Abstract
Alkyne-functionalized fullerenes (fullerynes) were designed and conveniently synthesized via Bingel reaction in one step with high yields. They were used to react with azido-functionalized polystyrene (PS) via Huisgen [3 + 2] cycloaddition ‘click’ chemistry to form two fullerene polymers: one with C60 tethered to the end of a PS chain (C60-1PS) and the other with C60 tethered at the junction point of two PS chains of identical molecular weight (C60-2PS). The fullerene polymers were characterized by 1H NMR, 13C NMR, FT-IR, MALDI-TOF mass spectrometry and SEC. The results showed that the fullerene polymers are well-defined with narrow polydispersity and high fullerene functionality. Besides, aggregation of C60 in THF was observed in the SEC traces. The optical properties of the fullerene polymers were studied by UV–Vis absorption spectroscopy, and the results suggested that the PS chain(s) on the fullerene core has no remarkable effect on the optic property of C60. The thermal properties of the fullerene polymers were studied by TGA and DSC, and the results indicated that the two fullerene polymers with different C60 content and distinct molecular topology may have different self-assemble architectures in the solid state. The well-defined fullerene polymers can be used as model compounds to study the self-assemble architecture of shape amphiphiles based on polymer-tethered molecular nanoparticles.
Introduction
Ever since its discovery and multi-gram availability, [60]fullerene(C60) has attracted intense research interest for its fascinating carbon nanostructures with unique photoelectronic and biological properties.[Citation1–3] However, the poor compatibility of pristine fullerenes with other materials leads to its strong tendency toward aggregation and low solubility in common solvents and thus limits their facile derivatization and application.[Citation1,4,5] To fully take advantage of its outstanding properties, it is critical to manipulate their interactions to form ordered structures in multi-dimensions across different length scales.[Citation6] Attaching a small number of polymer tethers to the surface of C60 core provides a convenient way to do so.[Citation7,8] Since C60 core are considered as a spherical molecular nanoparticles (MNP), the fullerene-polymer conjugates are model shape amphiphiles that have been predicted by computer simulation to exhibit rich phase behaviors and various unusual structures due to packing constraints and amphiphilic interactions.[Citation8–10]
Recently, Cheng et al. has reported a robust way to synthesize well-defined fullerene polymers by ‘click’ chemistry.[Citation5,11–13] The Cu-catalyzed Huisgen [3 + 2] cycloaddition reaction between the alkyne functionalized fullerene (thus named ‘fulleryne’) and azido-functionalized polymers proceeded efficiently at room temperature with no side reactions between azide and C60 core.[Citation14–23] With the development of living polymerization technology, polymer with controlled molecular weight, narrow polydispersity can be easily synthesized.[Citation24–28] However, the syntheses of fullerynes are usually nontrivial.[Citation5] Several methods have been reported,[Citation11,29–32] but most of them involves multi-step syntheses and drastic reaction conditions. In order for the ‘click’ chemistry to be viable for practical applications, it is important to find that fullerynes are readily available and highly reactive.[Citation11] Bingel–Hirsch reaction has been considered as one of the most versatile and efficient methods for C60 functionalization under mild reaction conditions to afford stable methanofullerene products with high yields.[Citation33–35] Nierengarten et al. [Citation36–46] prepared C60 derivatives bearing terminal alkyne, and used them as building blocks in the efficient preparation of ‘sugar balls’ and other conjugates. By varying the number and positions of the alkyne groups on the surface of the C60 in fullerynes, fullerene materials with different architecture can be obtained from simple common precursors.[Citation12,13,16]
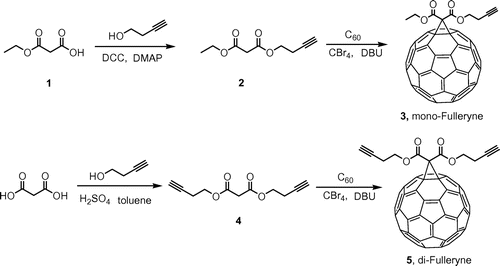
In this paper, we describe the use of Bingel reaction for the convenient syntheses of fullerynes in one step from C60 (Scheme ) and the application of these fullerynes in the syntheses of two fullerene polymers of distinct topology (Scheme ): one being C60 tethered with one polymer tail (C60-1PS) and the other being C60 tethered at the junction point between two identical polymer chains (C60-2PS). The fulleryne with one alkyne group is named mono-Fulleryne, and the fulleryne bearing two alkyne groups is named di-Fulleryne.
Experimental procedure
Materials and methods
C60 (MTR Ltd. >99.5%); malonic acid (SINOPHARM, ACS grade); 3-butyn-1-ol (J&K, 98%); diethyl malonate (SINOPHARM, ACS grade); carbon tetrabromide (CBr4, SINOPHARM, ACS grade); dicyclohexylcarbodiimide (DCC, J&K, 99%); dimethylaminopyridine (DMAP, J&K, 99%); 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, J&K, 98%); sodium azide (Alfa Aesar, 99%); N,N,N′,N′,N″-pentamethyldiethylenetriamine (PMDETA, Aldrich, >99%); petroleum ether (SINOPHARM, ACS grade); ethyl acetate (EtOAc, SINOPHARM, ACS grade); diethyl ether (SINOPHARM, ACS grade); methanol (SINOPHARM, ACS grade); acetone (SINOPHARM, ACS grade); cyclohexane (SINOPHARM, ACS grade); toluene (SINOPHARM, ACS grade); Tetrahydrofuran (THF, SINOPHARM, ACS grade) were used as received. Anhydrous dichloromethane (CH2Cl2, SINOPHARM, ACS grade), N,N′-dimethylformamide (DMF, SINOPHARM, ACS grade) were obtained by distillation from CaH2; anhydrous toluene were obtained by distillation from sodium. CuBr (SINOPHARM, ACS grade) was washed with 1 M sulfuric acid, anhydrous ethanol, anhydrous diethyl ether respectively before dried under vacuum. 3-Ethoxy-3-oxopropanoic acid (1), PS-N3 was prepared according to literature procedures.[Citation5,47]
All 1H and 13C NMR spectra were acquired in CDCl3 (Aldrich, 99.8% D) using a Varian Mercury spectrometer operating at 400 and 100 MHz respectively at room temperature. The 1H NMR spectra were referenced to the residual proton impurities in the CDCl3 at δ 7.27 ppm and 13C NMR spectra were referenced to 13CDCl3 at δ 77.00 ppm.
