Abstract
Aim of the present work was to develop alginate raft forming tablets for controlled release pantoprazole sodium sesquihydrate (PSS). Box behnken design was used to optimize 15 formulations with three independent and three dependent variables. Physical tests of all formulations were within pharmacopoeial limits. Raft was characterized by their strength, thickness, resilience, acid neutralizing capacity, floating lag time and total floating time. Raft strength, thickness and resilience of optimized formulation AR9 were 7.43 ± 0.019 g, 5.8 ± 0.245 cm and greater than 480 min, respectively. Buffering and neutralizing capacity were 11.2 ± 1.01 and 6.5 ± 0.56 meq, respectively. Dissolution studies were performed by using simulated gastric fluid pH 1.2 and cumulative percentage release of optimized formulation AR9 was found 98%. First order release kinetics were followed and non-fickian diffusion was observed as value of n was greater than 0.45 in korsmeyer-peppas model. PSS, polymers, tablets and rafts were further characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffractometry (XRD) and differential scanning calorimetry (DSC). FTIR spectra of PSS, polymers and raft of optimized formulation AR9 showed peaks at 3223.09, 1688.17, 1586.67, 1302.64 and 1027.74 cm−1 due to –OH stretching, ester carbonyl group (C=O) stretching, existence of water and carboxylic group in raft, C–N stretching and –OH bending vibration showed no interaction between them. XRD showed diffraction lines indicates crystalline nature of PSS. DSC thermogram showed endothermic peaks at 250 °C for PSS. The developed raft was suitable for controlled release delivery of PSS.
1. Introduction
The advancement of new materials built on polysaccharides is due to their benefits as low cost, freely available, biodegradable, non-toxic and sustainability. Biopolymers like sodium alginate, pectin and numerous others have been used in the field of GRDD. Sodium alginate, the sodium salt of alginic acid, is a biodegradable non-toxic naturally occurring macromolecule hydrate and swells in water but in acidic environment it produces gel after protonation.[Citation1] Alginate consists of linear copolymers of 1, 4-glycosidically linked β-D-mannuronic acid and α-L-guluronic acid. Sodium alginate, a pH sensitive polymer stable at acidic pH but unstable in alkaline medium because at higher pH a rapid dissolution occur that limits its application and can be crosslinked by physical and chemical mechanisms. Mono and divalent cations (sodium and calcium) can be used for crosslinking of sodium alginate to form three dimensional gel network.[Citation2,3] Hydroxypropyl methyl cellulose K100M (HPMC K100M) a hydrophilic polymer sustained the release of drug by increasing the viscosity of gel layer. HPMC K100M releases the drug from gel barrier by diffusion process.[Citation4]
Previously reported rafts such as alginate rafts of gaviscon liquid do not neutralize the gastric acid but inside the raft high pH was maintained for an extended period of time. Hampson et al. reported the alginate rafts and their various parameters used for the characterization such as raft resilience or resistance and buoyancy.[Citation5] The addition of antacids such as aluminum hydroxide have negative effect on structure and strength of raft but calcium carbonate have positive effect on raft thickness and strength. Hampson et al. reported the effect of antacid on raft structure and strength.[Citation6] In 2014 Jang et al. develop the risedronate sodium raft for reduction of esophageal irritation by using sodium alginate as raft forming polymer.[Citation7] Raft of curcumin-eudragit by using sodium alginate as gelling polymer and calcium carbonate for generating CO2 and Ca2+ reported by Kerdsakundee et al. [Citation8]
The objective of this research work is to develop and characterize alginate rafts for the treatment of peptic ulcer along with reflux disorders. In vitro modified balance method will be developed for measurement of strength of raft. Modified paddle mixer apparatus is developed for the determination of raft resilience. Acid neutralizing and buffering capacity of sodium bicarbonate and citric acid are measured by modified USP type II dissolution apparatus and effect of calcium on strength of raft is determined. The developed formulations are further characterized by determining the floating lag time (FLT) and total floating time (TFT) of raft. In vitro dissolution studies are performed to check the release pattern of pantoprazole sodium sesquihydrate (PSS). Fourier transform infrared spectroscopy (FTIR) studies are performed to check the interactions between drug, polymers and other excipients. X-ray diffractometry (XRD) is used to check the crystalline or amorphous nature of the drug and polymers and differential scanning calorimetry (DSC) is used to check the thermal behavior of drug and polymers.
