Abstract
Acryloyl based novel energetic monomers having nitro acrylates and nitro triazole acrylates were synthesized and further used for polymerization. Due to scavanging properties of nitro groups, syntheses of nitro aromatic polymers are not facile at normal conditions. In this regard, we report a simple protocol to synthesize these energetic group embeded acroloyl polymers. These polymers were characterized by FTIR, and NMR spectroscopic techniques. gel permeation chromatography (GPC) technique was employed in order to understand molecular mass of these polymers along with average molecular weight, number average weight and poly dispersity index. Glass transition temperature (Tg) was determined by using DSC analysis. It was observed that with increase in nitro groups in polymers there is a decrease in glass transition temperature. Two steps degradation were depicted in the TGA thermograph in nitro containing polymers. Heat release during this reaction was found up to 951 J/g. Increase in nitrogen content in polymer unit enhanced the heat release of polymers.
1. Introduction
Acrylate polymers have ubiquitous applications in areas such as leather,[Citation1] textile,[Citation2] pharmacological drugs,[Citation3] fiber optics,[Citation4] metal complexes, polymer supports and building materials.[Citation5] Studies of homopolymers, copolymers and blends acrylate have been reported in literature.[Citation6] Introduction of phenyl ring into acrylate polymers, enhances the thermal stability which is useful in industrial applications such as peptide synthesis. Copolymer of Methyl methacrylates and phenyl acrylates exhibit photoluminscent properties.[Citation7] Acrylamides are another class of acryloyl based polymers which are readily synthesized using primary amines.[Citation8] Amine is an important functional group having multiple applications. Controlled radical polymerization of primary amines containing homopolymers and copolymers have been tolerant to many functional groups and variety of polar monomers.[Citation9–11] Various types of controlled radical polymerization methods such as atom transfer radical polymerization (ATRP),[Citation12] initiator-transfer-terminator polymerization,[Citation13–15] nitroxide-mediated polymerization,[Citation16,17] and reversible addition–fragmentation transfer polymerization (RAFT) [Citation18] are reported in the literature. These acrylamide based polymers are useful in thermite application to bind the surface of nanoparticles.[Citation19] However, energetic groups tailored aromatic acrylamide are not reported in the literature.
In addition to this another class of GAP based energetic polymers containing triazole and tetrazole ring are well known energetic materials.[Citation20] Due to their sparing solubility in organic solvents, polymers containing tetrazoles as compared to triazoles are endeavored to a lesser extend in energetic applications. 1,2,3-triazole and 1,2,4-triazole compounds have directed recent attention as an energetic materials due to their high energy and nitrogen content. The heat of formation of 1,2,4-triazole and 1,2,3-triazole are 109 kJ/mol and 272 kJ/mol, respectively.[Citation21]
Copper catalyzed 13-cycloaddition reaction is a powerful tool for the formation of triazole based polymers which involves the mechanism of Hüisgen cycloaddition.[Citation22,23] Presently, few monomers have been synthesized in well-organized manner from organic azides and propargyl acrylate via copper catalyzed dipolar cyclo additions and further, polymerized using AIBN as a free radical initiator.
Energetic polymers are known to be useful in the field of nanoenergetic composites. Nanoenergetic materials are of great interest in combustion studies such as igniters and primers which are very useful for defence applications.[Citation24] However, these materials are sensitive to impact, friction and electrostatic discharge (ESD). Among various performance parameters, ESD is the most important tool for safe handling of energetic materials. To reduce ESD sensitivity, the nanoenergetic composite is embedded in polymer matrix. Polymer matrix also reduces the surface charge on the oxidizer surface which will contribute in decreasing the ESD ignition sensitivity of the energetic composites.[Citation24]
Polymer matrix not only lowers the sensitivity of nanoenergetic materials but also reduces the burn rate which is useful for controlled combustion. Incorporation of energetic groups like azido, nitro, N-nitro and tri fluoro in the polymer enhances the energetic properties of the nanocomposites.
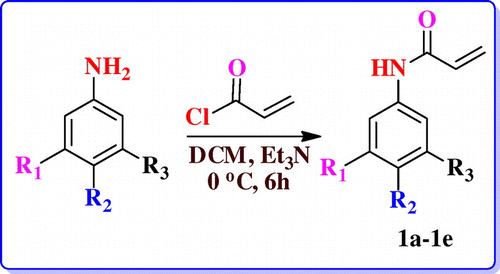
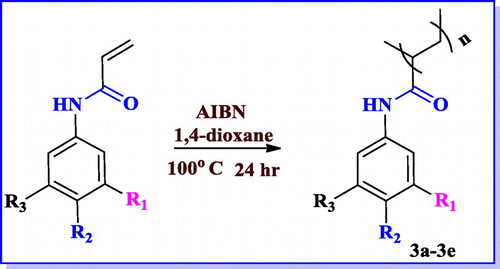
Herein we describe the short-step synthesis of energetic groups tailored acrylamide/acrylate based polymers. These polymers are cost effective, low vulnerable with high amount of energy. The energetic groups introduced are nitro, trifloromethyl benzene & triazoles (TrZ) in the polymer backbone. Radical polymerization techniques were applied in this regard. In–CF3 containing polymer, when –H present on the backbone of the polymer is replaced by fluorine containing moiety increases its density which in turn raises the oxygen balance and enhances the performance parameter of energetic molecules.[Citation25] However, there are few reports on synthesis of energetic groups having acrylamide or acrylate based polymers. These polymers were characterized by spectroscopic techniques and detailed thermal characterization was also performed. Our aim is to incorporate energetic groups containing aromatic ring in the acrylamide backbone to enhance the heat content of the polymers. Nitro and triazole containing polymers synthesized by us can be further explored in the area of nanoenergetic composites. The monomer (1a–1e) unit was prepared by condensation of aniline derivatives with acryol chloride.