Fourier transform infrared (FT-IR) spectra were taken using a Nicolet Magna-IR 550 FT-IR spectrometer in the range of 400–4000 cm−1 by making a KBr pellet from the mixture with the sample. The resolution was 4 cm−1 and 32 scans were averaged.
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra were recorded on a Kratos Axima CFR plus spectrometer (Shimadzu Biotech, Manchester, UK) with a 337 nm nitrogen laser and the acceleration voltage of 20 kv. The matrix was 2,5-dihydroxybenzoic acid (DHB). Mass spectra in positive ion mode were measured in both linear and reflection modes. Data analyses were conducted with Kompact software.
Size exclusion chromatography (SEC) analysis were performed using a Waters 515 Plus instrument equipped with three waters Styragel columns (103, 104, 105 Å) and three detectors (DAWN HELEOS, ViscoStar and Optilab rEX). THF was used as the eluent at a flow rate of 1.0 mL/min at 35 °C. The system was calibrated by a set of monodispersed standard polystyrenes.
UV–Vis spectra were measured on a CARY 100 UV–Vis spectrophotometer. Samples were prepared in THF at a concentration of 1 × 10−5 M, and the spectra were recorded between 200 and 600 nm.
Thermogravimetric analyses (TGA) were carried out with a NETZSCH STA 409 PC/PG thermogravimetric analyzer at a heating rate of 20 °C/min under a nitrogen atmosphere. Differential scanning calorimetry (DSC) was performed on DSC Q2000 V24.10 with a heating rate of 5 K min−1 under N2 flow.
Typical synthetic procedures
Ethyl-3-butyn-1-yl malonate (2)
To a mixture of 3-ethoxy-3-oxopropanoic acid (1, 36.16 g, 0.274 mol), DMAP (16.74 g, 0.137 mol) and dry CH2Cl2 (100 mL), DCC (67.81 g, 0.329 mol) was added at 0 °C. At this temperature, the mixture was stirred for 30 min before 3-butyn-1-ol (23.04 g, 0.329 mol) was added. After stirring at 0 °C for another 2 h, the mixture was allowed to warm up to room temperature, and stirred overnight. The mixture was then filtered, and the filtrate was evaporated. The residues were dissolved in cool EtOAc (−16 °C) and filtered again. After solvent removal, the solids were further purified by column chromatography on silica gel with petroleum ether/EtOAc (10/1, v/v) to afford compound 2 as a yellow, viscous oil (17.98 g, 27.7%). 1H-NMR (CDCl3, 400 MHz,): δ ppm 4.17–4.29 (m, 4H), 3.40 (s, 2H), 2.56 (td, J = 6.8, 2.7 Hz, 2H), 2.02 (t, J = 2.7 Hz, 1H), 1.29 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3, 100 MHz): δ ppm 166.28, 79.61, 70.11, 62.97, 61.52, 41.38, 18.76, 14.01. FT-IR (KBr) ν (cm−1): 3294.0 (≡C–H), 2986.0, 2929.7, 2855.3, 2117.2 (C≡C), 1725.3 (C=O). EI-MS: 184.1 ([M]+, calcd. for C9H12O4: 184.1).
Mono-Fulleryne (3)
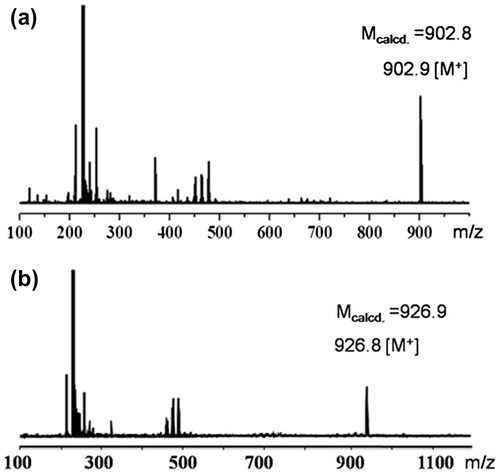
A 500 mL three-necked flask equipped with dropping funnel was charged with C60 (360 mg, 0.50 mmol), CBr4 (199 mg, 0.86 mmol), and dry degassed toluene (200 mL). After C60 was dissolved, compound 2 (138 mg, 0.75 mmol) was added, and a solution of DBU (152 mg, 1.0 mmol) in toluene (40 mL) was then added dropwise during a period of 1 h. After stirring for another 6 h at room temperature under nitrogen in the absence of light, the mixture was concentrated and eluted with cyclohexane/toluene (3/2,v/v) on silica gel column. After the first fraction containing unreacted C60, mono-Fulleryne (3) was obtained as a dark solid (187 mg, 41.5%). 1H-NMR (CDCl3, 400 MHz,): δ ppm 4.53–4.64 (m, 4H), 2.77 (td, J = 6.7, 2.7 Hz, 2H), 2.06 (t, J = 2.7 Hz, 1H), 1.50 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3, 100 MHz): δ ppm 163.69, 163.65, 145.54, 145.43, 145.30, 145.15, 144.94, 144.90, 144.13, 143.33, 143.22, 142.46, 142.18, 142.13, 141.20, 139.54, 139.11, 79.67, 71.69 (sp3 C), 70.81, 64.98, 63.85, 52.28, 19.27, 14.54. FT-IR (KBr) ν (cm−1): 3298.1 (≡C–H), 2956.3, 2924.2, 2828.5, 2124.8 (C≡C), 1748.1 (C=O), 1233.6, 527.6 (C60, C–C). MALDI-TOF-MS: 902.9 ([M]+, calcd. for C69H10O4: 902.8).
