Abstract
Synthesis, characterization, and properties of new thermally curable polysulfone containing benzoxazine moieties in the side chain were investigated. First, chloromethylation and subsequent azidation processes were performed to form polysulfone containing pendant clickable azide groups. Independently, antagonist 3,4-dihydro-3-(prop-2-ynyl)-2H-benzoxazine was prepared by using paraformaldehyde, phenol and propargylamine. The following copper(I) catalyzed azide-alkyne cycloaddition click reaction was applied to obtain self-curable polysulfone with pendant benzoxazine units. The polymer and intermediates at various stages were characterized by 1H-NMR, 13C-NMR and FT-IR spectroscopies. The thermal properties and curing behavior of final polymer were investigated by differential scanning calorimetry and thermal gravimetric analysis. Compared to the neat polysulfone, the obtained polymers exhibited thermally more stable polymers.
1. Introduction
The synthesis of novel materials for high-tech applications such as electronics, medical, automotive, and aerospace has received great deal in recent years. Researchers have prepared many different polymers displaying improved chemical and physical properties by reacting polymers with the functional groups on them or by modification of a polymer with a reactive group that can be used for addition of new polymeric units. As a commercially important polymer, polysulfones (PSU) are a class of engineering polymers with extra-ordinary features comprising thermal stability, durability in harsh conditions, oxidation, pH and temperature resistance, excellent mechanical strength, ease of processability and good film properties.[Citation1–3] They are widely utilized in many different applications such as, nano or micro filtration membranes, coatings, composites, microelectronic devices, thin-film technology, and biomaterials.[Citation4] Polysulfones can be converted to novel polymers having enhanced physical and chemical properties that fulfills the needs of market. New functional groups or polymeric units can be added by end chain or side chain (graft) chemical modification. In our previous works we studied the end chain modification of polysulfone oligomers and their usage in crosslinking network and nanocomposite formations.[Citation5–11] Other researches also applied different side chain modifications in order to obtain grafting polysulfones with different functional groups.[Citation12–15]
Benzoxazines are a novel class of thermosetting compounds extensively studied in polymer science due to their self-curable properties upon heating; i.e. there is no need for an additional curing agent.[Citation16–20] They were simply blended with or incorporated into various polymers such as polystyrenes, polyurethanes, and others.[Citation21–25] By opening of the benzoxazine ring at elevated temperatures, thermally and mechanically more durable polymeric networks were formed.[Citation26–30] The synthesis and curing behavior of benzoxazine chemistry and its potential applications were previously reviewed in the literature.[Citation16,17,19,29] There were limited studies for the combination of polysulfone with benzoxazine as either in the main chain or terminal groups. By simple heating of these polymers not only allowed to obtain new polymeric networks with enhanced properties such as high mechanical and thermal stabilities but also to get novel properties like self-healing property.[Citation31–33]
Due to its simplicity, versatility, efficiency and speed, the copper catalyzed azide-cycloaddition (CuAAC) click reaction was employed for the synthesis of many organic,[Citation34,35] inorganic and hybrid materials.[Citation36–38] It has been extensively utilized for synthesis or modification of thermoplastic and thermoset polymers.[Citation39–46] Several examples related to the application of the CuAAC click reaction for the modification of polysulfones were previously studied.[Citation13,14,24,47–51] However, there was no report on the introduction of benzoxazine units at the side-chain position of polysulfones using chemistry reactions or other efficient organic reactions. In this study, self-curable polysulfone containing pendant benzoxazine units was prepared via CuAAC click chemistry strategy. The thermal properties and curing behavior of the obtained polymer were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA).
2. Experimental
2.1. Materials
Polysulfone, (PSU UDEL P-1700, Mn = 30.000 g/mol) was obtained from Solvay Advanced Polymers and used without purification. Paraformaldehyde (Aldrich, 95%), tin(IV)chloride (SnCl4, Aldrich, 99%), and chlorotrimethylsilane ((CH3)3SiCl, Merck, 99%), chloroform (Aldrich, 99%), N,N-dimethylformamide (Merck, 99%), 1,4-dioxane (Aldrich, 99%), methanol (Aldrich, 99%), ethanol (technical grade), sodium azide (Merck, 99%), copper (I) bromide (Acros Organics, 98%), 2,2′-dipyridyl (Acros Organics, 99%) and propargylamine (Aldrich, 98%) were used without any further purifications.
