Abstract
Through metal-free protocol, hypercrosslinked porous polyporphyrin with permanent porosity was obatined via the Friedel–Crafts alkylation of tetracarbazolylporphyrin using formaldehyde dimethyl acetal as an external cross-linker. Its chemical structure and porosity was well characterized and confirmed. The BET specific surface area value of HCP-TCPP is 1050 m2 g−1 and related dominant pore size is centered at 0.63 nm. The adsorption amount of methanol by HCP-TCPP is high up to 800 mg g−1 (about 25.0 mmol g−1) at its saturated vapor pressure, which is higher than that of toluene (600 mg g−1, 6.5 mmol g−1). Further study indicates that polymer HCP-TCPP, possessing the high BET specific surface area and total pore volume, exhibits good hydrogen uptake of 3.44 wt % (77 K) and high carbon dioxide uptake of 41.1 wt % (298 K) at 18.0 bar. Besides, the obtained porous polymer can also be used as an effective heterogeneous catalyst for the Knoevenagel condensation between various aldehydes and malononitrile.
1. Introduction
As one of intrinsic nanoporous polymers, hypercrosslinked porous polymer represent a type of porous organic networks with high specific surface area and good thermal/chemical stability, which have been used for the adsorption of gas and organic vapors, the removal of organic compounds from water,[Citation1] and the heterogeneous catalyst of organic transformation.[Citation2] Friedel–Crafts alkylation is a commonly used reaction for preparation of hypercrosslinked porous polymers. Recently, a facile synthetic method to microporous polymers has been developed by Tan’s group, which is a simple one-step Friedel–Crafts alkylation of aromatic monomers using formaldehyde dimethyl acetal (FDA) as an external cross-linker.[Citation3] Through this approach, various hypercrosslinked porous heterocyclic polymers [Citation4,5] have been smoothly obtained for gas uptake & separation, and catalyst immobilization. Besides, various hypercrosslinked porous polymers made in metal-free condensation have reported recently,[Citation6,7] which will be of high importance toward future mass applications.
Owing to the large p-conjugated macrocyclic system containing basic pyrrole cavity, porphyrin may bind Lewis acid CO2 through strong interaction. Metal ions can also be readily incorporated into the porphyrin center because of the square-planar coordination site. In recent years, porous organic polymers based on porphyrin have drawn great attentions due to the rigid structure and special function of porphyrin.[Citation8–15] It is reported that metalloporphyrin-based porous materials can promote some important chemical transformation such as photosynthesis, oxygen transport, and catalytic oxidations.[Citation8,9,11,12] However, porphyrin-based porous organic polymers without built-in metal sites in the skeleton have been reported rarely until now.[Citation16,17] Compared with metalloporphyrin based porous materials, the porous polymer based on free-base porphyrin obtained with lower cost can display special properties and function derived from the basic pyrrole containing macrocyclic cavity with large p-conjugated system.
Efficient preparation and flexible molecular design of versatile porous polymers have been proven from the smoothly template-free preparation by selection of various building blocks and polymerization reactions.[Citation18,19] Herein, hypercrosslinked porous polyporphyrin with permanent porosity and special functions was prepared by the Friedel–Crafts alkylation of tetracarbazolylporphyrin in metal-free style using FDA as an external cross-linker. Its chemical structure and porosity was well characterized and confirmed. The related gas adsorption capacities under high pressure for hydrogen and carbon dioxide have been investigated and the capabilities for adsorption of solvent vapors such as toluene and methanol have also been explored. Its adsorption performance can be comparable with some conjugated porous polymers we reported before.[Citation20,21] Owing to the basic pyrrole containing macrocyclic cavities, the obtained porous polymer can also be used as a heterogeneous catalyst for the Knoevenagel condensation between various aldehydes and malononitrile.
2. Experimental
2.1. Materials
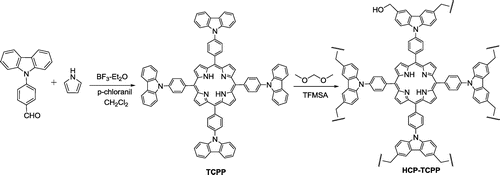
Trifluoromethane sulfonic acid (TFMSA), o-dichlorobenzene, and FDA were purchased from Alfa Aesar company. All chemicals and reagents were used without further purification unless otherwise stated. Tetrakis(carbazol-9-ylphenyl)porphyrin (TCPP) was prepared according to the reported method.[Citation22] The chemical structures of all monomers were fully confirmed.