Di(3-butyn-1-yl) malonate (4)
A 250 mL three-necked flask equipped with the dean-stark strap was charged with malonic acid (10.4 g, 0.10 mol), 3-butyn-1-ol (28 g, 0.40 mol), toluene (60 mL) and four drops of concentrated sulfuric acid as catalyst. The mixture was refluxed under nitrogen for 5 h before cooled to room temperature. Water and diethyl ether was added, and the organic layer was separated. The water layer was extracted with diethyl ether for three times, and then the combined organic layers were washed with brine. After dried over anhydrous Na2SO4, the solvent of the organic layer was removed under reduced pressure. The crude product was purified by column chromatography by using petroleum ether/EtOAc (5/1, v/v) as eluent to yield a colorless oil. (11.98 g, 57.7%). 1H-NMR (CDCl3, 400 MHz,): δ ppm 4.26 (td, J = 6.8, 1.3 Hz, 4H), 3.44 (s, 2H), 2.57 (dd, J = 8.9, 4.6 Hz, 4H), 2.04 (t, J = 2.7 Hz, 2H). 13C-NMR (CDCl3, 100 MHz): δ ppm 166.27, 79.90, 70.43, 63.30, 41.41, 18.99. FT-IR (KBr) ν (cm−1): 3294.9 (≡C–H), 2966.9, 2920.2, 2855.3, 2117.2 (C≡C), 1733.9 (C=O). EI-MS: 207.1 ([M-H]+, calcd. for C11H12O4: 208.1).
Di-Fulleryne (5)
The synthesis was performed similarly to that for 3 except using di(3-butyn-1-yl) malonate (4) instead of 2. Yield:41.6%. 1H-NMR (CDCl3, 400 MHz): δ ppm 4.54 (t, J = 6.7 Hz, 4H), 2.71 (m, 4H), 1.99 (t, J = 2.6 Hz, 2H). 13C-NMR (CDCl3, 100 MHz): δ ppm 163.48, 145.54, 145.45, 145.39, 145.28, 145.18, 144.95, 144.91, 144.87, 144.14, 143.34, 143.28, 143.25, 142.45, 142.15, 141.22, 139.36, 79.67, 71.54 (sp3 C) 70.83, 65.06, 51.92, 19.27. FT-IR (KBr) ν (cm−1): 3266.7 (≡C–H), 2957.0, 2924.2, 2849.2, 2117.0 (C≡C), 1748.1 (C=O), 1233.5, 524.8 (C60:C–C). MALDI-TOF-MS: 926.8 ([M]+, calcd. for C71H10O4: 926.9).
C60-1PS (6)
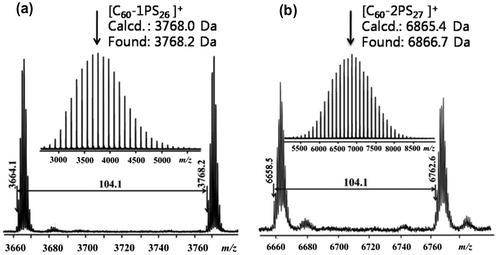
PS-N3 (Mn = 2900, 247.5 mg, 0.08 mmol), mono-Fulleryne (3, 45.1 mg, 0.05 mmol), CuBr (1 mg, 0.007 mmol) were combined in a 100 mL Schlenk flask before dry degassed toluene (50 mL) was added. The flask was subjected to three freeze-pump-thaw cycles and then two drop of PMDETA was added. The wine-red solution was stirred at room temperature for 2 days. The reaction mixture was concentrated, applied to the top of a short column of silica gel. The column was eluted with toluene/ethyl acetate (50/1, v/v) to give the product as a brown powder (161 mg, 80.6%). 1H-NMR (CDCl3, 400 MHz,): δ ppm 6.31–7.40 (m, 128H), 4.99–5.22 (m, 1H), 4.60–4.78 (m, 2H), 4.30–4.44 (m, 2H), 3.34–3.74 (m, 2H), 3.06–3.30 (m, 2H), 1.17–2.69 (m, 80H), 0.80–1.05 (m, 12H). 13C-NMR (CDCl3, 100 MHz): δ ppm 177.73, 163.72, 139.00–146.80 (C60 sp2), 125.60–129.40, 71.77 (C60 sp3), 66.08, 63.71, 60.19, 40.00–50.00, 32.21, 29.99, 25.81, 14.46. FT-IR (KBr) ν (cm−1): 3063.1, 3028.5, 2976.3, 2924.8, 2855.7, 1732.7, 1603.2, 1456.1, 1170.8, 1084.7, 1015.6, 755.9, 696.7, 531.7. SEC:PDI = 1.01. MALDI-TOF: 3768.2 ([26mer]+, calcd. C60-1PS26 for C283H229N3O6: 3768.0).