2. Experimental
2.1. Materials and methods
4-Nitroaniline (Sigma Aldrich), 3-Nitroaniline(Alfa Aesar), 3-(trifluoromethyl) aniline, 4-Nitro-2-(trifluoromethyl) aniline(Alfa Aesar), 3,5-dinitro aniline (Sigma Aldrich), 2,4-dinitro aniline (Sigma Aldrich), Sodium Azide (Sigma Aldrich), Copper Sulphate and Sodium ascorbate were used as received. Dichloromethane (Merck) was distilled. Triethyl amine was also distilled. Acryloyl chloride(Alfa Aesar) were used under inert conditions. AIBN (Sigma Aldrich) as obtained. All the solvents were purified by distillation prior to their use.
2.2. Instruments
1H-NMR and 13C-NMR spectra were recorded on a Bruker-500 and 125 MHz instrument, respectively using tetramethylsilane (TMS) as an internal reference and DMSO-d6 as solvent. Coupling constants (J values) are given in Hertz (Hz). Chemical shifts are expressed in parts per million (ppm) downfield from internal reference, tetramethylsilane. The standard abbreviations s, d, t, q, quin, m and dd refer to singlet, doublet, triplet, quartet, quintet, multiplet and doublet of doublet respectively. IR spectra were recorded on a BRUKER ATR FT-IR spectrometer as a neat sample in the range of 500 to 4000 cm−1. High resolution mass 35 spectra measurements were carried out on Micromass Q-Tof Mass spectrometer. Melting points were recorded on BUCHI M560. Differential Scanning Calorimetry (DSC) studies were carried out on a Perkin Elmer DSC-7 instrument operating at a heating rate of 10 °C/min in nitrogen atmosphere with 1 to 2 mg of sample. Thermal decomposition was also undertaken on a Perkin Elmer operating at a heating rate range from 10 °C/min.
2.3. General procedure for synthesis of N-(phenyl) acrylamide (1a–1e)
Substituted nitro aniline (36 mmol, 1 eq.) was dissolved in 100 ml of DCM in a two necked R.B. flask. Then the reaction mixture was kept for stirring 10–15 min under N2 atmosphere. Triethylamine (108 mmol, 3 eq.) was added dropwise at 0°–5 °C. Acroyl chloride (54.33 mmol, 1.5 eq.) was dissolved in 50 ml of dry DCM and was added dropwise with constant stirring. The reaction was continued for 1 h in cold condition and room temperature for 5 h. TLC was checked and the consumption of starting material was observed. Then the reaction mixture was diluted with the ethyl acetate (3 × 25 ml) and then washed with distilled water. Organic layer washed with brine solution and dried with anhydrous Na2SO4. The organic layer was concentrated under reduced pressure. The crude product was purified by column chromatography by using ethyl acetate/ hexanes mixture as eluent.
2.3.1. N-(3-nitrophenyl) acrylamide (1a)
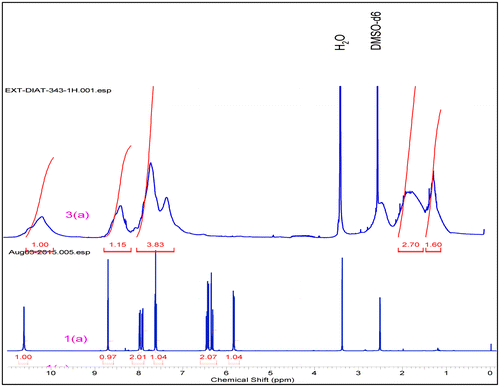
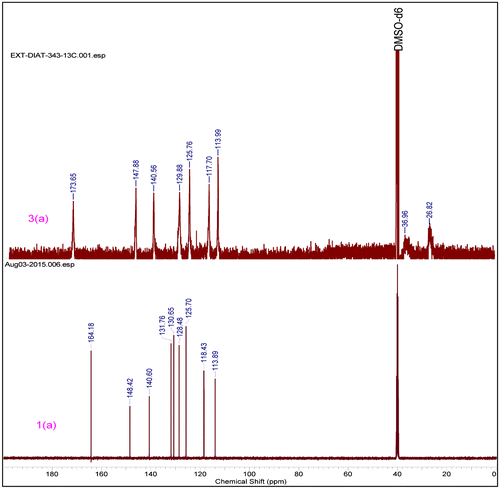
5.31 g, 75% yield, (20% EA+ n-Hexanes),m.p. 145 °C 1H NMR (500 MHz,DMSO-d6) δ = 10.64 (s, 1 H), 8.71 (t, J = 2.1 Hz, 1 H), 8.05–7.91 (m, 2 H), 7.64 (t, J = 8.2 Hz, 1 H), 6.54–6.25 (m, 2 H), 5.85 (dd, J = 10.11.8 Hz, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 164.2, 148.4, 140.6, 131.7, 130.7, 125.8, 118.6, 114.0. HRMS (Q-Tof) for C9H8N2O3 [M+H]+ calc. 192.0532 found 192.0535.
2.3.2. N-(4-nitrophenyl) acrylamide (1b)
4.58 g, 65% yield, (20% EA+ n-Hexanes), m.p. 172 °C 1H NMR (500 MHz, DMSO-d6) δ = 10.74 (br. s., 1 H), 8.24 (d, J = 8.5 Hz, 2 H), 7.91 (d, J = 9.2 Hz, 2 H), 6.56–6.25 (m, 2 H), 5.87 (d, J = 9.8 Hz, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 164.0, 145.7, 142.8, 131.7, 129.0, 125.5, 120.0. HRMS (Q-Tof) for C9H8N2O3 [M+H]+ calc. 192.0532 found 192.0535.
