. The compound was obtained by removing DCM in rotatory evaporator. 1HNMR (CDCl3): δ 1.45 (s, 9H); δ 1.86 (br, 4H); δ 3.35 (br, 2H); δ 4.00 & δ 4.11 (br, 2H); δ 4.23 (d, 1H); δ 5.84 (d, 1H), δ 6.15 (m, 1H), δ 6.42 (dd, 1H). 1H NMR spectra are provided in the Electronic Supplementary Information (Figure S2).
Synthesis of P2 (Scheme (B))
About 1.3 g (5 mmol, 25 eq) of (S)-1-Boc-2-pyrrolidinemethyl acrylate (3) was taken in a rb flask and to it 0.01 g (0.03 mmol, 0.3 eq) of AIBN as a free radical initiator and 0.065 g (0.2 mmol, 1 eq) of DDMAT as chain transfer agent (CTA) were added. 1,4-Dioxane (4 mL) was added to the mixture and the flask was sealed with a septum. The reactor vessel was then degassed and purged with nitrogen. The reaction mixture was heated at 70 °C for 8 h in an oil bath. The polymerization was quenched by cooling the solution in liquid nitrogen. Then, the reaction mixture was diluted with 5 mL THF and the product was obtained by precipitation from ice-cold hexane which produced Boc-protected polymer, P1 (Scheme (B)). P1 was vacuum dried and analyzed by 1H NMR spectroscopy to determine its final composition. 1H NMR spectra are provided in the Electronic Supplementary Information (Figure S3). For the Boc group deprotection, 1 g of polymer (P1) was dissolved in 10 mL of dry DCM in a 25 mL double-necked rb flask and 2 mL of trifluoroacetic acid (TFA) was added dropwise under a nitrogen atmosphere. The solution was kept at room temperature for 16 h with continuous stirring. After that, excess TFA was removed by azeotropic distillation with toluene, and finally the product was purified by precipitating in excess ice-cold diethyl ether followed by removal of diethyl ether, which yielded Boc-deprotected polymer, P2. The purified P2 was analyzed by 1H NMR spectroscopy to obtain its final composition (for 1H NMR spectra, see the Electronic Supplementary Information, Figure S4).
Synthesis of P3 (Scheme (B))
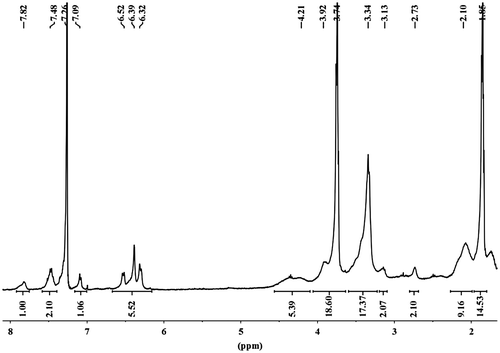
About 0.75 g (0.3 mmol, 1 eq) of P2 was reacted with 160 μL (0.15 g, 1.5 mmol, 5 eq) of ethyl acrylate in dry THF and was stirred for 48 h at room temperature under N2 atmosphere followed by in situ reaction with 0.75 g (1.5 mmol, 5 eq) ethylenediammine derivative of Rhodamine B. The stirring was continued for 24 h at 50 °C. After that, the reaction mixture was dialyzed against methanol through 3000 Da MWCO cellulose membrane for 24 h with replacement of the outside methanol at every 5 h interval for complete removal of unreacted starting materials. Thereafter, a vacuum drying of the purified methanol solution produced the targeted polymer, P3. The polymer was analyzed by 1H NMR spectroscopy (Figure ) to determine its final composition.
Figure 2. Change in (a) absorbance and (b) fluorescence spectra of the polymer P3 (20 μmol) in Tris buffer (10 mmol) at different pH.
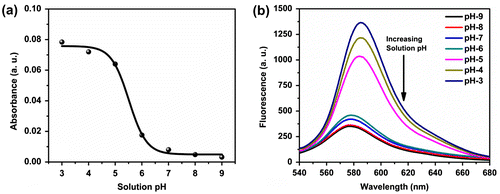
Figure 3. Variation in (a) absorbance and (b) fluorescence spectra (λex = 520 nm) of the polymer P3 (20 μmol) in Tris buffer (pH 7) at different concentrations of Cu2+ ion (0–30 μmol).