C60-2PS (7)
The synthesis was performed in a procedure similar to that for 6 except using 5 instead of 3. Yield: 78.7%. 1H-NMR (CDCl3, 400 MHz,): δ ppm 6.18–7.44 (m, 268H), 4.85–5.15 (m, 2H), 4.42–4.61 (m, 4H), 3.33–3.72 (m, 4H), 2.93–3.15 (m, 4H), 1.15–2.62 (m, 168H), 0.79–1.06 (m, 18H). 13C-NMR (CDCl3, 100 MHz): δ ppm 177.73, 163.57, 139.00–142.00 (C60 sp2), 125.60–129.40, 71.62 (C60 sp3), 68.05, 66.07, 63.68, 60.24, 40.00–50.00, 29.99, 31.19, 25.00–27.00, 14.20. FT-IR (KBr) ν (cm−1): 3064.1, 3028.5, 2921.8, 2859.7, 1721.0, 1596.9, 1490.1, 1445.5, 751.9, 698.2, 527.8. SEC:PDI = 1.01. MALDI-TOF: 6866.7 ([27mer·H]+, calcd. C60-2PS27 for C515H464N6O8: 6865.4).
Results and discussion
Design and synthesis of mono-fulleryne and di-fulleryne
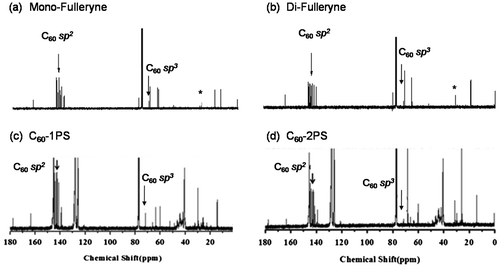
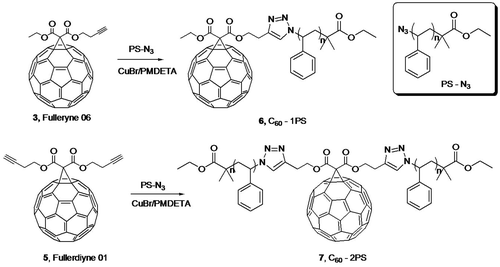
Fullerynes were synthesized in one step from C60 with high yield by reacting terminal-alkyne-functionalized malonates with fullerene via Bingel reaction (Scheme ). The synthesis of mono-alkyne-functionalized malonate could be achieved by reacting alcohol with Meldrum’s acid as reported in literature [Citation32,48] or the selective hydrolysis of diethylmalonate.[Citation47] Acid 1 was synthesized as described in literature [Citation47] and then reacted with 3-butyn-1-ol using Steglich esterification to afford ethyl-3-butyn-1-yl malonate (2) in a yield of 27.7%. Then, malonate 2 was directly reacted with fullerene in presence of CBr4 and DBU to give mono-Fulleryne (3) as a brown powder. The yield of 41.5% is good for fullerene functionalization. The synthesis of both malonate 2 and mono-Fulleryne were conveniently performed under mild reaction conditions. In literature, bifunctional fulleryne has been reported by Nierengarten and was synthesized via Bingel reaction. It was used as building blocks for copper-catalizedazide-alkyne cycloaddition (CuAAC) reaction.[Citation37] Here we report a similar structure, di-Fulleryne. Bifunctional malonate (4) was synthesized by Fisher esterification of malonic acid with 3-butyn-1-ol in a yield of 57.7%. Its reaction with C60 under Bingel–Hirsch condition gives a bifunctional fulleryne, the di-Fulleryne. The yields for mono-Fulleryne and di-Fulleryne are very close. Both fullerynes were fully characterized by 1H-NMR, 13C NMR, FT-IR and MALDI-TOF mass spectrometry. In the 1H-NMR spectra (Figure (a) and (b)), resonance signals attributed to the α-protons of the malonate (~3.5 ppm) disappeared completely, evidencing the success of the Bingel reaction. Signals attributed to the sp2 and sp3 carbons of C60 can be clearly observed in the 13C NMR spectra (Figure (a) and (b)). The characteristic absorbance for C–C vibration on C60 can be clearly observed at 525 cm−1 in the FT-IR spectra. The MALDI-TOF mass spectra (Figure ) further confirm the structures, where the observed molecular ion peaks have m/z values matching that of the calculated monoisotopic masses. All of the evidence clearly proves the chemical structure and purity of the new fullerynes.
Figure 1. 1H NMR spectra of mono-Fulleryne (a), di-Fulleryne (b), C60-1PS (c), and C60-2PS (d).
‘Clicking’ for fullerene polymers
Azido-functionalized polystyrene can be facilely synthesized by atom transfer radical polymerization (ATRP) and subsequent nucleophilic substitution as reported.[Citation5] ‘Click’ chemistry was then performed at room temperature with CuBr/PMDETA as the catalyst by reacting fullerynes with azido-functionalized polystyrene (Scheme ). Recently, Yu et al. reported the synthesis of fullerene polystyrene shape amphiphiles using hydrophilic fullerene as the polar head and polystyrene as hydrophobic tail.[Citation12] Versatile self-assembled micellar morphologies were observed and can be tuned by changing various parameters, such as molecular topology, polymer tail length and the molecular concentration. The C60 was used mainly as a structural scaffold in this work and the electronic properties of C60 were largely lost in these shape amphiphiles. It is of great interest to study the self-assemble behavior of shape amphiphiles containing pristine C60s. Thus, we try to synthesize well-defined fullerene polymers with distinct topologies as model shape amphiphiles.
The fullerene polymers synthesized using mono-Fulleryne and di-Fulleryne were also found to be a brown power that is readily soluble in common organic solvents such as THF, CH2Cl2 etc. The number and position of the tethered polymer tails are pre-determined by the location and number of alkyne groups on fullerynes, leading to shape amphiphiles of distinct architectures: C60-1PS and C60-2PS.