2.2. Synthesis of 3,4-dihydro-3-(prop-2-ynyl)-2H-benzooxazine monomer (Pg–Bz)
The Pg–Bz was prepared according to published procedure [Citation52]: phenol (36 mmol, 3.42 g), and propargyl amine (18 mmol, 1.00 g) were dissolved in 1,4-dioxane (30 mL) in a 50 ml three necked flask and then paraformaldehyde (72 mmol, 2.18 g) was slowly added into the mixture. After 10 min later, the mixture warmed to 110 °C and was refluxed this temperature for 12 h. The crude product was dissolved in diethyl ether and washed several times with 3 N sodium hydroxide solution and finally with distilled water. Then, the ether solution was dried over sodium sulfate anhydrous, followed by evaporation of solvent under vacuum to afford pale yellow viscous fluid.
FT-IR (ATR, cm−1): 3290 (≡C–H), 2120 (C≡C), 1490 (Ar) and 1230 (C–O–C).
1H-NMR (CDCl3, ppm): δ = 7.4–6.8 (m, Ar, benzoxazine), 4.9 (s, N–CH2–O, benzoxazine), 4.1 (s, N–CH2–C, propargyl), 3.6 (s, N–CH2–C, benzoxazine), and 2.3 (≡C–H, propargyl).
13C-NMR (CDCl3, ppm): δ = 44.4 (N–C–C≡, propargyl) 51.3 (Ar–C–N, benzoxazine), 73.1 (C≡C, propargyl), 78.2 (O–C–N, benzoxazine), 78.8 (C≡C, propargyl), 113.2, 120.4, 126.7, 129.1, 130.2 and 153.9 (Ar, benzoxazine).
2.3. Chloromethylation of polysulfone (PSU–CH2–Cl)
PSU (0.5 mmol, 15.00 g) was completely dissolved in 750 mL of chloroform in a round-bottom flask under inert atmosphere at 50 °C. After that trichloromethylsilane (CH3)3SiCl (225 mmol, 28.50 mL) were added slowly, drop by drop, to the flask. Paraformaldehyde (225 mmol, 6.70 g) was poured into the reaction mixture in one portion. Finally, tin (IV) chloride (SnCl4) (2.25 mmol, 0.26 mL) was added slowly by a syringe and the mixture was stirred under reflux for 72 h at 50 °C. At the end of this period, the reaction mixture was cooled to room temperature and poured into ethanol. The obtained polymer was collected by filtration and washed several times with ethanol. The polymer was then filtered and dried in a vacuum oven at room temperature for 24 h.
FT-IR (ATR, cm−1): 3200–3000 (Ar), 2975(–CH3), 2945(–CH3), 1320 and 1290 (O=S=O), 1240 (C–O–C), 1175 and 1150 (O=S=O) and 1015 (Ar).
1H-NMR (CDCl3, ppm): δ = 7.9–6.9 (m, Ar), 4.5 (s, CH2–Cl), 1.7 (s, CH3).
2.4. Azidation of chloromethylated polysulfone (PSU–CH2–N3)
The obtained PSU–CH2–Cl (0.1 mmol, 3.0 g) was dissolved in 50 mL of DMF in a round-bottom flask. Sodium azide (1.5 mmol, 0.1 g) was added to the solution and the reaction temperature was increased to 60 °C. After stirring for 24 h, the reaction mixture was concentrated and precipitated into technical ethanol. The product (PSU–CH2–N3) was dried in a vacuum oven for 24 h at room temperature, yielding a white amorphous solid.
FT-IR (ATR, cm−1): 3200–3000 (Ar), 3060 (C–H), 2120 (–N3), 1320 and 1290 (O=S=O), 1240 (C–O–C), 1175 and 1150 (O=S=O) and 1015 (Ar).