2. Experimental
2.1. Materials
PSS was obtained as a gift sample from Shrooq Pharmaceuticals Pvt. Ltd. Lahore, Pakistan. Sodium alginate and HPMC K100M were of analytical grade and purchased from Sigma–Aldrich Chemie Gmbh Germany. Sodium bicarbonate, citric acid, and calcium carbonate were obtained from KGaA Darmstadt, Germany. Pepsin was obtained from Scharlau Barcelona, Spain. Double distilled water was used in whole study and other chemicals/reagents used was of analytical grade.
2.2. Methods
2.2.1. Box behnken design
Box behnken response surface designs are used to require three levels, coded as −1, 0, and +1. Box behnken design (BBD) was used [Citation9] for optimization of tablets having three independent variables and three dependent variables using design expert (version 7.1 state-ease Inc., Minneapolis, MN). Independent variables were percentages of sodium alginate (X1), HPMC K100M (X2) and sodium bicarbonate (X3) while the dependent variables were % drug release of PSS at 2 h (Y2), 4 h (Y4) and at 8 h (Y8) as shown in Table . The nonlinear quadratic model by this design is given as [Citation10];(1)
Table 1. Independent and dependent variables and constrains in box-behnken design.
where Yi is the measured response of the dependent variables, b0 is the intercept, b1–b33 are the regression coefficients computed from the observed experimental values of Y. X1, X2 and X3 are the coded value of the independent variables. Xa Xb (a, b = 1, 2, 3) and (i = 1, 2, 3) represent the interaction and quadratic terms, respectively.
2.2.2. Preparation of tablets
Tablets were prepared by mixing PSS, sodium alginate, HPMC K100M (for sustained release effect), sodium bicarbonate, citric acid and calcium carbonate by using sigma mixer and passed through 20-mesh screen. Composition of 15 formulations are given in Table . Powder blend passed from the micromeritic limits were mixed thoroughly for 5 min by using sigma mixer. The mixture was granulated using 2% (w/w) HPMC E5 in a 90% ethanol solution. 2% (w/w) HPMC E5 in 90% ethanol used as granulating agent. The prepared granules were dried at 40 °C for 2 h, passed through 18-mesh screen.[Citation4,11] Granules were compressed by using minipress MII (pharma test Hainburg, Germany). Physical tests of tablets such as weight variation, hardness, thickness, diameter and friability were performed.
Table 2. Composition of alginate raft forming tablets.
2.2.3. Effect of pH on raft formation
Prepared tablets were added into 900 ml of simulated gastric fluid (SGF) having pH 1.2, 5.8, 1.0 N HCl pH 1.2 and 0.1 N HCl pH 5.7 and effect of pH on raft formation was observed.[Citation12]
2.2.4. Disintegration time of tablet in water
Disintegration time was measured by placing one tablet in 120 ml of distilled water at room temperature and evaluation of gas around the tablet or its fragments were observed. Tablet was fragmented if the evolution of gas around the tablet or its fragments stopped, being either dissolved or dispersed in water so that no agglomerate remains. The same process was repeated on four additional tablets.[Citation7,13]
2.2.5. Raft strength
Prepared tablet was transferred to 150 ml of SGF pH 1.2 at 37 °C. SGF was prepared with 2.0 g of sodium chloride, 3.2 g of purified pepsin and 7 ml of HCl in 1000 ml of distilled water. Raft was allowed to form around L-shaped wire probe (diameter: 1.2 mm) held straight in the beaker for 30 min. Raft strength was measured by using the modified balance method.[Citation13]
2.2.6. Volume, weight and thickness of raft