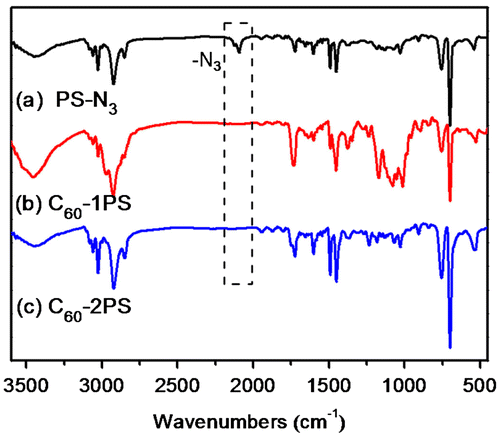
The fullerene polymers were fully characterized by 1H NMR, 13C NMR, FT-IR and MALDI-TOF mass spectrometry. The product exhibited the characteristic resonances of protons both near the 1,2,3-triazole (4.9–5.2, 3.0–3.3 ppm) and C60 unit (4.3–4.8 ppm) in the 1H-NMR spectra (Figure (c) and (d)). However, the signal attributed to the proton on the triazole ring cannot be observed, which may be overlapped with the peaks of the aromatic protons of PS.[Citation36,49] Nevertheless, the success of the ‘click’ reaction and the precisely defined structures of both C60-1PS and C60-2PS can be validated from the combination of other techniques. In the 13C NMR spectra (Figure (c) and (d)), the signals attributed to the alkyne carbons (79.8 and 70.8 ppm) on fullerynes disappeared completely, and the sp3 carbons as well as sp2 carbons of the C60 moiety can be observed. FT-IR spectra (Figure ) showed the complete disappearance of the azide group (2095.2 cm−1) and the appearance of a sharp peak at 525 cm−1, which is the characteristic for C60.[Citation1] The MALDI-TOF mass spectra (Figure ) showed a single Gaussian distribution with molecular weight in accordance to the proposed structure, confirming the stability and purity of the resulting fullerene polymers. The Gaussian distribution of the molecular weights was due to the different chain lengths of the polymers obtained during the polymerization. The difference in molecular weight between the two neighboring peaks was 104.1, which equals exactly to that of a single styrene repeating unit. The PDI calculated from MALDI-TOF is 1.01 for both C60-1PS and C60-2PS, which is very narrow. The results indicate that the fullerene polymers are pure with high C60 functionalization and well-defined as designed.
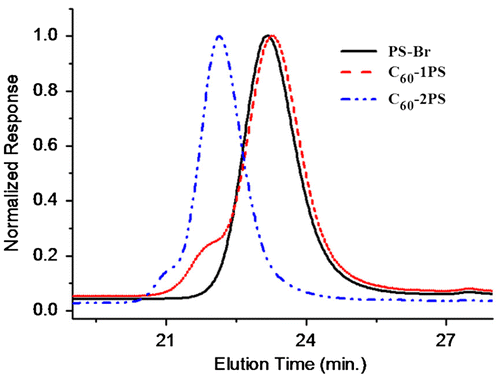
In the SEC traces (Figure ), the retention time of C60-1PS was slightly larger than that of PS–Br. Zhang et al. explained that the polystyrene chain tends to wrap around the fullerene ball due to the insoluble of C60 in THF, thus decreasing the hydrodynamic volume of the fullerene polymer.[Citation5] The C60-2PS showed a smaller retention volume, due to its larger molecular weight. A high molecular weight shoulder peak was observed in both fullerene polymers. The shoulder may be caused by the aggregation of C60 in THF,[Citation11,13,50] as the fullerene polymers were well defined without multi-addition product evidenced form the high sensitive MALDI-TOF. The shoulder peak of C60-2PS is smaller than that of C60-1PS indicating the tendency of aggregation in C60-2PS is weaker. Interestingly, previous study by Zhang et al. did not show the aggregation phenomena of the PS-C60 polymer.[Citation5] So we predicted that the aggregation of our C60-PS polymers may caused by the high concentration of the fullerene polymer solution or the low dissolve time in the SEC test, which need a systemic study in the further work. Ignoring the shoulder peak, the PDI calculated from SEC is 1.01 for both C60-1PS and C60-2PS, evidencing the well defined of the fullerene polymers.
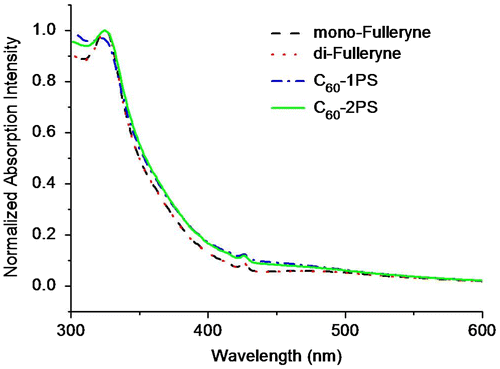
The steady state UV–Vis absorption spectra were measured in THF at room temperature. Figure displays the absorption spectra of the alkyne-functionalized fullerenes, and the fullerene polymers. In the UV–Vis spectra of fullerynes, the absorption peaks at ~330 and 430 nm were typical fingerprints of C60 monoadducts (methanofullerene).[Citation51–53] The UV–Vis absorption spectra of both C60-1PS and C60-2PS are very similar with that of fullerynes, indicated that the PS chain has no remarkable effect on the optic property of C60.
Figure 7. The steady-state UV–Vis absorption spectra of Mono-Fulleryne, Di-Fulleryne, C60-1PS and C60-2PS, measured in THF.