1H-NMR (CDCl3, ppm): δ = 7.9–6.9 (m, Ar), 4.4 (s, CH2–N3), 1.7 (s, CH3).
2.5. Synthesis of polysulfone containing pendant benzoxazine units (PSU–CH2–Bz) via CuAAC click chemistry
To a Schlenk tube equipped with a magnetic stirring bar, PSU–CH2–N3 (0.20 g, 26,000 g/mol, 1.0 equiv), P–Pa (0.182 g, 550 g/mol, 3.3 equiv), ligand (2,2′-dipyridyl, 0.33 mmol), catalyst (CuBr, 0.33 mmol) were dissolved in 10 mL of DMF. The tube was degassed by three freeze–pump–thaw cycles, left under vacuum, and placed in a thermostated oil bath. After stirring at 60 °C for 24 h, the mixture was diluted with THF and then passed through a column of neutral alumina to remove metal salt. The reaction mixture was concentrated and precipitated into methanol. The final product was washed with methanol and dried for 24 h in a vacuum oven at 25 °C, yielding a white amorphous solid.
2.6. Characterization
1H-NMR spectra of 5–10% (w/w) solutions of the intermediates and final polymers in CDCl3 and DMSO with Si(CH3)4 as an internal standard were recorded at room temperature at 500 MHz on a Bruker DPX 500 spectrometer. FT-IR spectra were recorded on PerkinElmer FT-IR Spectrum One spectrometer with an ATR Accessory (ZnSe, Pike Miracle Accessory) and cadmium telluride (MCT) detector. Resolution was 4 cm−1 and 24 scans with 0.2 cm/s scan speed. DSC was performed on a TA Q2000. Thermal gravimetric analysis (TGA) was performed on Perkin-Elmer Diamond TA/TGA with a heating rate of 10 °C min under nitrogen flow. Molecular weights and polydispersities of the polymers were measured by gel permeation chromatography (GPC) employing an Agilent 1100 instrument equipped with a differential refractometer by using tetrahydrofuran as the eluent at a flow rate of 0.5 ml min−1 at 30 °C. Molecular weights were determined using polystyrene standards.
3. Results and discussions
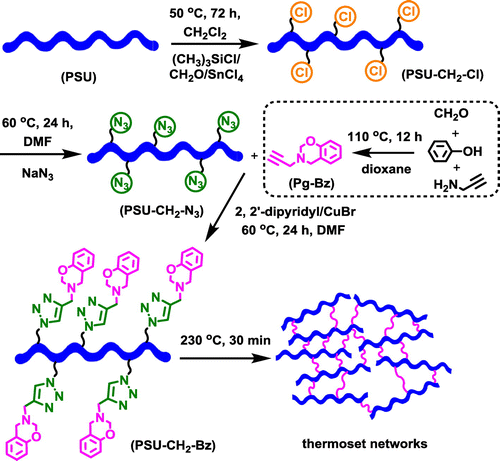
In practical applications, low molecular weight benzoxazine monomers were heated to elevated temperatures in order to enable the ring-opening reactions of oxazine without using any catalyst. However, the obtained thermoset networks suffered from the brittleness, which was the primary issue when exploring thin film applications. To overcome this problem, new strategies involving a combination of various thermoplastic polymers with benzoxazine units as curable precursors was developed. Generally, thermally curable benzoxazine moieties were located either main- or side- or end-chain of polymeric structures generating a processable and cross-linkable thermoplastic having flexibility and high cross-linking density after cure. In our case, the overall procedure involving the synthesis of chloromethylation and azidation of polysulfone and its click reaction with 3,4-dihydro-3-(prop-2-ynyl)-2H-benzooxazine monomer (Pg–Bz) to form self-curable polysulfone (PSU–CH2–Bz) was shown in Scheme . Firstly, chloromethylated polysulfone (PSU–CH2–Cl) was prepared with low degree of substitution (15%) according to modified halomethylation procedure.[Citation53]
The clickable alkyne-functional benzoxazine Pg–Bz, and azide-functional polysulfone (PSU–CH2–N3) were formed by reaction of paraformaldehyde, phenol and propargylamine and nucleophilic substitution reaction of the chlorine atoms of PSU–CH2–Cl with excess sodium azide, respectively. Thereafter, the successful CuAAC click reaction between PSU–CH2–N3 and Pg–Bz monomer led to self-curable polysulfone possessing pendant benzoxazine groups, PSU–CH2–Bz. Finally, the thermoset networks, where the ring-opening of benzoxazine units of resulting polymer could convert partial linear structures to the network structures, were obtained by simple heating without curing agents.