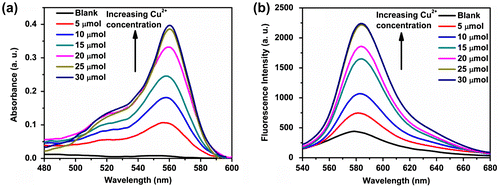
Figure 4. (a) Intensity-normalized emission spectra of polymer P3 (20 μmol) in the presence of different concentration of Cu2+ ion in Tris buffer at pH 7. (b) Photograph showing colour change of the polymer P3 (20 μmol) upon addition of 30 μmol Cu2+ ions in tris buffered solution (pH 7).
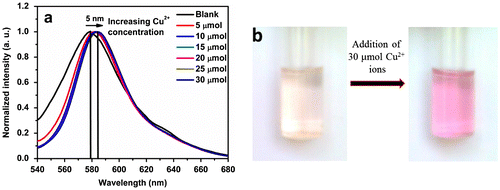
Figure 5. Variation of normalized fluorescence intensity enhancement at 584 nm of the polymer P3 (20 μmol) in Tris buffer (pH 7) after 30 min on addition of 30 μmol of various metal ions.
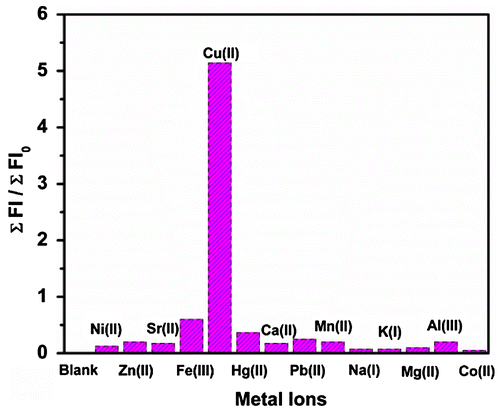
Figure 6. Variation in (a) absorbance and (b) fluorescence spectra of the polymer P3 (20 μmol)-Cu2+(30 μmol) in Tris buffer (pH 7) at different concentrations of SCN− (0–35 μmol).
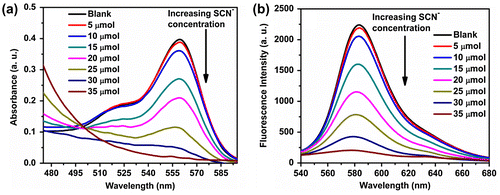
Figure 7. Fluorescence spectrum, of initial and after extraction using DCM, of polymer P3 (20 μmol)-Cu2+ (20 μmol) complex in Tris buffer (pH 7) and also addition of SCN− (20 μmol) to the P3-Cu2+ complex (redissolved in Tris buffer after extraction using DCM) showing quantitative estimation of Cu2+ extraction.
Preparation of solutions of metal ions and anions
About 0.01 mol Tris and appropriate amount of HCl were utilized for the preparation of Tris–HCl buffer solution (pH 7.0). The above-mentioned buffer solution was used for the preparation of stock solutions (10−2 mol) of different metal ions as well as P3. The stock solutions were diluted using same buffer to prepare solutions of desired concentrations as needed. Two milligrams of P3 were dissolved in 10 mL of Tris buffer for the preparation of polymer stock solution in which the concentration of rhodamine was ~2 × 10−4 mol.
Results and discussion
The main problem associated with rhodamine-based sensors is their low solubility in water and their poor complexation with Cu2+ at low concentrations due to high degree of hydration of Cu2+ ion. There are reports where rhodamine-containing polymers were used to sense Cu2+ ion in water–organic mixed solvents.[Citation20] Moreover, the colour produced on complexation of Cu2+ with the polymers was too faint to be detected by naked eye in aqueous environment. These problems hindered the application of such rhodamine-based sensors in biological systems. In this work, a polymer bearing rhodamine units was synthesized and its selectivity and sensitivity towards Cu2+ ion in aqueous media were investigated.