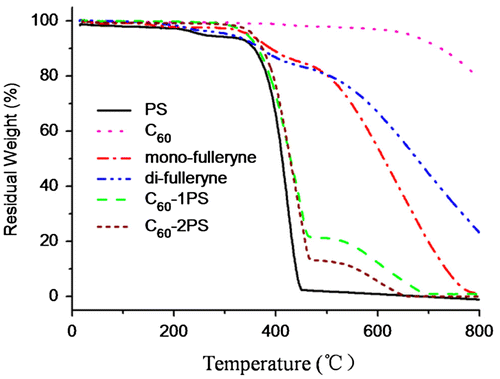
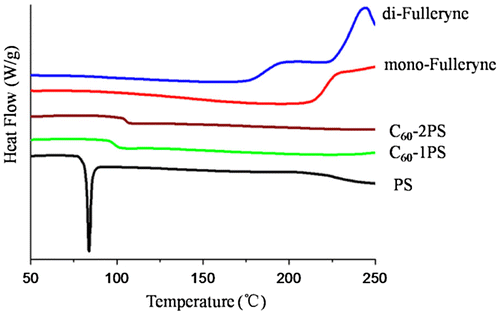
The thermal properties of the alkyne-functionalized fullerenes and the fullerene polymers were studied by TGA and DSC (Figures and ). C60 and polystyrene were used as controls. The pristine fullerene exhibits outstanding thermal stability when heated to 550 °C. In comparison, the residue of mono-fulleryne and di-fulleryen at this temperature is 80.9 and 79.7% respectively, which is in good agreement with the theoretical estimation if assuming that the ‘non fullerene’ moiety had been completely decomposed and removed (79.7% for mono-fulleryen and 77.7% for di-fulleryne). The thermal decomposition of the alkyne-functionalized fullerene occurred at 330 and 328 °C respectively. Before this temperature, mono-fulleryne showed two distinctive exotherms process between the region 150–300 °C in the DSC profile, which may be attributed to the cross-linking reaction of the alkyne group. Di-fulleryne showed a similar DSC profile (thermal behavior) as that of mono-fulleryne, except that the exothermal peaks appeared at a lower temperature, indicating the lower thermal stability of di-fulleryene. Polystyrene undergoes a thermal decomposition between 386 and 450 °C. The onset decomposition temperature of the fullerene polymers (374 °C for C60-1PS and 382 °C for C60-2PS) are very similar with that of PS, indicated that the existence of C60 has no remarkable effect on the thermal stability of the PS chain. The residue of the fullerene polymer at 550 °C (18.9% for C60-1PS and 10.7% for C60-2PS) can be considered as the fullerene part, which is in good agreement with the theoretical content of fullerene in the polymer (19.1% for C60-1PS and 10.5% for C60-2PS). In the DSC profile, PS showed a melting point at 84 °C. In contrast, only one glass transition process was detected for fullerene polymers, indicated that the incorporation of C60 restricts the crystal of the PS chain. The Tg of fullerene polymers was reported to be varied with C60 content.[Citation24,54–57] In our case, the Tg of C60-1PS (99 °C) is lower than the Tg of C60-2PS (105 °C), whereas the C60 content in C60-1PS is higher. This indicated that the two fullerene polymers with different C60 content and distinct molecular topology may have different self-assemble architectures in the solid state.
Conclusions
In summary, mono-Fulleryne and di-Fulleryne were designed and conveniently synthesized in one step via Bingel reaction. These fullerynes were employed for ‘click’ into two fullerene polymers of distinct topology: one being C60 tethered with one polymer tail (C60-1PS) and the other being C60 tethered at the junction point between two polymer chains (C60-2PS). The fullerene polymers were well defined with narrow polydispersity and high degree of C60 functionalization. The PS chain(s) on the fullerene core has no remarkable effect on the optic property of C60. Aggregation of C60 in THF was observed in the SEC traces. The thermal behavior studies indicated that the two fullerene polymers with different C60 content and distinct molecular topology may have different self-assemble architectures in the solid state. The well-defined fullerene polymers can be used as model compounds to study the self-assemble architecture of shape amphiphiles based on polymer-tethered molecular nanoparticles.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the International Science & Technology Cooperation Program of China [grant number 2010DFB70470].
Acknowledgements
The authors thank Wen-bin Zhang at Peking University for the helpful discussions.
References
- Hirsch A, Brettreich M. Fullerenes: chemistry and reactions. Weinheim: WILEY-VCH Verlag Gmbh & Co. KGaA; 2005.
- Langa F, Nierengarten J-F. Fullerenes: principles and applications. Cambridge: The Royal Society of Chemistry; 2007.10.1039/9781847557711
- Krätschmer W, Lamb LD, Fostiropoulos K, et al. Solid C60: a new form of carbon. Nature. 1990;347:354–358.10.1038/347354a0
- Ouhib F, Khoukh A, Ledeuil J-B, et al. Diblock and random donor/acceptor “double cable” polythiophene copolymers via the GRIM method. Macromolecules. 2008;41:9736–9743.10.1021/ma801934g
- Zhang W-B, Tu Y, Ranjan R, et al. “Clicking” fullerene with polymers: synthesis of [60]fullerene end-capped polystyrene. Macromolecules. 2008;41:515–517.10.1021/ma702345r
- Damasceno PF, Engel M, Glotzer SC. Predictive self-assembly of polyhedra into complex structures. Science. 2012;337:453–457.10.1126/science.1220869
- Glotzer SC. Some assembly required. Science. 2004;306:419–420.10.1126/science.1099988
- Iacovella CR, Horsch MA, Zhang Z, et al. Phase diagrams of self-assembled mono-tethered nanospheres from molecular simulation and comparison to surfactants. Langmuir. 2005;21:9488–9494.