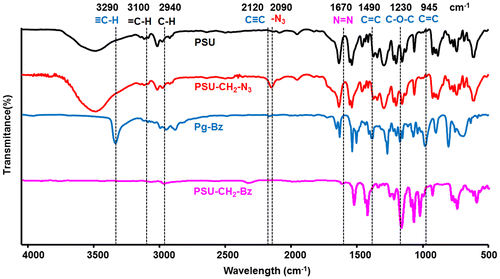
The synthesis of PSU–CH2–Bz was monitored by FT-IR analysis following characteristic azide, alkyne and aromatic bands of starting compounds (PSU–CH2–N3 and Pg–Bz) (Figure ). The FT-IR spectrum of Pg–Bz showed ≡C–H and C≡C stretching vibrations at 3290 and 2120 cm−1, whereas the C=C stretching vibration derived from the 1,2,4- substitution of the benzene ring appeared at 1490 cm−1 and the asymmetric stretching of C–O–C at 1230 cm−1. In the case of PSU–CH2–N3, azidation of chloromethylated polysulfone was followed by appearance of a strong azide peak arising at 2090 cm−1 indicating the presence of azide groups. After the CuAAC ‘click’ reaction, the disappearance of the azide and alkyne peaks and appearance of new peaks from the benzene ring of the Pg–Bz in the in the FT-IR spectrum of PSU–CH2–Bz confirmed the structure of desired polysulfone bearing pendant benzoxazine units. Also, the emergence of the N=N peak of triazole ring at 1670 cm−1 and the disappearance of ≡C–H peak at 3290 cm−1 indicated the successful CuAAC ‘click’ reaction.
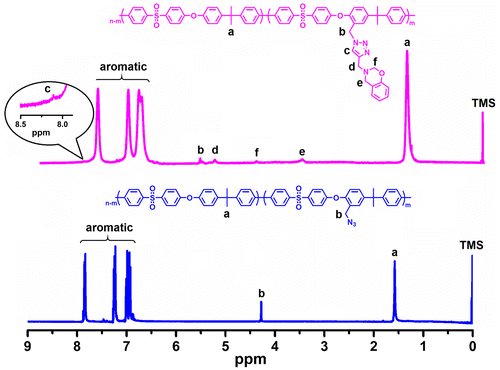
The structures of the PSU–CH2–Bz was further confirmed by 1H-NMR analysis as shown in Figure . The proportion of chloromethylated repeating units on PSU chains (degree of chloromethylation) was determined by the ratio of the integration values of the signals corresponding to the peak at 4.5 to the peak at 7.8 belonging to meta protons on the benzene ring near to the sulfonyl group. The degree of chloromethylation was determined to be 15%. After azidation reaction, the signals corresponding to methylene protons shifted from 4.5 to 4.4 ppm indicating successful azidation with 100% conversion (Figure S1). Successful click reaction was also proven by 1H-NMR spectra of the PSU–CH2–Bz that exhibited complete removal of the signal 4.4 ppm shifting to the position at 5.3 ppm. The attachments of the benzoxazine units were further proved by the appearance of the characteristic peaks at 3.6 and 4.9 ppm, corresponding to protons in the methylene bridge of the oxazine (Figure ). Moreover, the structure of PSU–CH2–Bz was also confirmed by 13C-NMR spectroscopy. The disappearance of the propargyl group signals at 73.1 and 78.2 ppm, and the appearance of triazole signals of PSU–CH2–Bz at 121.4 and 144.6 ppm indicated the successful CuAAC click reaction (Figure S2).[Citation54]
The molecular weight characteristics of the pure PSU and modified PSU were also monitored by GPC analysis (Table ). The molecular weight and molecular weight distribution of the modified PSU were slightly increased after the synthesis steps and no degradation products with small molecular weights were detected during the whole process.