Synthesis of the targeted polymer (P3, Scheme 1(B))
Our main goal was to synthesize a polymer which would be completely soluble in water without the help of any organic solvent and would be able to sense Cu2+ ion selectively with high sensitivity. For this purpose, L-proline moiety was utilized to produce a completely water-soluble polymer backbone. Thereafter, ethylenediamine derivative of rhodamine B was covalently attached to the polymer backbone via Aza-Michael type condensation reaction using ethyl acrylate and the resulting polymer bearing rhodamine units (P3) as pendant moiety was used for sensing of Cu2+ ion. For the synthesis of P3, as shown in Scheme (B), we first protected the α-amino group of L-proline using di-tert-butyl dicarbonate followed by reduction of the acid functionality producing (S)-1-Boc-2-pyrrolidinemethanol. Then, the primary alcohol of (S)-1-Boc-2-pyrrolidinemethanol was subjected to acrylation using acryloyl chloride which yielded the monomer (S)-1-Boc-2-pyrrolidinemethyl acrylate. The corresponding polymer containing L-proline moiety (S-1-Boc-2-pyrrolidinemethyl acrylate) was synthesized by RAFT polymerization technique in 1,4-dioxane medium, using DDMAT as a chain transfer agent and AIBN as a free radical initiator. For the determination of number-average molecular weight as well as the composition of the functional groups attached during post-polymerization modification of the synthesized polymers, we have utilized end-group analysis technique by 1H NMR spectroscopy as it provides absolute and more reliable data for quantification.[Citation33,45] The molecular weight of the poly(S-1-Boc-2-pyrrolidinemethyl acrylate) (P1) was quantified by integration of the relative intensities of three protons present in the terminal methyl group of dodecyl unit of DDMAT (at 0.86 ppm) with respect to the intensities of two methylene protons adjacent to nitrogen atom of the pyrrolidine unit of the polymer chain (for 1H NMR spectra, please see the Electronic Supplementary Information, Figure S3). P1 was found to consist of 20 pyrrolidine units that corresponded to a Mn of ∼4400 g mol−1. Successful removal of the Boc group from P1 using TFA yielded the polymer, P2, with free secondary amine functionality (Figure S4, Electronic Supplementary Information). The complete attenuation of the peak corresponding to the protons of tert-butyl group present in P1 (at 1.45 ppm) confirmed the formation of P2 with free secondary amine groups. Now secondary amine groups present in P2 was subjected to Aza-Michael type reaction with ethyl acrylate and then charging of ethylenediamine derivative of rhodamine B (1) yielded the final polymer P3. Number of (1) units present in the polymer chains after post-polymerization modification was determined using 1H NMR spectroscopy by comparing relative integration of one proton at 7.82 ppm ((1) unit) with respect to one proton at 4.21 ppm (pyrrolidine unit of polymer chain) and found to be ~4 (Figure ).
Sensing studies in water with P3
pH-dependent solution properties of P3
The blank solution of P3 (2 × 10−5 mol in terms of rhodamine unit) in 10 mmol Tris buffer (pH 7.0) was almost colourless and showed very weak fluorescence with emission maxima at ~579 nm (λex = 520 nm). Further, in order to investigate whether a change in pH affects the absorbance and fluorescence properties of P3, we measured the absorbance as well as the fluorescence of P3 by changing the solution pH from 3 to 9 (shown in Figure ). Figure (a) and (b) shows no significant change in absorbance or fluorescence intensity at alkaline pH (7 and above) but at lower pH range (pH ≤ 6), a gradual increase in colour and fluorescence intensity was observed with the decrease in the pH of the medium. This observation can be explained on the basis of the stabilization of the spirolactam ring of the rhodamine unit in the solution.[Citation20] In alkaline pH, the spirolactam form of the rhodamine unit present in P3 predominated, whereas under acidic pH, the ring-opened amide form was preferred over the spirolactam in the rhodamine derivatives due to protonation.