2.3.3. N-(3-(trifluoromethyl) phenyl) acrylamide) (1c)
5.21 g, 78% yield, (23% EA+ n-Hexanes)m.p. 87 °C, 1H NMR (500 MHz, DMSO-d6) δ = 10.46 (s, 1 H), 8.19 (s, 1 H), 7.87 (d, J = 8.9 Hz, 1 H), 7.54 (t, J = 7.9 Hz, 1 H), 7.42–7.34 (d, J = 10 Hz, 1 H), 6.52–6.40 (m, 1 H), 6.37–6.24 (m, 1 H), 5.79 (dd, J = 2.1, 10.1 Hz, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 164.0, 140.3, 131.9, 130.4, 130.3, 130.16, 130.03 (q, J = 31.25 Hz), 129.66, 127.9, 125.6, 122.4(JC-F = 271.25 Hz), 121.27, 120.1, 120.7115.9. 19F NMR (CDCl3, 376 MHz) δ −62.7 (3F,s, CF3). HRMS (Q-Tof) for C10H8F3NO [M+H]+ calc. 215.0558 found 215.0559.
2.3.4. N-(4-nitrophenyl)-3-(trifluoromethyl) phenyl) acrylamide) (1d)
5.16 g, 80% yield, (40% EA+ n-Hexanes)m.p. 153 °C, 1H NMR (500 MHz,DMSO-d6) δ = 10.88 (br. s., 1 H), 8.28 (s, 1 H), 8.19–8.01 (m, 2 H), 6.48–6.28 (m, 2 H), 5.86 (d, J = 9.8 Hz, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 164.5, 144.1, 141.8, 131.3, 129.3, 128.01, 125.7, 123.9, 123.46 (q, J = 33.7 Hz,) 123.53, 123.46 (q, J = 33.7 Hz), 123.07, 122.73, 122.31(q, JC-F = 253.3 Hz), 121.4, 119.2, 117.8. 19F NMR (CDCl3, 376 MHz) δ −60.0 (3F, s, CF3). HRMS (Q-Tof) for C10H7F3N2O3 [M+H]+ calc. 260.0432 found 260.0409.
2.3.5. N-(35-dinitro phenyl acrylamide) (1e)
1.71 g, 65% yield, (52% EA+ n-Hexane)m.p. 179 °C, 1H NMR (500 MHz,DMSO-d6) δ = 11.04 (s, 1 H), 8.92 (d, J = 2.1 Hz, 2 H), 8.52 (t, J = 2.1 Hz, 1 H), 6.53–6.24 (m, 2 H), 6.05–5.75 (m, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 164.7, 148.7, 141.5, 131.2, 129.7, 119.1, 113.0. HRMS (Q-Tof) for C9H7N3O5 [M+H]+ calc. 237.0398 found 237.0386.
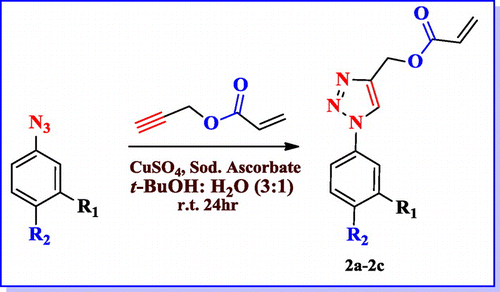
2.4. General procedure for synthesis of 1(1-(phenyl)-1H-1,2,3- triazol-4-yl) acrylate (2a–2c)
1-azido-3-nitrobenzene (5.44 mmol, 1 eq.) was taken in 30% t-butanol-water mixture. In this reaction mixture of prop-2-yn-1-yl acrylate (6.53 mmol, 1.2 eq.) and copper sulphate (1.08 mmol, 0.2 eq.), and sodium ascorbate (0.54 mmol, 0.1 eq.) were added. Then the reaction mixture was stirring at room temperature for overnight. TLC was analyzed and it showed consumption of starting material. The reaction mixture was diluted with ethyl acetate (3 × 25 ml) and washed with distilled water, brine solution and dried using anhydrous Na2SO4. The organic layer was concentrate under reduced pressure. The crude products were purified by column chromatography by using ethyl acetate/hexanes mixture as the eluent.
2.4.1. (1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl) acrylate (2a)
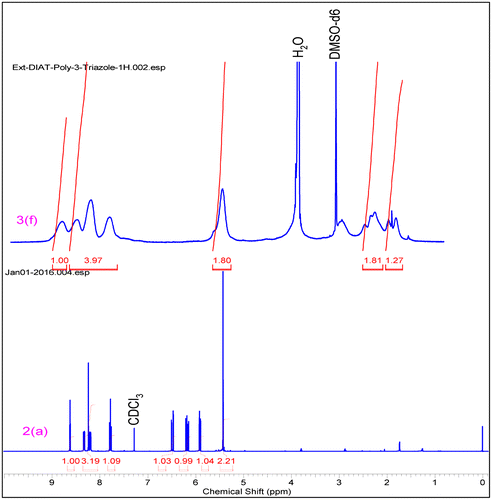
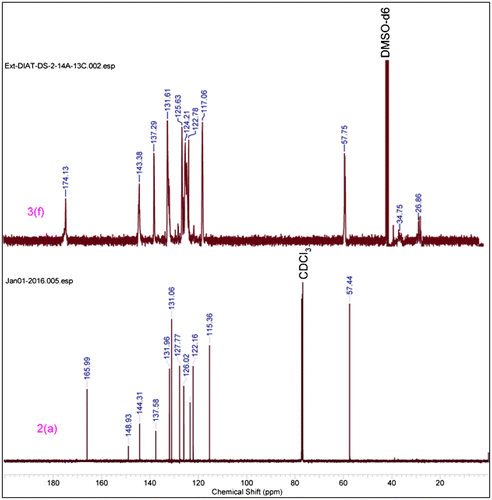
1.72 g, 68% yield, (57% EA+ n-Hexanes) m.p. 88 °C, 1H NMR (500 MHz, CHLOROFORM-d) δ = 8.63 (t, J = 2.1 Hz, 1 H), 8.33 (ddd, J = 0.9, 2.1, 8.2 Hz, 1 H), 8.24 (s, 1 H), 8.20 (ddd, J = 0.9, 2.1, 8.2 Hz, 1 H), 7.78 (t, J = 8.2 Hz, 1 H), 6.49 (dd, J = 1.4, 17.2 Hz, 1 H), 6.18 (dd, J = 10.4, 17.4 Hz, 1 H), 5.91 (dd, J = 1.410.5 Hz, 1H), 5.43(s, 2H).13CNMR(125 MHz,CDCl3) 165.9, 148.9, 144.3, 137.6, 131.9, 131.1, 127.8, 126.0, 123.4, 122.2, 115.4, 57.4 .HRMS (Q-Tof) for C12H10N4O4 [M+H]+ calc. 274.0702 found 274.0692.