Tablet was transferred to 150 ml of SGF pH 1.2 maintained at 37 °C and wait of 30 min until the raft was formed. Beaker used for raft formation was pre-weighed (W1). Top of each raft was observed from outer surface of beaker. The whole weight of beaker and filling was obtained after raft formation (W2). Raft was removed from the beaker by pouring off the subnatant liquor and weighed (W3). Remaining liquid was removed from the beaker and it was refilled with water to the noticeable position and weighed (W4). The volume of each raft was measured in ml and weight was measured in grams.[Citation5] Thickness of raft was measured by placing tablet in 150 ml of SGF. Raft was allowed to form for 10 min and thickness of the raft was measured at three places around the cylinder by using digital vernier caliper (Shandong, China) and expressed as mean value.[Citation7]
2.2.7. Raft resilience
Place one tablet in 150 ml of SGF pH 1.2 at 37 °C in 250 ml glass jar and wait for 30 min until the raft was completely developed. Glass jar was capped and positioned in modified tumble mixer, set to revolve at 20 rpm, to simulate gastric agitation. Raft was assessed visually for such time that a raft could no longer be noticed. A raft was distinct or dispersed into two or more hovering gels at least 15 mm in diameter.[Citation6]
2.2.8. FLT and TFT
USP dissolution apparatus II (pharma test Hainburg, Germany) was used for the determination of FLT and TFT. Add one tablet in 900 ml SGF pH 1.2 maintained at 37 ± 0.5 °C and set at 50 rpm. The time required for raft to rise to the surface and float was determined as FLT. TFT is the total time for which the raft floats in dissolution medium including FLT.[Citation8]
2.2.9. Acid neutralization capacity
The acid neutralization ability of raft forming tablet was estimated using an in vitro method. The dissolution apparatus II (paddle method) was operated with a paddle speed of 125 rpm and with 250 ml of 0.02 M HCl solution at 37 °C. Tablet formulation dissolved solution 120 ml was added into the medium and the pH of the medium was checked continuously, after 20 min the burette started with continuous titration of 0.1 M HCl solution at a continual speed of 2.0 ml/min until the acidity of medium reached pH 2.5.Neutralizing and total buffering capacity from pH 2.5–4.5 was calculated by following equations.(2)
(3)
where VHCl is the volume of HCl in the vessel, THCl the titer of HCl in the vessel, Vtr−2 the added volume of HCl from the burette until pH 2.5, Ttr the titer of HCl in the burette, W1 the weight of intact formulation and W2 the weight of tested quantity of formulation. Vtr−1 is the added volume of HCl from the burette between pH 2.5 and 4.5, Ttr the titer of HCl in the burette.[Citation7]
2.2.10. In vitro drug release studies
The in vitro drug release study was carried in 900 ml SGF of pH 1.2 at 37 ± 0.5 °C from 0 to 8 h by using USP dissolution apparatus II at 50 rpm. 5 ml aliquot was pipette out at regular interval and replaced with fresh medium of same volume. The aliquot was filtered by 0.45 μm filter and concentration of drug was determined by UV spectrophotometer (PerkinElmer Inc. New York, USA) at 290 nm.[Citation14]
2.2.11. Drug release kinetics
The mechanisms of controlled release alginate raft forming formulations were determined by different in vitro kinetics models such as zero order (Equation 4), first order (Equation 5), higuchi (Equation 6) and Korsmeyer-peppas model (Equation 7).[Citation15](4)
(5)
(6)
(7)
where F is fraction of drug release in time t, K0 is rate constant for zero order release equation, K1 is first order release constant, K2 is higuchi constant, Mt is amount of drug release at time t, M∞ is amount of drug release at infinity and n is diffusion constant.
2.2.12. Fourier transform infrared spectroscopy
FTIR of PSS, sodium alginate, HPMC K100M and raft of optimized formulation AR9 were obtained by FTIR spectrophotometer (Bruker Alpha, Germany) and compared. The spectra was recorded at wavelength range of 800–3500 cm−1.