Table 1. Molecular weight characteristics of pure PSU and modified PSU derivatives.
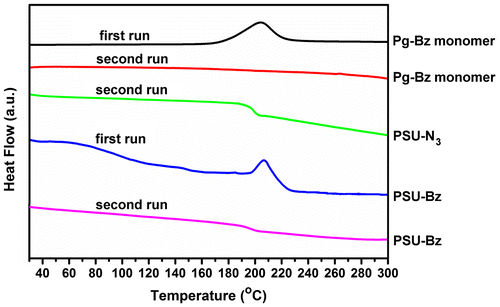
The curing behavior of Pg–Bz and PSU–CH2–Bz was investigated by DSC analysis and obtained thermograms were presented in Figure . The PSU–CH2–N3 exhibited a glass transition temperature at 189 °C due to amorphous nature of polysulfone. The ring-opening reaction of Pg–Bz monomer had an exotherm with a maximum at 206 °C. The amount of the exotherm reaction heat of Pg–Bz was calculated 943 J/g and it disappeared completely after the 230 °C cure. In the second run there was no thermal transition indicating the complete ring-opening reactions and the formation of polybenzoxazine network. In the case of PSU–CH2–Bz, the sample displayed an exotherm at 206 °C, which could be assigned to the ring-opening reaction of benzoxazine moieties. In the second run, there was no exotherm as the result of the formation of thermoset and completion of ring opening reaction. Due to the linear polymer chains, the sample still had a broad Tg at 196 °C, which increased by the addition of hard benzoxazine units and triazole linkages.
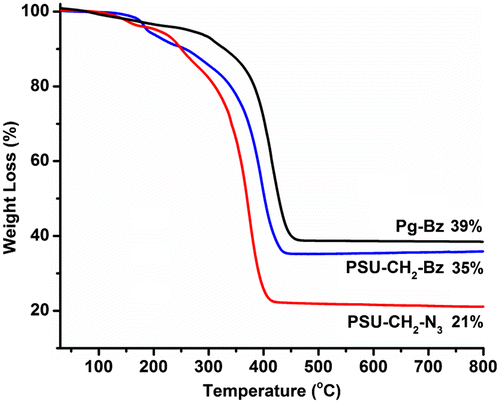
TGA thermograms of Pg–Bz, PSU–CH2–N3 and PSU–CH2–Bz samples were shown in Figure . The thermograms involve preheating cycle in which the samples were heated up to 230 °C, allowed to stay at this temperature for 30 min and then cooled down to ambient temperature followed by heating to 800 °C. The thermal degradation of cured Pg–Bz started at 270 °C and completed at 450 °C, which was well above that of polybenzoxazine. In case of PSU–CH2–N3, the main degradation occurred between 300 and 470 °C with a single step. When polysulfone and benzoxazine groups were combined in the network, thermal stability was increased with onset degradation temperature was 440 °C. Compared to the PSU–CH2–N3, the char yield of cured PSU–CH2–Bz was increased with the addition of benzoxazine units. At high temperatures, the cured PSU–CH2–Bz sample exhibited greater thermal stability, which might be due to the hydrogen bonding interactions between phenolic groups of benzoxazine and sulfoxide groups on the main chain.[Citation10] Similar results were also obtained from previously reported study, in which thermosets prepared from benzoxazine end-functional polysulfone.
4. Conclusion
In conclusion, a self-curable polysulfone bearing pendant benzoxazine units was successfully prepared by CuAAC click chemistry. By simple heating of this polymer allowed to form thermoset network containing polysulfone and benzoxazine groups in the structure. The benzoxazine moieties contribute to enhance the thermal properties of the polysulfone networks. This work may be useful for the obtainment of new polymeric networks that can be used in membrane science as ultrafiltration membranes or coating materials.