2.2. Structure characterization and analysis
Solid-state cross polarization magic angle spinning (CP/MAS) NMR spectrum was obtained on a Bruker Avance III 400 NMR spectrometer. The infrared (IR) spectra were obtained from a PerkinElmer Spectrum One Fourier transform infrared (FTIR) spectrometer. Thermogravimetric analysis (TGA) was carried out on a Pyris Diamond thermogravimetric/differential thermal analyzer by heating (10 °C min−1) the samples to 900 °C under the nitrogen or air atmosphere. Scanning electron microscopy (SEM) observation was performed using a Hitachi S-4800 microscope without sputter coating. Transmission electron microscopy (TEM) observation was carried out with a FEI Tecnai G2 20S-TWIN microscope at an accelerating voltage of 200 kV. The SEM and TEM samples were prepared by placing a drop of the sonicated suspension of the as-prepared material in ethanol on a silica wafer and lacey support film, respectively, followed by drying under ambient condition.
2.3. Porosities studies and adsorption measurements
Nitrogen sorption isotherms were recorded with Micromeritics ASAP 2020 M+C accelerated surface area and porosimetry analyzers at certain temperature (all the samples were degassed at 120 °C for 12 h before measurement). BET specific surface area and micropore surface area were evaluated based on the obtained adsorption–desorption isotherms. The PSD of materials was calculated from the related adsorption branch by the nonlocal density function theory (NLDFT) approach. Total pore volume was calculated from nitrogen adsorption–desorption isotherms at P/P0 = 0.99. The vapor (methanol and toluene) adsorption–desorption isotherms and gas (H2 and CO2) uptake capacities were measured on an IGA-100B intelligent gravimetric analyzer.
2.4. Preparation of HCP-TCPP
TCPP (0.10 g, 0.081 mmol) was dissolved in anhydrous o-dichlorobenzene (20 mL), and then FDA (450 µL, 0.3 mmol), catalyst TFMSA (3.0 mmol) were added to the reaction mixture The mixture was stirred for 1 h under nitrogen at room temperature, and then the mixture was heated to 100 °C and kept at the temperature for 12 h. The crude product was filtered off from the hot reaction mixture and carefully washed with water, ethanol, acetone, and chloroform to remove reagents, solvents and possible low molecular weight by-products. After extracted in a Soxhlet extractor with methanol for 24 h, and then with tetrahydrofuran for another 24 h, the desired polymer HCP-TCPP (yield: 95%) was collected and dried in vacuum oven at 110 °C overnight.
2.5. General procedure for Knoevenagel condensation
To a suspension of HCP-TCPP (1 mol% of the substrates) and dioxane (0.2 mL)/water (0.2 mL) in a 5 mL round bottomed flask were added aromatic aldehyde (0.1 mmol) and malononitrile (0.1 mmol). The reaction mixture was stirred at the room temperature. The formation of the products was monitored by TLC. After 5 h, the mixture was filtered through a short silica gel flash column and purified using petroleum ether/dichloromethane as eluent to afford the desired product.
3. Results and discussion
The synthetic route to porphyrin monomers and corresponding porous polymer is shown in Scheme . Condensation of 4-(9-carbazolyl)benzaldehyde with pyrrole was performed under Lindsey conditions to produce the porphyrin derivative TCPP,[Citation23,24] in which the Lewis acid catalysis is at high dilution followed by addition of oxidant. Using the TCPP as monomers and FDA as the crosslinker, the Friedel–Crafts crosslinking polymerization was promoted smoothly by TFMSA in dry o-dichlorobenzene under nitrogen atmosphere at 100 °C. This modified metal-free protocol can furnish the desired hypercrosslinked porphyrin-based porous organic polymer HCP-TCPP in high yield. The obtained polymer is stable and insoluble in common solvents as a result of the cross-linking nature.
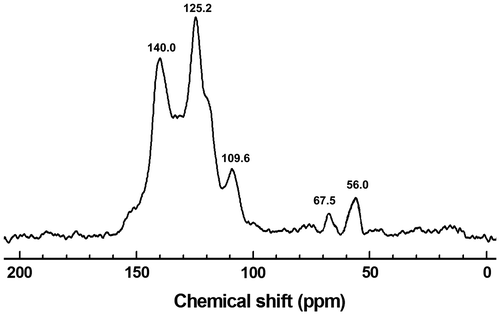
Polymer HCP-TCPP is characterized at the molecular level by the solid-state 13C CP/MAS NMR spectrum as shown in Figure . Generally, the broad peaks at 140–105 ppm are ascribed to the peak of aromatic carbons, in which assignment of the signal resonances related to porphyrin macrocycle and carbazolyphenylene are consistent with reported data. In details, the peak at about 140.0 ppm corresponds to the substituted phenyl carbons binding with nitrogen atom. The high-intensity peak for other substituted phenyl carbons is located at about 125.2 ppm, the signal peak at about 109.6 ppm is ascribed to the unsubstituted phenyl carbons, which are perfectly consistent with the previous work about carbazole-based porous organic polymers.[Citation25–27] Peaks at 68–55 ppm are ascribed to the peak of methylene carbons from the linker FDA. The methylene carbons binding with phenyl, which are derived from FDA reacted with the monomer completely, are located at 56.0 ppm. Meanwhile, the peak at about 67.5 ppm corresponds to methylene carbons binding with oxygen atom, which are ascribed to imperfect reaction of the linker.[Citation28] As we known, there are some methylene carbons that just bind with the monomer partially in the material.