2.4.2. (1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl) acrylate (2b)
1.77 g, 71% yield, (55% EA+ n-Hexanes) 1H NMR (500 MHz,CHLOROFORM-d) δ = 8.48–8.42 (m, 2 H), 8.22 (s, 1 H), 8.04–7.96 (m, 2 H), 6.47 (d, J = 5 Hz, 1 H), 6.23–6.13 (m, 1 H), 5.92(d, J = 10 Hz, 1 H), 5.43 (s, 2 H).
13C NMR (125 MHz CDCl3) δ = 166.0, 147.4, 144.4, 141.0, 131.9, 127.8, 125.6, 122.1, 120.6, 57.5. HRMS (Q-Tof) for C12H10N4O4 [M+H]+ calc. 274.0702 found 274.0688.
2.4.3. (1-(3-trifloromethyl phenyl)-1H-1,2,3-triazol-4-yl) acrylate (2c)
1.35 g, 66% yield, (51% EA+ n-Hexanes) m.p. 110 °C, 1H NMR (500 MHz,CHLOROFORM-d) δ = 8.18 (s, 1 H), 8.03 (s, 1 H), 7.97 (d, J = 5.0 Hz, 1 H), 7.76–7.65 (m, 2 H), 6.46 (dd, J = 1.4, 17.2 Hz, 1 H), 6.16 (dd, J = 10.4, 17.4 Hz, 1 H), 5.89 (dd, J = 1.4, 10.5 Hz, 1 H), 5.41 (s, 2 H).
13 C NMR (125 MHz CDCl3) δ = 165.9, 143.9, 137.2, 132.4 (q, J = 33.7 Hz,) 131.8, 130.8, 127.8 (q, JC-F = 281.25 Hz), 125.7, 124.4, 123.6, 122.2, 117.5, 57.6. 19F NMR (CDCl3, 376 MHz) δ −62.81 (3F, s, CF3). HRMS (Q-Tof) for C13H10F3N3O2 [M+H]+ calc. 297.0725 found 297.0722.
2.5. General procedure for polymerization (3a–3h)
(5.25 mmol, 1 eq.) of N-(phenyl) acrylamide and (0.105 mmol, 0.2 eq.) of AIBN were dissolved in 1,4-dioxane, flushed with nitrogen gas for 20 min and then reaction mixture was heated to 100 °C and reaction continued for 24 h under inert atmosphere. The color of the solution changed from grey to brown. During monitoring of reaction mixture by TLC, it was observed that starting material was consumed and a new spot was noticed at the bottom of the TLC. Methanol was slowly added to the reaction mixture to precipitate the polymer. The precipitate was further washed 2–3 times with methanol and then dried in oven at 50 °C.
2.5.1. Poly (N-(3-nitrophenyl) acrylamide) (3a)
0.310 g, 62% yield, 1H NMR (500 MHz, DMSO-d6) δ = 10.63–9.96 (m, 1 H), 8.39 (br. s., 1 H), 7.97–7.50 (m, 2 H), 7.46–7.10 (m, 1 H), 2.58–2.22 (m, 1 H), 2.05–1.49 (m, 2 H), 1.36–1.06 (m, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 173.7, 147.9, 140.6, 129.9, 125.8, 117.7, 113.9, 35.9, 26.9.
2.5.2. Poly (N-(4-nitrophenyl) acrylamide) (3b)
0.321 g, 64% yield, 1H NMR (500 MHz, DMSO-d6) δ = 10.77–10.10 (m, 1 H), 8.27–7.32 (m, 3 H), 2.51 (d, J = 1.7 Hz, 1 H), 2.12–1.53 (m, 1 H), 1.38–0.76 (m, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 174.1, 145.6, 142.4, 124.9, 119.6, 35.6, 26.9.
2.5.3. Poly (N-(3-(trifluoromethyl) phenyl) acrylamide) (3c)
0.354 g, 75% yield, 1H NMR (400 MHz, DMSO-d6) δ = 10.16–9.71 (m, 1 H), 8.14–7.43 (m, 2 H), 7.36–7.02 (m, 1 H), 2.61–2.17 (m, 1 H), 2.06–1.55 (m, 1 H), 1.45–1.13 (m, 1 H). 13CNMR (125 MHz, DMSO-d6) δ = 173.6, 140.2, 129.5, 125.8, 123.5, 123.09, 120.4, 119.6, 116.3, 35.4, 26.9.