2.2.13. X-ray diffractometry
Crystalline or amorphous nature of drug, polymers, prepared tablets and rafts were evaluated from their diffractograms. Diffractograms of PSS, sodium alginate, HPMC K100M, tablet of optimized formulation AR9 and raft of AR9 optimized formulation were obtained using an XRD diffractometer D/max-2500pc, Rigaku Co, Japan. Tube voltage was 40 kV, current was mA, and scanning rate was 50 over a range of 80–800 diffraction angle.
2.2.14. Differential scanning calorimetry
DSC was used to analyze the thermal characteristics of the powdered sample of drug and polymers, physical mixture, prepared tablets and raft. DSC thermograms of PSS, sodium alginate, HPMC K100M, tablet of optimized formulation and raft of AR9 optimized formulation were obtained by using differential scanning calorimeter DSC-60 Shimadzu, Germany. 5.5 mg sample was placed in aluminum pans, sealed and analyzed under a stream of nitrogen gas of 100 ml/min and heated from 50 to 350 °C.
3. Results and discussion
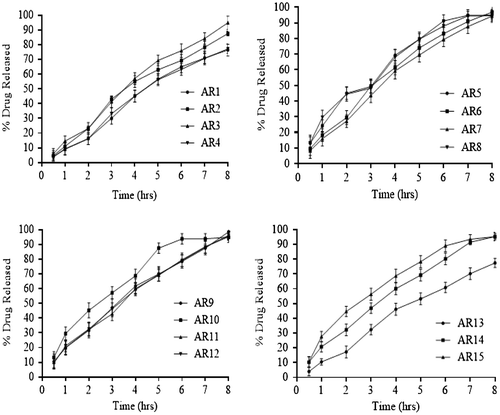
Interaction between independent and dependent variables (Table ) were studied and three dimensional graphs were developed as shown in Figure . Disintegration time of tablets and strength, weight, volume and thickness of rafts were within pharmacopoeial limits are mentioned in Table . Effect of different pH medium on raft formation was studied successfully. Buffering capacity, neutralizing capacity, resilience, FLT and TFT of rafts of all formulations were successfully determined and are shown in Table . The release pattern of PSS form pectin rafts were determined and are shown in Figure . FTIR spectra of PSS, sodium alginate, HPMC K100M and raft of optimized formulation AR9 showed compatibility of PSS with polymers and are shown in Figure . DSC thermograms and X-ray diffractograms of PSS, sodium alginate, HPMC K100M, tablet of optimized formulation AR9, and raft of optimized formulation AR9 showed compatibility of drugs with polymers and are shown in Figures and , respectively.
Table 3. Observed responses for alginate rafts forming tablets (n = 6).
Figure 1. 3D response surface graph showing effects of sodium alginate (X1), HPMC K100M (X2) and sodium bicarbonate (X3) on (A) % drug release at 2 h (Y2), (B) % drug release at 4 h (Y4) and (C) % drug release at 8 h (Y8).
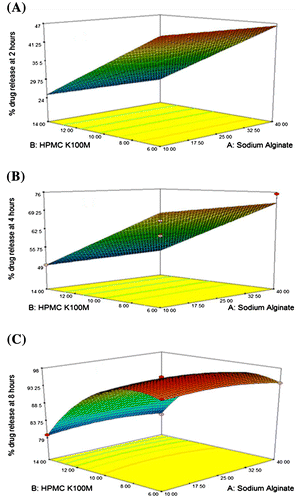
Table 4. Disintegration time of tablets and strength, weight, volume and thickness of raft (n = 6).
Table 5. Buffering capacity, neutralizing capacity, resilience, FLT and TFT of raft forming tablets (n = 6).
Figure 3. FTIR spectra of (A) PSS, (B) sodium alginate, (C) HPMC K100M and (D) alginate raft of optimized formulation AR9.