- Zhang Z, Horsch MA, Lamm MH, et al. Tethered nano building blocks: toward a conceptual framework for nanoparticle self-assembly. Nano Lett. 2003;3:1341–1346.10.1021/nl034454g
- Glotzer SC, Horsch MA, Iacovella CR, et al. Self-assembly of anisotropic tethered nanoparticle shape amphiphiles. Curr Opin Colloid Interface Sci. 2005;10:287–295.10.1016/j.cocis.2005.09.011
- Zhang W-B, He J, Dong X, et al. Improved synthesis of fullerynes by Fisher esterification for modular and efficient construction of fullerene polymers with high fullerene functionality. Polymer. 2011;52:4221–4226.10.1016/j.polymer.2011.07.026
- Yu X, Zhang W-B, Yue K, et al. Giant molecular shape amphiphiles based on polystyrene-hydrophilic [60]fullerene conjugates: click synthesis, solution self-assembly, and phase behavior. J Am Chem Soc. 2012;134:7780–7787.10.1021/ja3000529
- Dong X-H, Zhang W-B, Li Y, et al. Synthesis of fullerene-containing poly(ethylene oxide)-block-polystyrene as model shape amphiphiles with variable composition, diverse architecture, and high fullerene functionality. Polym Chem. 2012;3:124–134.10.1039/C1PY00435B
- Rousseau G, Fensterbank H, Baczko K, et al. Synthesis of clickable water-soluble poly(amidoamine) fullerodendrimers and their use for surface functionalization of azido-coated polymer nanoparticles. Chempluschem. 2013;78:352–363.10.1002/cplu.201300045
- Segura JL, Castelaín M, Salavagione H. Synthesis and properties of [60]fullerene derivatives functionalized through copper catalyzed huisgen cycloaadition reactions. Curr Org Synth. 2013;10:724–736.10.2174/1570179411310050004
- Muñoz A, Illescas BM, Sánchez-Navarro M, et al. Nanorods versus nanovesicles from amphiphilic dendrofullerenes. J Am Chem Soc. 2011;133:16758–16761.10.1021/ja206769a
- Steinmetz NF, Hong V, Spoerke ED, et al. Buckyballs meet viral nanoparticles: candidates for biomedicine. J Am Chem Soc. 2009;131:17093–17095.10.1021/ja902293w
- Pereira GR, Santos LJ, Luduvico I, et al. ‘Click’ chemistry as a tool for the facile synthesis of fullerene glycoconjugate derivatives. Tetrahedron Lett. 2010;51:1022–1025.10.1016/j.tetlet.2009.12.050
- Inglis AJ, Pierrat P, Muller T, et al. Well-defined star shaped polymer-fullerene hybrids via click chemistry. Soft Matter. 2010;6:82–84.10.1039/B920806M
- Castelaín M, Salavagione H, Segura JL. “Click”-functionalization of [60]fullerene and graphene with an unsymmetrically functionalized diketopyrrolopyrrole (DPP) derivative. Org Lett. 2012;14:2798–2801.10.1021/ol301028r
- Le Ho K-H, Hijazi I, Rivier L, et al. Host-guest complexation of [60]fullerenes and porphyrins enabled by “click chemistry”. Chem Eur J. 2013;19:11374–11381.
- Mahmud IM, Zhou N, Wang L, et al. Triazole-linked dendro [60] fullerenes: modular synthesis via a ‘click’ reaction and acidity-dependent self-assembly on the surface. Tetrahedron. 2008;64:11420–11432.10.1016/j.tet.2008.08.083
- Gubskaya VP, Latypov SK, Sibgatullina FG, et al. Synthesis and properties of new triazole methanofullerenes under the “click-chemistry” conditions. Russ Chem Bull. 2013;61:1169–1175.
- Li L, Wang C, Long Z, et al. Synthesis of C60 end-capped polymers from azide functional polystyrene via atom transfer radical polymerization. J Polym Sci Part A: Polym Chem. 2000;38:4519–4523.10.1002/(ISSN)1099-0518
- Ayres N. Atom transfer radical polymerization: a robust and versatile route for polymer synthesis. Polym Rev. 2011;51:138–162.10.1080/15583724.2011.566402
- Grishin DF. Synthesis of functional polymers under conditions of controlled atom-transfer radical polymerization. Polym Sci Ser C. 2011;53:3–13.10.1134/S1811238211030015
- Matyjaszewski K. Atom transfer radical polymerization: from mechanisms to applications. Isr J Chem. 2012;52:206–220.10.1002/ijch.201100101
- Li Y, Dong X-H, Guo K, et al. Synthesis of shape amphiphiles based on POSS tethered with two symmetric/asymmetric polymer tails via sequential “grafting-from” and thiol−ene “click” chemistry. ACS Macro Lett. 2012;1:834–839.10.1021/mz300196x
- Li C, Hu J, Yin J, et al. Click coupling fullerene onto thermoresponsive water-soluble diblock copolymer and homopolymer chains at defined positions. Macromolecules. 2009;42:5007–5016.10.1021/ma900788k
- Miyanishi S, Zhang Y, Hashimoto K, et al. Controlled synthesis of fullerene-attached poly(3-alkylthiophene)-based copolymers for rational morphological design in polymer photovoltaic devices. Macromolecules. 2012;45:6424–6437.10.1021/ma300376m
- Bu H-B, Götz G, Reinold E, et al. Efficient post-polymerization functionalization of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) via ‘click’-reaction. Tetrahedron. 2011;67:1114–1125.10.1016/j.tet.2010.12.022
- Li J, Benicewicz BC. Synthesis of well-defined side chain fullerene polymers and study of their self-aggregation behaviors. J Polym Sci Part A: Polym Chem. 2013;51:3572–3582.10.1002/pola.v51.17
- Bingel C. Cyclopropylation of fullerenes. Chem Ber. 1993;126:1957–1959.10.1002/(ISSN)1099-0682
- Kessinger R, Crassous J, Herrmann A, et al. Preparation of Enantiomerically Pure C76 with a general electrochemical method for the removal of di(alkoxycarbonyl)methano bridges from methanofullerenes: the retro-bingel reaction. Angew Chem Int Ed. 1998;37:1919–1922.10.1002/(ISSN)1521-3773
- Taillemite S, Aubert C, Fichou D, et al. Synthesis of a linear benzo[3]phenylene–[60] fullerene dyad. Tetrahedron Lett. 2005;46:8325–8328.10.1016/j.tetlet.2005.09.174
- Iehl J, Freitas RPD, Nierengarten J-F. Click chemistry with fullerene derivatives. Tetrahedron Lett. 2008;49:4063–4066.10.1016/j.tetlet.2008.04.064
- Nierengarten J-F. Copper-catalyzed alkyne-azide cycloaddition for the functionalization of fullerene building blocks. Pure Appl Chem. 2012;84:1027–1037.