2.5.4. Poly(N-(4-nitrophenyl)-3-(trifluoromethyl) phenyl) acrylamide) (3d)
0.278 g, 58% yield, 1H NMR (500 MHz, DMSO-d6) δ = 10.84–10.33 (m, 1 H), 8.35–7.49 (m, 2 H), 2.61–2.20 (m, 1 H), 2.04–1.35 (m, 2 H), 1.29–0.90 (m, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 178.8, 148.8, 146.2, 132.3, 128.1, 127.4, 125.9, 123.8, 122.5, 41.1, 29.1.
2.5.5. Poly (N-(35-dinitro phenyl acrylamide) (3e)
0.256 g, 51% yield, 1H NMR (500 MHz, DMSO-d6) δ = 11.17–10.65 (m, 1 H), 9.18–8.08 (m, 2 H), 2.23–1.90 (m, 1 H), 1.87–0.90(m, 3H). 13C NMR (125 MHz, DMSO-d6) δ = 172.7, 140.4, 132.1, 130.5, 123.5, 119.91, 116.06, 29.51, 21.6.
2.5.6. Poly (1-(3-nitrophenyl)-1H-123-triazol-4-yl) acrylate (3f)
0.362 g, 62% yield, 1H NMR (500 MHz, DMSO-d6) δ = 9.14–8.62 (m, 1 H), 8.57–7.50 (m, 3 H), 5.45–4.79 (m, 2 H), 2.43–2.06 (m, 1 H), 1.92–1.42 (m, 2 H), 1.34–0.97 (m, 1 H). 13C NMR (125 MHz, DMSO-d6) δ = 174.1, 148.6, 143.6, 137.2, 131.8, 126.1, 123.4, 114.9, 57.635.025.8.
2.5.7. Poly (1-(4-nitrophenyl)-1H-123-triazol-4-yl) acrylate (3g)
0.367 g, 63% yield1H NMR (500 MHz, DMSO-d6) δ = 9.08–8.62 (m, 1 H), 8.44–7.85 (m, 2 H), 5.44–4.96 (m, 1 H), 2.60–2.23 (m, 1 H), 2.07–1.56 (m, 1 H), 1.45–0.99 (m, 1 H). 13C NMR (125 MHz,DMSO-d6) δ = 174.2, 147.02, 143.78, 140.83, 125.83, 123.3, 120.9, 57.8, 34.3, 25.9.
2.5.8. Poly (1-(3-trifloromethyl phenyl)-1H-123- triazol-4-yl) acrylate (3h)
0.421 g, 80% yield, 1H NMR (500 MHz, DMSO-d6) δ = 9.13–8.56 (m, 1 H), 8.36–7.92 (m, 2 H), 7.88–7.50 (m, 2 H), 5.38–4.92 (m, 2 H), 2.48–2.11 (m, 1 H), 1.94–1.50 (m, 2 H), 1.37–0.95 (m, 2 H). 13C NMR (125 MHz, DMSO-d6) δ = 174.1, 143.4, 137.3, 131.6, 127.2, 125.6, 124.2, 123.6, 122.8, 120.6, 117.1, 57.6, 35.2, 26.6.
3. Results and discussion
Attempt was made in this study to synthesize acrylate monomers having various substituents. Initially, various reactions parameters were examined using different kinds of reaction conditions for synthesis of acrylate monomers (Table ). For the reaction between aromatic amine and acryloyl chloride, we attempted reaction with triethyl amine as a base and DCM as solvent to get the product as substituted acrylamide (Scheme ). By using the above mentioned optimized method, five aromatic substituted acrylamide were synthesized. The substitution was done with energetic groups such as –NO2, and –CF3. The structure of the aromatic acrylamide monomers was characterized. Yield of the nitro containing polymers are observed to be very less due to their electron withdrawing nature. In–CF3 molecule when –H is replaced by fluorine, density of the hydrocarbon will be increased. While –CF3 possessing monomers yield is high. Finally, the desired products of monomers were obtained after optimizing the conditions with good amount of yield. The structure and the yield of various monomers are described below in Table .
Table 1. Different conditions to optimized the reaction.
Table 2. Structure and yield of compounds (1a–1e).
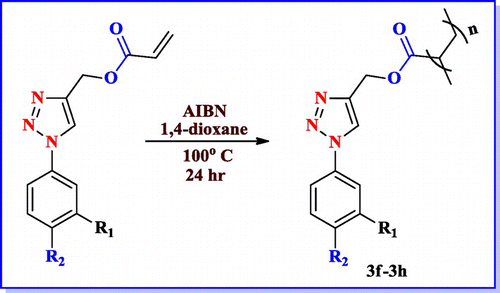
After synthesizing energetic group embedded acrylamide monomers, we ventured upon to synthesize triazole based acrylesters. The formation of the acrylic monomers (2a–2c) was accomplished by using copper-catalyzed 13-dipolar cycloaddition of the organic azides [Citation26] with propargyl acrylate. The reaction was carried out under mild conditions for 8–10 h at room temperature in water and t-butanol mixture (Scheme ) and structure of different triazole acrylate monomers are mentioned in Table .
Table 3. Structure and yield of compounds (2a–2c).
3.1. Polymerization
After accomplishing acrylamide (1a–1e) and acrylate monomers (2a–2c), we attempted radical polymerization reaction by various methods. In polymerization step, different conditions were endeavoured (Table ), but the yield of the desired polymer was low. After several efforts, changing the solvent to dioxane & AIBN as radical initiator, better yields were obtained as listed in Table . After 12 h the color of the reaction mixture turned brown & then product formed were precipitated in the methanol. The yield of the polymerization ranged from good to better. The good yield of the –CF3 possessing polymer was observed as mentioned above. Reactions of the acrylamide and acrylate polymers are shown in Schemes and .