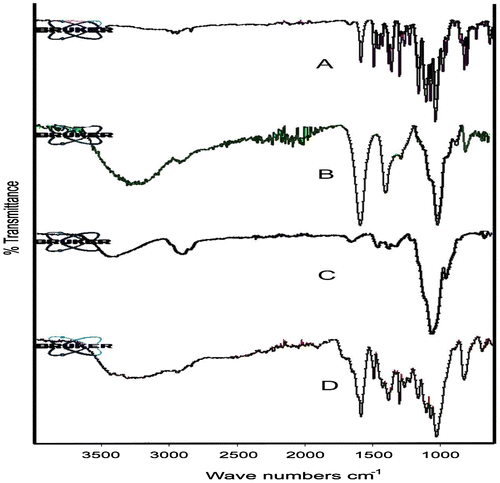
Figure 4. DSC thermograms of (A) PSS, (B) sodium alginate, (C) HPMC K100M and (D) tablet of optimized formulation AR9 and (E) alginate raft of optimized formulation AR9.
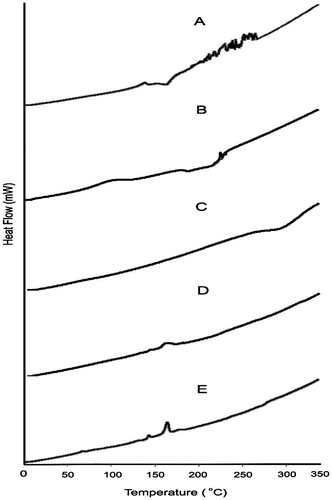
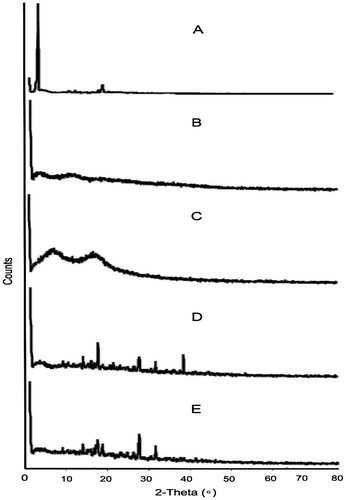
Figure 5. XRD of (A) PSS, (B) sodium alginate, (C) HPMC K100M and (D) tablet of optimized formulation AR9 and (E) alginate raft of optimized formulation AR9.
The outcome of independent variables on dependent variables were studied and 3D plots were developed. Values of % drug release at 2 h were ranged from 21.53 ± 0.987 to 47.76 ± 0.456%. % drug release at 4 and 8 h were found between 46.55 ± 0.654–75.29 ± 0.087 and 79.12 ± 0.098–98.32 ± 0.911%, respectively. All responses were fitted to the quadratic models using BBD. Data of Y2, Y4 and Y8 was observed and best fitted model was quadratic and regression Equations (8–10) were generated.(8)
(9)
(10)
In regression equations positive sign favors the optimization while negative sign indicates an inverse relationship between independent and dependent variables. The amount of sodium alginate (X1) HPMC K100M (X2) and sodium bicarbonate (X3) have different effects on % drug release (Y2), (Y4) and (Y8). Tested formulations showed that percentage of drug release were more when the polymers concentration were less as compared to the formulation contained high amount of polymers. When the concentration of sodium alginate and HPMC K100M were 25 and 10%, respectively the sustained release effect of drug from alginate rafts were good but below that concentrations the drug was rapidly released and above that concentrations the release of drug was slow. The interaction of X1 and X2 was insignificant and showed negative effect on Y2, Y4 and Y8 and X1 and X3 possessed negative value and have insignificant effect on Y2, Y4 and Y8. Interaction of X2 and X3 was significant and have positive effect on Y2, Y4 and Y8. Optimized formulation AR9 was selected on the basis of better release pattern of drug at 2, 4 and 8 h. Rapolu et al. studied the effect of different polymer concentrations on release profile of GRRD of metronidazole by using BBD.[Citation9]
At pH 1.2 of SGF and 1.0NHCl tablets rapidly disintegrated and rafts were formed on the top of medium but at pH 5.7 of 0.1N HCl and 5.8 of SGF, tablets were reside at the bottom of the medium and rafts were not formed. Elliot et al. studied the effect of different pH medium on raft forming alginate-antacids combined formulations.[Citation12]
Disintegration time of tablets of 15 formulations were ranged from 53 to 67 s. Effects of concentration of sodium alginate and sodium bicarbonate on disintegration time of tablets were observed. Formulations (AR1, AR4, AR11 and AR14) containing less amount of sodium alginate showed less disintegration time of tablets as compared to the formulations (AR3, AR6, AR8 and AR9) containing high amount of sodium alginate as shown in Table . Formulations (AR3, AR10, AR13 and AR14) containing higher concentration of sodium bicarbonate rapidly disintegrate as compared to the formulations (AR5, AR6 and AR7) having less amount of sodium bicarbonate. Jang et al. studied the disintegration time of risedronate sodium tablets in water containing sodium alginate as a raft forming polymer and sodium bicarbonate as gas generating substances.[Citation7]