Binding of Cu2+ ion to the polymer P3
Now, in order to observe whether the spirolactam form of the rhodamine unit present in P3 also acted as a signal switcher when Cu2+ ion was bound and envisioned to fluorescence turn-on like previously observed protonation, we have gradually added Cu2+ ions to a solution of P3 (2 × 10−5 mol in terms of rhodamine unit) at neutral pH (pH 7.0, 10 mmol Tris buffer) and measured the absorbance and the fluorescence of P3 at 556 and 579 nm, respectively. We observed a significant enhancement in absorbance and fluorescence intensity of P3 in Tris buffer (pH 7) with gradual addition of Cu2+ ions (Figure (a) and (b)). This was indicative of Cu2+-induced opening of spirolactam ring of the pendant rhodamine units present in P3 on complexation at a fixed solution pH ~ 7.0, where the spirolactam form of the rhodamine unit predominated, as previously observed from the pH-dependent spirolactam to ring-opened amide equilibrium study of rhodamine units present in P3. The increase in absorbance as well as fluorescence intensity occurred linearly till ~25 μmol of Cu2+ ions before levelling off, indicating nearly 1:1 stoichiometry of complex formation between Cu2+ ion and rhodamine units at this concentration. Here, it is noteworthy that the enhancement in absorbance of P3 upon addition of 30 μmol of Cu2+ ions was much higher (~50-fold) as compared to the enhancement of fluorescence intensity (~5-fold) in presence of the same amount of Cu2+ ions. This differential intensity enhancement may be attributed to the fluorescence quenching of rhodamine units by paramagnetic Cu2+ ions.[Citation46] Furthermore, the emission spectra of P3 showed a red shift of the emission maxima from 579 to 584 nm (Figure (a)) upon gradual addition of Cu2+ ions which confirms the formation of the Cu2+-induced ring-opened structure of rhodamine from the corresponding non-fluorescent spirolactam structure present in purely aqueous buffer (pH 7) solution of P3. This showed the potential of P3 for sensing Cu2+ ions in practical applications where the Cu2+ ion concentrations are similar to the concentrations studied here, e.g. in blood systems (15–25 μmol) as well as in drinking water (~20 μmol) according to US EPA limit.[Citation40]
It was also observed that, a change in absorbance and fluorescence spectra of P3 by the addition of Cu2+ ions was found to be similar for the three Cu(II) salts used here, i.e. Cu(NO3)2, CuCl2 and CuSO4, suggesting a negligible effect of the corresponding anions on the P3-Cu2+ complexation. The high enhancement (~50-fold) of absorbance of P3 upon binding of Cu2+ ions resulted in an easily noticeable colour change from nearly colourless to pink (shown in Figure (b)). Therefore P3 served as a ‘naked-eye’ chemosensor for Cu2+ ions. In this work, we have shown that P3 was able to sense Cu2+ ions in completely aqueous media, and this was possible due to incorporation of rhodamine probe into the amino acid (L-proline) containing water-soluble polymer backbone, as against previously reported rhodamine-based non-polymeric chemosensors where 1:1(v/v) organic solvent–aqueous buffer were used.[Citation46–48]
Selectivity of P3 for Cu2+ sensing in water
In order to validate the high selectivity of the spirolactam form of the rhodamine units present in P3 towards Cu2+ ions, we measured the fluorescence intensity of P3 at pH 7 in presence of some transition metal cations viz. Al3+, Co2+, Fe3+, Hg2+, Mn2+, Ni2+, Pb2+ and Zn2+ as well as in presence of some ions viz. Na+, K+, Ca2+, Mg2+ and Sr2+ commonly encountered in environmental and biological analysis. We have investigated the effect of a series of these metal ions by adding 30 μmol solution of each of the metal ions to a solution of P3 (20 μmol) as in the case of Cu2+ solution. It has been observed that none of these metal ions have the ability to provide any significant change in the normalized fluorescence intensity (Figure ).
However among these metal ions, only Fe3+ showed very small enhancement in fluorescence intensity, after incubation for a relatively long time (~30 min) as compared to Cu2+ ions, which indicated that the Fe3+ ion induced the opening of the spirolactum ring of rhodamine only to a little extent, after incubation for a long time. This observation clearly revealed that P3 was much more selective to Cu2+ ion as compared to other commonly encountered metal ions.
Reversing the binding of Cu2+ to the polymer using SCN–
We have investigated the reversible nature of the P3-Cu2+ complexation by disintegrating the polymer–Cu2+ complex with SCN− ion, which is very essential for chemosensor applications. For this purpose, 20 μmol of P3 solution was made in Tris buffer (pH 7) and a 30 μmol Cu2+ solution was added to it. The solution became pink and to this pink solution, different concentrations of SCN− were gradually added and incubated for 30 min after each addition. Upon addition of SCN− ion to the solution of P3-Cu2+ complex, the pink colour of the solution gradually diminished and a significant decrease in absorbance at 560 nm was observed, due to stronger binding affinity of Cu2+ towards SCN−.[Citation20,48] Figure shows that the absorbance as well as fluorescence intensity of P3-Cu2+ complex decreased steadily with the increase in concentration of SCN− till 35 μmol. Thus, it may be concluded that the binding nature of P3 with Cu2+ is reversible and it can also act as a fluorescence turn ‘on–off’ biosensor for selective determination of Cu2+ under physiological condition.