- Nierengarten J-F, Iehl J, Oerthel V, et al. Fullerene sugar balls. Chem Commun. 2010;46:3860–3862.10.1039/c0cc00034e
- Cecioni S, Oerthel V, Iehl J, et al. Synthesis of dodecavalent fullerene-based glycoclusters and evaluation of their binding properties towards a bacterial lectin. Chem Eur J. 2011;17:3252–3261.10.1002/chem.v17.11
- de Freitas RP, Iehl J, Delavaux-Nicot B, et al. Synthesis of fullerene building blocks bearing alkyne or azide groups and their subsequent functionalization by the copper mediated Huisgen 1,3-dipolar cycloaddition. Tetrahedron. 2008;64:11409–11419.10.1016/j.tet.2008.09.047
- Iehl J, Freitas RPd, Delavaux-Nicot B, et al. Click chemistry for the efficient preparation of functionalized [60] fullerene hexakis-adducts. Chem Commun. 2008:2450–2452.
- Iehl J, Nierengarten J-F. A click-click approach for the preparation of functionalized 5: 1 -hexaadducts of C60. Chem Eur J. 2009;15:7306–7309.10.1002/chem.v15:30
- Iehl J, Osinska I, Louis R, et al. A stable fullerene-azide building block for the construction of a fullerene-porphyrin conjugate. Tetrahedron Lett. 2009;50:2245–2248.10.1016/j.tetlet.2009.02.185
- Iehl J, Nierengarten J-F. Sequential copper catalyzed alkyne-azide and thiol-ene click reactions for the multiple functionalization of fullerene hexaadducts. Chem Commun. 2010;46:4160–4162.10.1039/c0cc00252f
- Yoosaf K, Iehl J, Nierengarten I, et al. A supramolecular photosynthetic model made of a multiporphyrinic array constructed around a C60 core and a C60-imidazole derivative. Chem Eur J. 2014;20:223–231.10.1002/chem.201303481
- Iehl J, Vartanian M, Holler M, et al. Photoinduced electron transfer in a clicked fullerene–porphyrin conjugate. J Mater Chem. 2011;21:1562–1573.10.1039/C0JM02310H
- Breslow DS, Baumgarten E, Hauser CR. A new synthesis of β-Keto esters of the type RCOCH2COOC2H5. J Am Chem Soc. 1944;66:1286–1288.10.1021/ja01236a022
- Felder D, Nierengarten H, Gisselbrecht J-P, et al. Fullerodendrons: synthesis, electrochemistry and reduction in the electrospray source for mass spectrometry analysis. New J Chem. 2000;24:687–695.10.1039/b003345f
- Yue K, Liu C, Guo K, et al. Sequential “click” approach to polyhedral oligomeric silsesquioxane-based shape amphiphiles. Macromolecules. 2012;45:8126–8134.10.1021/ma3013256
- Song T, Dai S, Tam KC, et al. Aggregation behavior of C60-end-capped poly(ethylene oxide)s. Langmuir. 2003;19:4798–4803.10.1021/la026992z
- Segura JL, Martín N, Guldi DM. Materials for organic solar cells: the C60/p-conjugated oligomer approach. Chem Soc Rev. 2005;34:31–47.10.1039/B402417F
- Cardullo F, Seiler P, Isaacs L, et al. Bis- through tetrakis-adducts of C60 by reversible tether-directed remote functionalization and systematic investigation of the changes in fullerene properties as a function of degree, pattern, and nature of functionalization. Helv Chim Acta. 1997;80:343–371.10.1002/(ISSN)1522-2675
- Ohno T, Moriwaki K, Miyata T. Intramolecular charge-transfer interaction in a new dyad based on C60 and bis(4′-tert-butylbiphenyl-4-yl)aniline(BBA) donor. J Org Chem. 2001;66:3397–3401.10.1021/jo001100q
- Wang C, He J, Fu S, et al. Synthesis and characterization of the narrow polydispersity fullerene-end-capped polystyrene. Polym Bull. 1996;37:305–311.10.1007/BF00318062
- Wu H-X, Cao W-M, Cai R-F, et al. C60 end-functionalized four-armed polymers: synthesis and optical limiting properties. J Mater Sci. 2007;42:6515–6523.10.1007/s10853-007-1566-1
- Wang C, Pan B, Fu S, et al. Synthesis and photoconductivity study of polystyrene-fullerene. Macromol Chem Phys. 1996;197:3783–3790.10.1002/macp.1996.021971123
- Hawker CJ. A simple and versatile method for the synthesis of C60 copolymers. Macromolecules. 1994;27:4836–4837.10.1021/ma00095a027