Raft strength was ranged from 3.10 ± 0.097 to 7.32 ± 0.047 g measured by modified balance method as shown in Table . Raft weight and volume was ranged from 1.11 ± 0.012 to 2.20 ± 0.013 g and 5.5 ± 0.15 to 8.9 ± 0.05 ml, respectively. Hampson et al. measured the strength, weight and volume of rafts of sodium alginate.[Citation5] Raft thickness ranged from 3.4 ± 0.236 to 5.8 ± 0.245 cm as mentioned in Table . AR14 formulation showed less thickness of raft due to less amount of polymers but AR9 formulation has highest raft thickness because of maximum amount of polymers.[Citation7] Thickness of raft was increased when the concentration of the polymers were increased. Raft resilience of all formulations were greater than 480 min as shown in Table .[Citation16] Hampson et al. measured the resilience of alginate rafts and studied the effect of polymer concentration on resilience of rafts.[Citation5] FLT was ranged from 48 to 55 s, AR9 formulation showed maximum FLT and AR7 showed the minimum value as shown in Table . TFT of all prepared formulations was found to be greater than 8 h.
An in vitro method reported by Jang et al. [Citation7] were used to check the buffering and neutralizing capacity. pH values after 4 and 20 min were recorded as mentioned in Table . The formulations containing the maximum amount of sodium citrate and citric acid possessed higher buffering between pH 2.5–4.5 and neutralizing capacity. The pH after 4 and 20 min checks an un-physiologically high pH and neutralizing capacity between 2.5 and 4.5 is sign for the efficacy in the physiological environment.
The drug release from alginate rafts forming formulations AR1–AR15 were investigated. The concentration of sodium alginate was ranged from 10 to 40% have an effect on release of drug from raft. When the amount of sodium alginate was increased the release of PSS from raft was decreased. PSS is freely water soluble, a retardant HPMC K100M was added to sustain the release pattern of drug from raft. HPMC K100M form a gel barrier around raft that allows the drug to be released by diffusion process. HPMC K100M was used the concentration ranges of 6–14%. As predictable, on increasing the concentration of HPMC K100M, the thickness of gel barrier was increased that delayed the release of PSS from raft. He et al. studied the effect of effect of HPMC K100 on release profile of metformin.[Citation4] Sodium bicarbonate 20–40% a gas generating substance also have effect on drug release from raft.[Citation17] Sodium bicarbonate generate carbon dioxide after reacting with acidic dissolution medium and resulted in the form of gel like raft system at the surface of the medium. The carbon dioxide is entrapped in the gel cause obstruction of diffusion pathway of drug release from raft. This effect was more observed at low polymer concentrations in the formulation (AR1, AR13). When the polymer concentrations were increased in the formulation (AR2, AR5, AR9) the effect of sodium bicarbonate on drug release from alginate raft was decreased. Jiménez-Martínez et al. studied the effect of sodium bicarbonate on release profile of captopril from floating matrix tablets.[Citation18] The drug release percentages after 2, 4 and 8 h were mentioned in Table . The optimized formulation AR9 showed optimum drug release i.e. 98%.