Extraction of Cu2+ using P3
After establishing the turn ‘on–off’ nature of P3 due to complexation of Cu2+ ions, we wanted to investigate the possibility of separating Cu2+ ion from aqueous media using our polymer. For this, 1 mL solution of P3 (20 μmol in terms of rhodamine B group) in Tris buffer (pH 7) was taken and 20 μmol of Cu2+ ions was added to it. Then the solution of P3-Cu2+ complex was taken in an eppendorf tube and 1 mL of DCM was added to it. Then the mixture was shaken vigorously and allowed to stand for 30 min. Thereafter, the DCM was separated and dried under vacuum which yielded a pink residue. The residue was then dissolved in water, and to it, addition of SCN− was done gradually until it reached saturation. The fluorescence intensity of the P3-Cu2+ complex in Tris buffer (pH 7) was recorded initially as well as after extraction using DCM, and the data are shown in Figure . The fluorescence intensity of the solution after extraction was slightly less than that of the initial solution from which the extraction was carried out, indicating that the majority of the Cu2+-complexed polymer had been recovered. This suggested that P3 can be utilized for easy separation of toxic Cu2+ ions from aqueous solution. Further, in a separate experiment, gradual addition of SCN− was done to the P3-Cu2+ solution which was extracted using DCM and then redissolved in the buffer solution. It was observed that 20 μmol SCN− was required for fluorescence quenching of this solution and it reverted back to the intensity which was nearly same as that of the initial solution containing only P3. This proves quantitative estimation of Cu2+ after extraction.
Conclusions
In conclusion, we have successfully synthesized a rhodamine-based polymeric chemosensor which is capable of selective and sensitive detection of Cu2+ ions in the presence of variety of other metal ions in completely aqueous medium without the involvement of any organic solvent. Limitations of low solubility and poor complexation with Cu2+ ion in purely aqueous environment as described in previously reported rhodamine-based sensors have been successfully overcome in this work by introducing L-proline moiety to produce water-soluble polymer backbone. This polymeric chemosensor was synthesized by versatile RAFT polymerization method followed by functional group modification via Aza-Michael type condensation reaction. The equilibrium between spirolactam and ring-opened amide forms of rhodamine was utilized as a special feature for rapid survey and prompt colorimetric estimation of Cu2+ ions in biological medium. We have also investigated the binding nature of this polymer with Cu2+ ions and found it to be reversible which is very essential for chemosensor applications. This polymeric chemosensor can also be used for the removal of Cu2+ ions from drinking water. Additionally, by attaching appropriate cell-targeting biomarkers into this polymer, it can be potentially utilized for sensing of toxic ions inside the living cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
TDMP_1199111_Supporting_Information.pdf
Download PDF (469.1 KB)Acknowledgements
The authors thanks SRIC, IIT Kharagpur for financial support (project code NPA, institute approval number – IIT/SRIC/CHY/NPA/2014-15/81). C. Maiti and R.Banerjee acknowledge UGC, New Delhi, and S. Maiti acknowledges CSIR, New Delhi, for Senior Research Fellowships.
Funding
This work was supported by the Indian Institute of Technology Kharagpur [grant number IIT/SRIC/CHY/NPA/2014-15/81].
References
- Carter KP, Young AM, Palmer AE. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014;114:4564–4601.10.1021/cr400546e
- Butcher DJ. Review: recent advances in optical analytical atomic spectrometry. Appl. Spectrosc. Rev. 2013;48:261–328.10.1080/05704928.2012.717570
- Lin TW, Huang SD. Direct and simultaneous determination of copper, chromium, aluminum, and manganese in urine with a multielement graphite furnace atomic absorption spectrometer. Anal. Chem. 2001;73:4319–4325.10.1021/ac010319h
- Cerchiaro G, Manieri TM, Bertuchi FR. Analytical methods for copper, zinc and iron quantification in mammalian cells. Metallomics. 2013;5:1336–1345.10.1039/c3mt00136a
- Andria SE, Seliskar CJ, Heineman WR. Simultaneous detection of two analytes using a spectroelectrochemical sensor. Anal. Chem. 2010;82:1720–1726.10.1021/ac902243u
- Becker JS, Zoriy M, Matusch A, et al. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010;29:156–175.