Table 4. Different conditions to optimized polymer reaction.
Table 5. Structure and yield of compounds (3a–3e).
The triazole based polymers (3f–3h) were synthesized by the same procedure and the yields of the polymers are mentioned in the Table .
Table 6. Structure and yield of compounds (3f–3h).
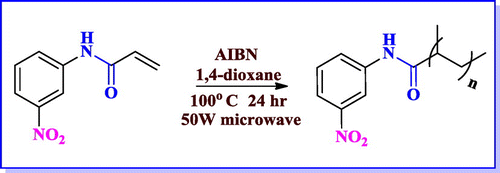
Polymerization reaction for substrate (1b) was also attempted using microwave. Wherein, monomer was dissolved in 14 dioxane, followed by addition of AIBN to the reaction mixture under inert atmosphere at 80 °C, 50 W varying the heating duration from 10, 15, 30 and 60 min. After 60 min. the polymerization reaction was completed, but the yield obtained was not equivalent with that of conventional heating method as described above and also scaling up of this reaction was difficult. Hence we concluded that conventional heating gave better yield of poly nitro acrylamide as compared to microwave heating.
Attempts to do polymerization at lower temperature (less than 70 °C) showed lower yield because fewer radicals were generated at low temperature leading to incomplete conversion to polymer. Generally, polymerization step is reported to be carried out at 70 °C, but in our case presence of nitro and di nitro polymerization took place at relatively higher temperature ~100 °C. Increasing the number of nitro group in aromatic ring retards the reactivity of monomer. In 35 dinitro acrylamide (3e) presence of two nitro groups lowers the yield of the polymer. On the other hand, 246 trinitro acrylamide did not show polymerization reaction due to the presence of three nitro groups which act as scavenger to free radicals thereby inhibiting the polymerization reaction.[Citation27]
The structure of compounds (3a–3h) was confirmed by different techniques. FTIR spectra of compound (1a–1e) exhibit the presence of peaks in the range cm−1, 1665–1690 (–CONH), 1493–1595(Aromatic –C=C–), 1327& 1520(–NO2), ~1240 (–C=C). During polymerization a new peak was generated at ~2940 cm−1, which confirms that the double bond peaks has been reduced to as happening during the polymerization step was successfully done. (Supporting Information) In similar way, 1H NMR spectra of polymer (3a–3h) ~6.50–6.25(s, 1H) and ~5.8(s, 1H) peaks disappeared and a new peak ~1.26–2.55(–CH2) was observed, this peak indicates that –CH = CH2 changes into methylene group. In carbon NMR, two additional new peaks were observed around 25 and 37 ppm and corresponding to saturated carbon atoms. (Figure ). In Figure , carbon NMR depicts two additional new peaks around 26 and 37 ppm which are assigned to saturated carbon atoms. (Supporting Information)
In IR spectrum formation of triazole based monomer (3f–3h), 2100 cm−1 strong absorption peak of azide –N3 disappeared when it reacted with propargyl acrylate and the triazole and propargyl acrylate peak appeared at ~1647 and ~1728 cm−1 respectively. Cu catalyzed [2+3] cycloadditions reaction mechanism is involved in this reaction. Similar results were obtained from triazole polymers (Figures and ).
3.2. Molecular weight
Molecular weight of polymers was determined by GPC. Number and weight average molecular weight, polydispersity index were determined and are represented in Table . The average polydispersity indexes of the polymers are in the range of 1.00 to 1.74. Molecular weights of nitro based polymers (3a–3h) (2700–9000) are in good agreement with the reports present in the literature.[Citation28] Nitro group acts as a radical scavenger and hence the presence of nitro groups in polymer backbone retards the growth of polymer chain. Further, the decrease in polymer weight in 3(c) and 3 (d) was observed. The presence of bulky and electron withdrawing group (–CF3) in both the monomers prevents attaining high molecular weight, which may be due to reduced approach of monomers and also the electron withdrawing effect weakening –NH bond for favored chain transfer to monomer. In the case of polymer (3 h) even in presence of –CF3 group, the molecular weight is enhanced. The presence of –CF3 group being far away from the double bond may not interfere by reducing the reactivity. Further, the hydrogen bonding increases the viscosity. Under this condition, the rate of termination of polymer radicals becomes diffusion controlled. As highly viscous medium is present the termination rate becomes very low making the overall rate of increase of polymerization higher (Equation Equation1(1) ). Further, the monomer being a small molecule, the mobility is not much affected and molecular weight continues to increase. Moreover it reduces the rate of termination of polymer radicals which also leads to increase of molecular weight. The rate of polymerization Rp is expressed as:
(1)
Table 7. Molecular weight of polymers.
Where M is the monomer, f is the mole fraction of free radical initiator which initiate the polymerization, I is the initiator, kp, kd, and kt are the constants for chain propagation, initiator dissociation, and termination.