In kinetic release models R2 values of zero order release were ranged from 0.711 to 0.981 while in first order release it was 0.957–0.990 and which observed the concentration dependent release of PSS. In korsmeyer-peppas model value of n was found to be 0.57 which was greater than 0.45 showing a non-fickian drug release mechanism in prepared formulations. Jose et al. studied the release pattern of insulin from tablets of crosslinked chitosan microspheres by using korsmeyer-peppas.[Citation19]
FTIR spectra of PSS showed its features peaks at 1587.83, 1301.87, 1104.89 cm−1 due to C=C stretching, C–N stretching and C–F stretching, respectively.[Citation20] Sodium alginate showed peaks at 1583.96, 1406.68 cm−1 provide information about water and carboxylic group of alginate and 1022.38 cm−1 due to –OH bending vibration.[Citation21] HPMC K100M showed two peaks at 1054.87 and 3400.03 cm−1 due to C–O and O–H stretching vibrations respectively.[Citation22]). Raft of AR9 formulation showed peaks at 3223.09, 1688.17, 1586.67−1, 1302.64−1, 1162.01−1,and 1027.74 cm−1 due to –OH stretching, ester carbonyl group (C=O) stretching, existence of water and carboxylic group in raft, C–N stretching, C–F stretching and –OH bending vibration. The FTIR spectra of drug, polymers and raft of AR9 formulation showed no notable interaction between them. Spectra of raft of AR9 formulation showed the presence of sodium alginate, HPMC K100M and drug were effectively entrapped in the pectin raft.
DSC thermograms of PSS, sodium alginate, HPMC K100M, tablet of optimized formulation AR9 and raft of AR9 formulation are shown in Figure . Thermogram of PSS showed an endothermic peaks at 250 °C which was the indication of melting point of PSS.[Citation23] Thermograms of sodium alginate, HPMC K100M, tablet of optimized formulation AR9 and raft of AR9 optimized formulation showed no peaks indicating that PSS was dispersed in the tablet and raft effectively.
XRD diffractograms showed characteristics diffraction lines of PSS at 2θ of 6°and 22° due to its crystalline nature are shown in Figure .[Citation23] Sodium alginate showed well defined peaks at 3° (2θ) related to its crystallinity due to strong intermolecular hydrogen bonding [Citation24] and HPMC K100M at 3°, 9° and 18° (2θ).[Citation25] The diffractograms of tablet of optimized formulation AR9 and raft of AR9 optimized formulation showed many characteristics peaks at 15°, 17°, 19°, 27°, 32° and 40° (2θ) but disappearance of the peaks of PSS, sodium alginate and HPMC K100M were observed. This indicated that the crystalline nature of PSS was decreased after tablet preparation and raft formation of AR9 optimized formulation.
4. Conclusion
Raft forming tablets were successfully developed using sodium alginate as raft forming polymers, HPMCK100M for sustained effect, sodium bicarbonate and citric acid as gas generating agents and neutralizing agent calcium carbonate. This novel oral dosage form rapidly disintegrate and formed floating raft on the surface of SGF, preventing reflux disorders associated with peptic ulcer and release the PSS up to 8 h. The raft floats on the surface of SGF for up to 24 h with 1 min of FLT. In vitro modified balance method for measurement of raft strength was developed successfully. Optimized formulation AR9 showed good strength, thickness and resilience of raft.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
All authors are grateful to Bahauddin Zakariya University Multan, Pakistan for providing research facilities.
References
- Diós P, Nagy S, Pál S, et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015;96:196–206.10.1016/j.ejpb.2015.07.020
- Mukhopadhyay P, Sarkar K, Soam S, et al. Formulation of pH-responsive carboxymethyl chitosan and alginate beads for the oral delivery of insulin. J. Appl. Polym. Sci. 2013;129:835–845.10.1002/app.38814
- Ghayempour S, Mortazavi SM. Preparation and investigation of sodium alginate nanocapsules by different microemulsification devices. J. Appl. Polym. Sci. 2015;132:41904–41911.