Supplemental data
The Supplemental data for this article is available online at http://dx.doi.org/10.1080/15685551.2016.1257379.
Disclosure statement
No potential conflict of interest was reported by the authors.
TDMP_1257379_Supplemental_Material.zip
Download Zip (182.9 KB)References
- Dizman C, Tasdelen MA, Yagci Y. Recent advances in the preparation of functionalized polysulfones. Polym Int. 2013;62:991–1007.
- Van der Bruggen B. Chemical modification of polyethersulfone nanofiltration membranes: a review. J Appl Polym Sci. 2009;114:630–642.10.1002/app.v114:1
- Sur GS, Sun HL, Lyu SG, et al. Synthesis, structure, mechanical properties, and thermal stability of some polysulfone/organoclay nanocomposites. Polymer. 2001;42:9783–9789.10.1016/S0032-3861(01)00527-4
- Nady N, Franssen MCR, Zuilhof H, et al. Modification methods for poly(arylsulfone) membranes: a mini-review focusing on surface modification. Desalination. 2011;275:1–9.10.1016/j.desal.2011.03.010
- Dizman C, Uyar T, Tasdelen MA, et al. Synthesis and characterization of polysulfone/POSS hybrid networks by photoinduced crosslinking polymerization. Macromol Mater Eng. 2013;298:1117–1123.
- Dizman C, Kahveci MU, Yagci Y. Synthesis of polysulfone-b-polystyrene block copolymers by mechanistic transformation from condensation polymerization to free radical polymerization. Polym Bull. 2013;70:2097–2109.10.1007/s00289-013-0931-2
- Dizman C, Ates S, Uyar T, et al. Polysulfone/clay nanocomposites by in situ photoinduced crosslinking polymerization. Macromol Mater Eng. 2011;296(12):1101–1106.10.1002/mame.v296.12
- Dizman C, Demirkol DO, Ates S, et al. Photochemically prepared polysulfone/poly(ethylene glycol) amphiphilic networks and their biomolecule adsorption properties. Colloids Surf, B. 2011;88:265–270.10.1016/j.colsurfb.2011.06.042
- Sangermano M, Roppolo I, Camara VHA, et al. Polysulfone/metal nanocomposites by simultaneous photoinduced crosslinking and redox reaction. Macromol Mater Eng. 2011;296:820–825.10.1002/mame.v296.9
- Ates S, Dizman C, Aydogan B, et al. Synthesis, characterization and thermally activated curing of polysulfones with benzoxazine end groups. Polymer. 2011;52:1504–1509.10.1016/j.polymer.2011.01.051
- Dizman C, Ates S, Torun L, et al. Synthesis, characterization and photoinduced curing of polysulfones with (meth)acrylate functionalities. Beilstein J Org Chem. 2010;6: Article no. 56.
- Ates S, Tatar-Guner P, Yagci Y, et al. Synthesis and characterization of polysulfone-g-poly(2-alkyl-2-oxazoline)s. Des Monomers Polym. 2013;16:137–144.10.1080/15685551.2012.705500
- Yilmaz G, Toiserkani H, Demirkol DO, et al. Polysulfone based amphiphilic graft copolymers by click chemistry as bioinert membranes. Mater Sci Eng C. 2011;31:1091–1097.10.1016/j.msec.2011.03.010
- Yilmaz G, Toiserkani H, Demirkol DO, et al. Modification of polysulfones by click chemistry: amphiphilic graft copolymers and their protein adsorption and cell adhesion properties. J Polym Sci, Part A Polym Chem. 2011;49:110–117.10.1002/pola.v49.1