3.3. Thermal studies
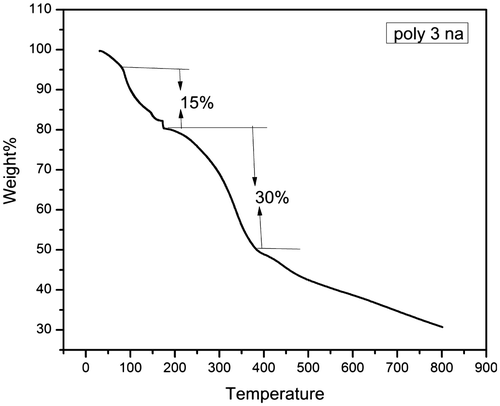
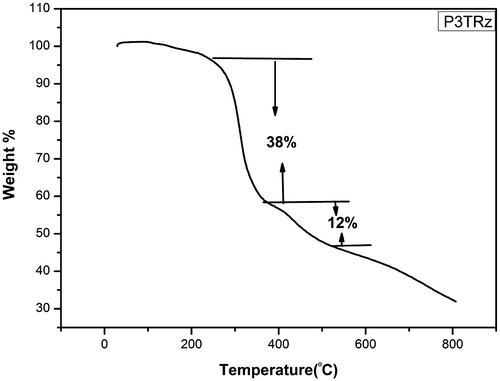
The thermograms of all the synthesized polymers except poly 3NA (3a), poly 3TrZ(3f) and poly 4 TrZ (3 g) clearly indicates that they undergo single stage decomposition. The two stage decomposition of poly 3NA, poly 3TrZ and poly 4TrZ can be explained (Figures and ) by taking into consideration the following mechanism. The first step of decomposition implicates the loss of CO2, breaking of the weak linkage in the backbone of the polymer thereby leading the low molecular moiety to volatilize. The second step is mainly due to loss of the benzene ring and rupture of the pendant group (–NO2) from the backbone leading to the cleavage of the main chain.[Citation29] TGA curves for these polymer samples are depicted in supporting information.
TGA curves shows the variation of enthalpy (∆H) (3a–3h) from 81 to 951.59 J/g. Nitrogen containing (–NO2, N3 and triazole) compounds are found to possess high amount of energy, so incorporation of nitro and triazole ring in polymer backbone will led to high amount of heat in the form of exothermic reaction. Poly 4TrZ (3g) showed maximum enthalpy (∆H) i.e. 951.59 J/g, which is mainly due to presence of both nitro group and triazole ring.
The glass transition temperatures (Tg) of the synthesized polymers are evaluated using DSC and represented in the Table . Tg values varies in the range from 78 to 125 °C. The observed suppression of Tg values is due to presence of electron withdrawing groups (–NO2, –CF3) in the polymer backbone.[Citation18]
Table 8. Thermal studies of polymers.
3.4. Oxygen balance (O.B.)
Oxygen balance is an important parameter to explain the sensitivity and degree of explosion, where an explosive material can be oxidized. Oxygen balance (O.B.) is expressed as,(2)
Where X = number of atoms of carbon, Y = number of atoms of hydrogen, Z = number of atoms of oxygen and M = number of atoms of metal (if metallic oxide produced).
Mostly energetic molecules possess negative oxygen balance, it means these materials require oxygen to combust. Oxygen is needed to convert all C into CO2 and H into H2O. O.B. of the polymers which we have described in the present work lies in the range of −111.37 to −174.95% (Table ) which is almost similar to energetic binders which are used in propellant formulations such as GAP(−121%), Poly-BAMO(−124%), Poly-AMMO(−170%), and Poly-NIMMO (−114%).[Citation30] But polymers which we are describing here possess explosophores groups such as nitro and triazole, hence these types of polymers are comparable to other energetic materials.
Table 9. O.B. of polymers.
4. Conclusions
Herein, we report the synthesis of novel energetic acrylamide/acrylate polymers which consist of energetic groups like –NO2,–CF3, and nitropheyl substituted triazole. Generally, the synthesis of these polymers may be difficult under normal conditions because of electron withdrawing nature. We have adopted a simple methodology to synthesize these molecules. These molecules demonstrate high heat release and better thermal stability. In addition, low value of glass transition temperature and increase in enthalpy of formation were found due to the increase of nitrogen content in polymer backbone. Molecular weights of the polymers were reduced due to the nitro group present in polymer network. Nitro group behaved like a scavenger in free radical polymerization. Nitro group containing polymers decomposed in two steps whereas others are found to be single step decomposition. All these properties of the nitro and triflouro containing polymers make then a good candidates for energetic applications.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/15685551.2016.1258977.
TDMP_1258977_Supplementary_Material.pdf
Download PDF (1.9 MB)Acknowledgements
The authors would like to thank the Vice Chancellor, DIAT for providing the infrastructural facilities. Financial support from ER&IPR, DRDO, New Delhi through ‘DRDO-DIAT programme on Nano Materials’ is gratefully acknowledged.
References
- Thamizharasi S, Srinivas G, Sulochana N, et al. Copolymerization of 4-chlorophenyl acrylate with methyl acrylate: synthesis, characterization, reactivity ratios, and their applications in the leather industry. J Appl Polym Sci. 1999;73:1153–1160.10.1002/(ISSN)1097-4628
- Mahmud S, Habib MA, Pervez MN, et al. Organic silicone based poly-acrylate binder synthesis for textile pigment printing. Am J Polym Sci Eng. 2015;3:119–128.