- Becker JS, Matusch A, Depboylu C, et al. Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (slugs−genus arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2007;79:6074–6080.10.1021/ac0700528
- Soylak M. Determination of trace amounts of copper in high-purity aluminum samples after preconcentration on an activated carbon column. Fresenius Environ. Bull. 1998;7:383–387.
- Soylak M, Ercan O. Selective separation and preconcentration of copper (II) in environmental samples by the solid phase extraction on multi-walled carbon nanotubes. J. Hazard. Mater. 2009;168:1527–1531.10.1016/j.jhazmat.2009.03.032
- Royzen M, Dai ZH, Canary JW. Ratiometric displacement approach to Cu(II) sensing by fluorescence. J. Am. Chem. Soc. 2005;127:1612–1613.10.1021/ja0431051
- Zhao Y, Zhang XB, Han ZX, et al. Highly sensitive and selective colorimetric and off–on fluorescent chemosensor for Cu2+ in aqueous solution and living cells. Anal. Chem. 2009;81:7022–7030.10.1021/ac901127n
- Lee S J, Lee JE, Seo J, et al. Optical sensor based on nanomaterial for the selective detection of toxic metal ions. Adv. Funct. Mater. 2007;17:3441–3446.10.1002/(ISSN)1616-3028
- Posokhov YO, Kyrychenko A, Ladokhin AS. Steady-state and time-resolved fluorescence quenching with transition metal ions as short-distance probes for protein conformation. Anal. Biochem. 2010;407:284–286.10.1016/j.ab.2010.07.035
- White BR, Holcombe JA. Fluorescent peptide sensor for the selective detection of Cu2+. Talanta. 2007;71:2015–2020.10.1016/j.talanta.2006.09.009
- Mokhir A, Kiel A, Herten DP, et al. Fluorescent sensor for Cu2+ with a tunable emission wavelength. Inorg. Chem. 2005;44:5661–5666.10.1021/ic050362d
- Wegner SV, Arslan H, Sunbul M, et al. Dynamic copper(I) imaging in mammalian cells with a genetically encoded fluorescent copper(I) sensor. J. Am. Chem. Soc. 2010;132:2567–2569.10.1021/ja9097324
- Liu M, Zhao H, Chen S, et al. A ‘turn-on’ fluorescent copper biosensor based on DNA cleavage-dependent graphene-quenched DNAzyme. Biosens. Bioelectron. 2011;26:4111–4116.10.1016/j.bios.2011.04.006
- Zhang JF, Zhou Y, Yoon J, et al. Naphthalimide modified rhodamine derivative: ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Org. Lett. 2010;12:3852–3855.10.1021/ol101535s
- Chen J, Liu W, Zhou B, et al. Coumarin- and rhodamine-fused deep red fluorescent dyes: synthesis, photophysical properties, and bioimaging in vitro. J. Org. Chem. 2013;78:6121–6130.10.1021/jo400783x
- Banerjee R, Pal DS, Dhara D. Synthesis of a new rhodamine-containing block copolymer for highly selective and sensitive detection of Cu2+ and CN− ions in aqueous media. Polym. Int. 2014;63:1974–1981.10.1002/pi.2014.63.issue-11
- Kwon JY, Jang YJ, Lee YJ, et al. A highly selective fluorescent chemosensor for Pb2+. J. Am. Chem. Soc. 2005;127:10107–10111.10.1021/ja051075b
- Guo Z, Zhu W, Xiong Y. Multiple logic fluorescent thermometer system based on N-isopropylmethacrylamide copolymer bearing dicyanomethylene-4H -pyran moiety. Macromolecules. 2009;42:1448–1453.10.1021/ma802660e
- Zeng Q, Cai P, Li Z, et al. An imidazole-functionalized polyacetylene: convenient synthesis and selective chemosensor for metal ions and cyanide. Chem. Commun. 2008;1094–1096.