- Toiserkani H, Yilmaz G, Yagci Y, et al. Functionalization of polysulfones by click chemistry. Macromol Chem Phys. 2010;211:2389–2395.10.1002/macp.v211.22
- Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines - new high performance thermosetting resins: synthesis and properties. Prog Polym Sci. 2007;32:1344–1391.10.1016/j.progpolymsci.2007.07.002
- Kiskan B, Ghosh NN, Yagci Y. Polybenzoxazine-based composites as high-performance materials. Polym Int. 2011;60:167–177.10.1002/pi.v60.2
- Takeichi T, Kawauchi T, Agag T. High performance polybenzoxazines as a novel type of phenolic resin. Polym J. 2008;40:1121–1131.10.1295/polymj.PJ2008072
- Yagci Y, Kiskan B, Ghosh NN. Recent advancement on polybenzoxazine-a newly developed high performance thermoset. J Polym Sci, Part A Polym Chem. 2009;47:5565–5576.10.1002/pola.v47:21
- Lligadas G, Tüzün A, Ronda JC, et al. Polybenzoxazines: new players in the bio-based polymer arena. Polym Chem. 2014;5:6636–6644.10.1039/C4PY00914B
- Kiskan B, Yagci Y, Ishida H. Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J Polym Sci, Part A Polym Chem. 2008;46:414–420.10.1002/(ISSN)1099-0518
- Kiskan B, Yagci Y. Synthesis and characterization of naphthoxazine functional poly(epsilon-caprolactone). Polymer. 2005;46:11690–11697.10.1016/j.polymer.2005.09.061
- Yildirim A, Kiskan B, Demirel AL, et al. Synthesis, characterization and properties of naphthoxazine-functional poly(propyleneoxide)s. Eur Polym J. 2006;42:3006–3014.10.1016/j.eurpolymj.2006.07.019
- Karadag M, Yilmaz G, Toiserkani H, et al. Polysulfone/pyrene membranes: a new microwell assay platform for bioapplications. Macromol Biosci. 2011;11:1235–1243.10.1002/mabi.v11.9
- Aydogan B, Sureka D, Kiskan B, et al. Polysiloxane-containing benzoxazine moieties in the main chain. J Polym Sci, Part A Polym Chem. 2010;48:5156–5162.10.1002/pola.v48:22
- Ergin M, Kiskan B, Gacal B, et al. Thermally curable polystyrene via click chemistry. Macromolecules. 2007;40:4724–4727.10.1021/ma070549j
- Kiskan B, Demiray G, Yagci Y. Thermally curable polyvinyl chloride via click chemistry. J Polym Sci, Part A Polym Chem. 2008;46:3512–3518.10.1002/(ISSN)1099-0518
- Kukut M, Kiskan B, Yagci Y. Self-curable benzoxazine functional polybutadienes synthesized by click chemistry. Des Monomers Polym. 2009;12:167–176.10.1163/156855509X412108
- Demir KD, Kiskan B, Aydogan B, et al. Thermally curable main-chain benzoxazine prepolymers via polycondensation route. React Funct Polym. 2013;73:346–359.10.1016/j.reactfunctpolym.2012.04.016
- Demir KD, Kiskan B, Latthe SS, et al. Thermally curable fluorinated main chain benzoxazine polyethers via ullmann coupling. Polym Chem. 2013;4:2106–2114.10.1039/c2py21029k
- Demir KD, Tasdelen MA, Uyar T, et al. Synthesis of polybenzoxazine/clay nanocomposites by ın situ thermal ring-opening polymerization using ıntercalated monomer. J Polym Sci Part A Polym Chem. 2011;49:4213–4220.