- Williams VA, Ribelli TG, Chmielarz P, et al. Elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J Am Chem Soc. 2015;137:1428–1431.10.1021/ja512519j
- Thamizharasi S, Reddy BS. Synthesis and characterization of thermally stable naphthyl acrylate polymers. Eur Polymer J. 2000;36:993–1000.10.1016/S0014-3057(99)00159-7
- Arshady R. A new synthetic approach for the preparation of polymer supports based on beaded copolymers of styrene and 2, 4, 5-trichlorophenyl acrylate: Synthesis and swelling behaviour of poly (styrene-co-acrylamide) resins. Die Makromolekul Chem. 1984;185:2387–2400.10.1002/macp.1984.021851116
- Takada K, Ito T, Kitano K, et al. Synthesis of homopolymers, diblock copolymers, and multiblock polymers by organocatalyzed group transfer polymerization of various acrylate monomers. Macromolecules. 2015;48:511–519.10.1021/ma502298v
- Jiang G, Jiang T, Chen H, et al. Preparation of multi-responsive micelles for controlled release of insulin. Colloid Polym Sci. 2015;293:209–215.10.1007/s00396-014-3394-6
- Roos K, Planes M, Hassani CB, et al. Solvent-free anionic polymerization of acrylamide: a mechanistic study for the rapid and controlled synthesis of polyamide-3. Macromolecules. 2016;49:2039–2045.10.1021/acs.macromol.5b02410
- Zhou Y, Jiang K, Chen Y, et al. Gold nanoparticle-incorporated core and shell crosslinked micelles fabricated from thermoresponsive block copolymer of N-isopropylacrylamide and a novel primary-amine containing monomer. J Polym Sci Part A Polym Chem. 2008;46:6518–6531.10.1002/pola.v46:19
- Yamamoto K, Serizawa T, Muraoka Y, et al. Synthesis and functionalities of poly (N-vinylalkylamide). 13. Synthesis and properties of thermal and pH stimuli-responsive poly (vinylamine) copolymers. Macromolecules. 2001;34:8014–8020.10.1021/ma0102969
- Xu G, Chung TC. Synthesis of syndiotactic polystyrene derivatives containing amino groups. Macromolecules. 2000;33:5803–5809.10.1021/ma000303d
- Adzima BJ, Taylor SC, He H, et al. Vinyl-triazolium monomers: Versatile and new class of radically polymerizable ionic monomers. J Polym Sci Part A Polym Chem. 2014;52:417–423.10.1002/pola.27016
- Thibault RJ, Takizawa K, Lowenheilm P, et al. A versatile new monomer family: functionalized 4-vinyl-1, 2, 3-triazoles via click chemistry. J Am Chem Soc. 2006;128:12084–12085.10.1021/ja0648209
- Hetzer M, Chen G, Barner-Kowollik C, et al. Neoglycopolymers based on 4-vinyl-1, 2, 3-triazole monomers prepared by click chemistry. Macromol Biosci. 2010;10:119–126.10.1002/mabi.v10:2
- Beghdadi S, Abdelhedi Miladi I, Ben Romdhane H, et al. RAFT polymerization of bio-based 1-vinyl-4-dianhydrohexitol-1,2,3-triazole stereoisomers obtained via click chemistry. Biomacromolecules. 2012;13:4138–4145.10.1021/bm301435e
- Nulwala H, Takizawa K, Odukale A, et al. Synthesis and characterization of isomeric vinyl-1,2,3-triazole materials by azide–alkyne click chemistry. Macromolecules. 2009;42:6068–6074.10.1021/ma900892h
- Nulwala H, Burke DJ, Khan A, et al. N-vinyltriazoles: a new functional monomer family through click chemistry. Macromolecules. 2010;43:5474–5477.10.1021/ma100011x
- Andjouh S, Bressy C, Blache Y. RAFT polymerization of bromotyramine-based 4-acryloyl-1,2,3-triazole: a functional monomer and polymer family through click chemistry. RSC Adv. 2016;6:14496–14504.10.1039/C5RA27578D
- Gangopadhyay S, Shende R, Subramanian S, et al. Ordered nanoenergetic composites and synthesis method. U.S. Patent 2011;437:7927.
- Shin JA, Lim YG, Lee KH. Synthesis of polymers including both triazole and tetrazole by click reaction. Bull Korean Chem Soc. 2011;32:547–552.10.5012/bkcs.2011.32.2.547
- (a) Ostrovskii VA, Pevzner MS, Kofmanand TP, et al. Targets Heterocyclic Syst. 1999;3:467–562; (b) Drake G, Hawkins T, Brand A, et al. Energetic, low‐melting salts of simple heterocycles. Propellants Explos Pyrotech. 2003;28:174–180.
- Beghdadi S, Miladi IA, Addis D, et al. Synthesis and polymerization of C-vinyl-and N-vinyl-1, 2, 3-triazoles. Polym Chem. 2012;3:1680–1692.10.1039/C1PY00446H
- Mori H, Ishikawa K, Abiko Y, et al. Synthesis of triazole-based amphiphilic block copolymers containing carbazole moiety By RAFT polymerization. Macromol Chem Phys. 2012;213:1803–1814.10.1002/macp.201200176
- Shende R, Subramanian S, Hasan S, et al. Nanoenergetic composites of CuO nanorods, nanowires, and Al‐nanoparticles. Propellants Explos Pyrotech. 2008;33:122–130.10.1002/(ISSN)1521-4087
- Hudlicky M. Chemistry of organic fluorine compounds. Halsted Press; 1976.
- Roy S, Chatterjee T, Islam SM. Polymer anchored Cu(ii) complex: an efficient and recyclable catalytic system for the one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles starting from anilines in water. Green Chem. 2013;15:2532–2539.10.1039/c3gc41114a
- Kadoma Y, Toida T, Takeda K, et al. Polymerization of methacrylates. I. Polymerization reactivity of picryl methacrylate. J. Polym. Sci Polym Chem. 1975;3:707–716.10.1002/pol.1975.170130313
- Dikusar MA, Kubrakova IV, Chinarev AA, et al. Polymerization of 4-nitrophenyl acrylate under microwave heating conditions. Russ J Bioorg Chem. 2001;27:408–412.10.1023/A:1012949005183
- Thamizharasi S, Gnanasundaram P, Rao KV, et al. Copolymers of 4-nitrophenyl acrylate with styrene: Synthesis, characterization and monomer reactivity ratios. Eur Polymer J. 1996;32:105–109.10.1016/0014-3057(95)00113-1
- Badgujar DM, Talawar MB, Mahulikar PP, et al. Advances in science and technology of modern energetic materials: an overview. J Hazard Mater. 2008;151:289–305.10.1016/j.jhazmat.2007.10.039