- Ma B, Wu S, Zeng F. Reusable polymer film chemosensor for ratiometric fluorescence sensing in aqueous media. Sens. Actuators B. 2010;145:451–456.10.1016/j.snb.2009.12.054
- Moad G, Rizzardo E, Thang SH. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005;58:379–410.10.1071/CH05072
- Huang Z, Peng Y, Chen H, et al. Synthesis and RAFT polymerization of a novel vinyl monomer containing both triarylimidazole and triazole moieties. Des. Monomers Polym. 2014;17:601–609.10.1080/15685551.2014.907612
- Matyjaszewski K, Xia J. Atom transfer radical polymerization. Chem. Rev. 2001;101:2921–2990.10.1021/cr940534g
- Maiti C, Banerjee R, Maiti S, et al. pH-induced vesicle-to-micelle transition in amphiphilic diblock copolymer: investigation by energy transfer between in situ formed polymer embedded gold nanoparticles and fluorescent dye. Langmuir. 2015;31:32–41.10.1021/la504165e
- Lowe AB, McCormick CL. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007;32:283–351.10.1016/j.progpolymsci.2006.11.003
- Banerjee R, Maiti S, Dhara D. Synthesis of soluble core cross-linked polystyrene star polymer by application of acrylate-nitrile oxide ‘click chemistry’ using metal-free reagents. Green Chem. 2014;16:1365–1373.10.1039/C3GC41722K
- Dey D, Maiti C, Maiti S, et al. Interaction between calf thymus DNA and cationic bottle–brush copolymers: equilibrium and stopped-flow kinetic studies. Phys. Chem. Chem. Phys. 2015;17:2366–2377.10.1039/C4CP03309D
- Jia X, Zhao X, Tian K, et al. Fluorescent copolymer-based prodrug for pH-triggered intracellular release of DOX. Biomacromolecules. 2015;16:3624–3631.10.1021/acs.biomac.5b01070
- Maiti C, Dey D, Mandal S, et al. Thermoregulated formation and disintegration of cationic block copolymer vesicles: fluorescence resonance energy transfer study. J. Phys. Chem. B. 2014;118:2274–2283.
- Qiu F, Wang D, Zhu Q, et al. Real-time monitoring of anticancer drug release with highly fluorescent star-conjugated copolymer as a drug carrier. Biomacromolecules. 2014;15:1355–1364.10.1021/bm401891c
- Banerjee R, Maiti C, Dutta S, et al. Size- and distance-dependent excitation energy transfer in fluorophore conjugated block copolymer-gold nanoparticle systems. Polymer. 2015;59:243–251.10.1016/j.polymer.2015.01.004
- Krupanidhi S, Sreekumar A, Sanjeevi CB. Copper & biological health. Indian J. Med. Res. 2008;128:448–461.
- Stern BR, Solioz M, Krewski D, et al. Copper and human health: biochemistry, genetics, and strategies for modeling dose-response relationships. J. Toxicol. Environ. Health, Part B. 2007;10:157–222.10.1080/10937400600755911
- Kim YR, Kim HJ, Kim JS, et al. Rhodamine-based ‘turn-on’ fluorescent chemodosimeter for Cu(II) on ultrathin platinum films as molecular switches. Adv. Mater. 2008;20:4428–4432.10.1002/adma.v20:23
- Zietz BP, Dieter HH, Lakomek M, et al. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci. Total Environ. 2003;302:127–144.10.1016/S0048-9697(02)00399-6
- Liu JW, Lu Y. A DNAzyme catalytic beacon sensor for paramagnetic Cu2+ ions in aqueous solution with high sensitivity and selectivity. J. Am. Chem. Soc. 2007;129:9838–9839.10.1021/ja0717358
- Kiaune L, Singhasemanon N. Pesticidal copper (I) oxide: environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 2011;213:1–26.
- Desai V, Kaler SG. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008;88:855–858.
- Lai JT, Filla D, Shea R. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient RAFT agents. Macromolecules. 2002;35:6754–6756.10.1021/ma020362m
- Zhang X, Shiraishi Y, Hirai T. Cu(II)-selective green fluorescence of a rhodamine−diacetic acid conjugate. Org. Lett. 2007;9:5039–5042.10.1021/ol7022714
- Banerjee R, Gupta S, Dey D, et al. Synthesis of PEG containing cationic block copolymers and their interaction with human serum albumin. React. Funct. Polym. 2014;74:81–89.10.1016/j.reactfunctpolym.2013.11.001
- Xiang Y, Tong A, Jin P, et al. New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org. Lett. 2006;8:2863–2866.10.1021/ol0610340
- Xiang Y, Tong A. A new rhodamine-based chemosensor exhibiting selective FeIII-amplified fluorescence. Org. Lett. 2006;8:1549–1552.10.1021/ol060001h
- Lou X, Qiang L, Qin J, et al. A new rhodamine-based colorimetric cyanide chemosensor: convenient detecting procedure and high sensitivity and selectivity. ACS Appl. Mater. Interfaces. 2009;1:2529–2535.10.1021/am900467b