- Tasdelen MA, Durmaz H. Thermally curable polyoxanorbornene by ring opening metathesis polymerization. Macromol Chem Phys. 2011;212:2121–2126.10.1002/macp.v212.19
- Tasdelen MA, Kiskan B, Yagci Y. Photoinitiated free radical polymerization using benzoxazines as hydrogen donors. Macromol Rapid Commun. 2006;27:1539–1544.10.1002/(ISSN)1521-3927
- Kose MM, Onbulak S, Yilmaz II, et al. Orthogonally, “clickable” biodegradable dendrons. Macromolecules. 2011;44:2707–2714.10.1021/ma200593r
- Yigit S, Sanyal R, Sanyal A. Fabrication and functionalization of hydrogels through “click” chemistry. Chem Asian J. 2011;6:2648-2659.10.1002/asia.v6.10
- Nandivada H, Jiang XW, Lahann J. Click chemistry: versatility and control in the hands of materials scientists. Adv Mater. 2007;19:2197–2208.10.1002/(ISSN)1521-4095
- Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–2021.10.1002/(ISSN)1521-3773
- Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem Soc Rev. 2007;36:1249–1262.10.1039/B613014N
- Fournier D, Hoogenboom R, Schubert US. Clicking polymers: a straightforward approach to novel macromolecular architectures. Chem Soc Rev. 2007;36:1369–1380.10.1039/b700809k
- Binder WH, Sachsenhofer R. ‘Click’ chemistry in polymer and materials science. Macromol Rapid Commun. 2007;28:15–54.10.1002/(ISSN)1521-3927
- Tasdelen MA, Yagci Y. Light-ınduced click reactions. Angew Chem Int Ed. 2013;52:5930–5938.10.1002/anie.201208741
- Tasdelen MA, Kiskan B, Yagci Y. Externally stimulated click reactions for macromolecular syntheses. Prog Polym Sci. 2016;52:19–78.10.1016/j.progpolymsci.2015.09.003
- Arslan I, Tasdelen MA. POSS-based hybrid thermosets via photoinduced copper-catalyzed azide-alkyne cycloaddition click chemistry. Des Monomers Polym. 2016;19:155–160.10.1080/15685551.2015.1124323
- Demirci G, Tasdelen MA. Synthesis and characterization of graft copolymers by photoinduced CuAAC click chemistry. Eur Polym J. 2015;66:282–289.10.1016/j.eurpolymj.2015.02.029
- Tasdelen MA. Poly(epsilon-caprolactone)/clay nanocomposites via “click” chemistry. Eur Polym J. 2011;47:937–941.10.1016/j.eurpolymj.2011.01.004
- Tinmaz HB, Arslan I, Tasdelen MA. Star polymers by photoinduced copper-catalyzed azide-alkyne cycloaddition click chemistry. J Polym Sci, Part A Polym Chem. 2015;53:1687–1695.10.1002/pola.v53.14
- Xu CH, Gu SJ, Huang JJ, et al. Modification of polysulfones by click chemistry: zwitterionic graft complex and their antiprotein fouling property. J Appl Polym Sci. 2015;132: Article no. 41327.
- Meng JQ, Yuan T, Kurth CJ, et al. Synthesis of antifouling nanoporous membranes having tunable nanopores via click chemistry. J Membr Sci. 2012;401:109–117.10.1016/j.memsci.2012.01.049
- Yuan T, Meng JQ, Hao TY, et al. Polysulfone membranes clicked with poly (ethylene glycol) of high density and uniformity for oil/water emulsion purification: effects of tethered hydrogel microstructure. J Membr Sci. 2014;470:112–124.10.1016/j.memsci.2014.07.013
- Norris BC, Li W, Lee E, et al. ‘Click’-functionalization of poly(sulfone)s and a study of their utilities as proton conductive membranes in direct methanol fuel cells. Polymer. 2010;51:5352–5358.10.1016/j.polymer.2010.09.041
- Gaina C, Gaina V, Ionita D. Chemical modification of chloromethylated polysulfones via click reactions. Polym Int. 2011;60:296–303.10.1002/pi.v60.2
- Wu YC, Kuo SW. Synthesis and characterization of polyhedral oligomeric silsesquioxane (POSS) with multifunctional benzoxazine groups through click chemistry. Polymer. 2010;51:3948–3955.10.1016/j.polymer.2010.06.033
- Ioan S, Filimon A, Avram E. Influence of the degree of substitution on the solution properties of chloromethylated polysulfone. J Appl Polym Sci. 2006;101:524–531.10.1002/(ISSN)1097-4628
- Sood R, Donnadio A, Giancola S, et al. 1,2,3-triazole-functionalized polysulfone synthesis through microwave-assisted copper-catalyzed click chemistry: a highly proton conducting high temperature membrane. ACS Appl Mater Interfaces. 2016;8:16897–16906.10.1021/acsami.6b02713