- He W, Li Y, Zhang R, et al. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014;476:223–231.10.1016/j.ijpharm.2014.09.056
- Hampson F, Farndale A, Strugala V, et al. Alginate rafts and their characterisation. Int. J. Pharm. 2005;294:137–147.10.1016/j.ijpharm.2005.01.036
- Hampson FC, Jolliffe IG, Bakhtyari A, et al. Alginate–antacid combinations: raft formation and gastric retention studies. Drug Dev. Ind. Pharm. 2010;36:614–623.10.3109/03639040903388290
- Jang SW, Lee JW, Ryu DS, et al. Design of pH-responsive alginate raft formulation of risedronate for reduced esophageal irritation. Int. J. Biol. Macromol. 2014;70:174–178.10.1016/j.ijbiomac.2014.06.048
- Kerdsakundee N, Mahattanadul S, Wiwattanapatapee R. Development and evaluation of gastroretentive raft forming systems incorporating curcumin-Eudragit® EPO solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Biopharm. 2015;94:513–520.10.1016/j.ejpb.2015.06.024
- Rapolu K, Sanka K, Vemula PK, et al. Optimization and characterization of gastroretentive floating drug delivery system using Box-Behnken design. Drug Dev. Ind. Pharm. 2013;39:1928–1935.10.3109/03639045.2012.699068
- Sharma D, Maheshwari D, Philip G, et al. Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using Box-Behnken design: in vitro and in vivo evaluation. BioMed Res. Int. 2014;2014:1–14.
- Mandal U, Pal TK. Formulation and in vitro studies of a fixed-dose combination of a bilayer matrix tablet containing metformin HCl as sustained release and glipizide as immediate release. Drug Dev. Ind. Pharm. 2008;34:305–313.10.1080/03639040701657487
- Elliott BM, Steckbeck KE, Murray LR, et al. Rheological investigation of the shear strength, durability, and recovery of alginate rafts formed by antacid medication in varying pH environments. Int. J. Pharm. 2013;457:118–123.10.1016/j.ijpharm.2013.09.034
- Prajapati ST, Mehta AP, Modhia IP, et al. Formulation and optimisation of raft-forming chewable tablets containing H2 antagonist. Int. J. Pharm. Invest. 2012;2:176–182.10.4103/2230-973X.106988
- Prajapati VD, Jani GK, Khutliwala TA, et al. Raft forming system – an upcoming approach of gastroretentive drug delivery system. J. Controlled Release. 2013;168:151–165.10.1016/j.jconrel.2013.02.028
- Bose A, Wong TW, Singh N. Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics. Saudi Pharm. J. 2013;21:201–213.10.1016/j.jsps.2012.03.006
- Dettmar P, Hampson F, Farndale A, et al. Alginate rafts and their characterization. Int. J. Pharm. 2005;294:137–147.
- Bhandari PN, Jones DD, Hanna MA. Characterization of sodium starch glycolate prepared using reactive extrusion and its comparisons with a commercial brand VIVASTAR® P. Ind. Crops Prod. 2013;41:324–330.10.1016/j.indcrop.2012.04.050
- Jiménez-Martínez I, Quirino-Barreda T, Villafuerte-Robles L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008;362:37–43.10.1016/j.ijpharm.2008.05.040
- Jose S, Fangueiro J, Smitha J, et al. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013;60:249–253.10.1016/j.ejmech.2012.12.011
- Reddy GM, Bhaskar BV, Reddy PP, et al. Structural identification and characterization of potential impurities of pantoprazole sodium. J. Pharm. Biomed. Anal. 2007;45:201–210.10.1016/j.jpba.2007.05.032
- Borba PAA, Pinotti M, de Campos CEM, et al. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydr. Polym. 2016;137:350–359.10.1016/j.carbpol.2015.10.070
- Ding C, Zhang M, Li G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015;119:194–201.10.1016/j.carbpol.2014.11.057
- Zupančič V, Ograjšek N, Kotar-Jordan B, et al. Physical characterization of pantoprazole sodium hydrates. Int. J. Pharm. 2005;291:59–68.10.1016/j.ijpharm.2004.07.043
- Seeli DS, Dhivya S, Selvamurugan N, et al. Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016;91:45–50.10.1016/j.ijbiomac.2016.05.057
- Meneguin AB, Cury BSF, Evangelista RC. Films from resistant starch-pectin dispersions intended for colonic drug delivery. Carbohydr. Polym. 2014;99:140–149.10.1016/j.carbpol.2013.07.077