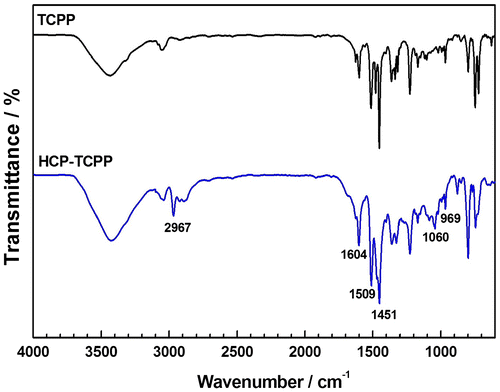
A comparison of the FTIR spectra of monomer and polymer is shown in Figure , in which signal peak at about 969 cm−1 in the spectra of both monomer and polymer is ascribed to N–H in-plane bending vibration of the pyrrole group. The peaks at about 1604, 1509, and 1451 cm−1 are attributed to aromatic ring skeleton vibrations, which were consistent with the structure of monomer and polymer. Besides, as for the spectrum of prepared polymer, we found that the broad absorption peaks at about 3422 cm−1 (O–H band stretching vibrations), the weak absorption peaks at about 2967 cm−1 (C–H band stretching vibrations) and the peaks at about 1060 cm−1 (C–O stretching vibrations) indicate the structure of –CH2– and a handful of tail end group such as –CH2OH and –CH2OCH3 in the hypercrosslinked network.[Citation28]
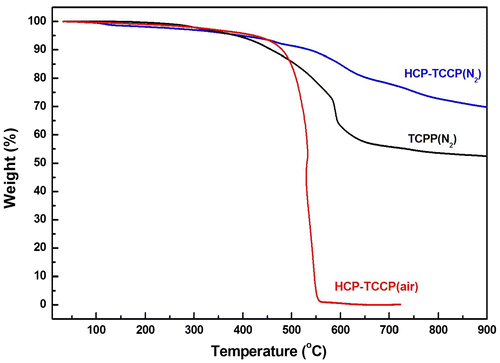
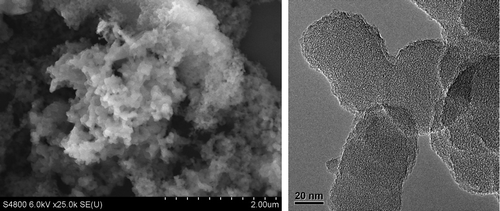
The thermal stability of the polymer was characterized by TGA (Figure ). Five percent of mass loss was observed at about 420 °C in the air, even similar to that under nitrogen. When the temperature rises to 800 °C, there is less than 30% of mass loss under nitrogen. It has no evidence for distinct glass transition for these polymers below the thermal decomposition temperature due to the nature of their cross-linking structures. For monomer TCPP, it appears 35% mass loss at around 635 °C, and the cross-linked polymer HCP-TCPP retains the residual masses of 71.8% at 800 °C. From comparing the thermogravimetric results of the network and corresponding monomer, it can be inferred that the formation of hypercrosslinked network take place. The SEM and TEM images of the obtained polymer are shown in Figure . Material morphology was observed to be solid submicrometer particles with different size between 70 and 150 nm.
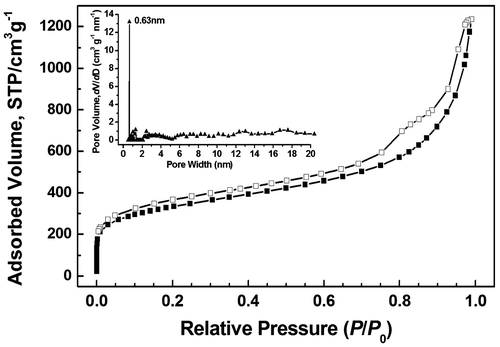
Porosity of the obtained polymer was studied by sorption analysis using nitrogen as the sorbate molecule. Nitrogen adsorption–desorption isotherms of polymer measured at 77 K are shown in Figure , which exhibit a combination of type I and II nitrogen sorption isotherms according to the IUPAC classification.[Citation29] The increase in the nitrogen sorption at a high relative pressure above 0.9 may arise in part from interparticulate porosity associated with the meso-/macrostructures and interparticular voids of the sample.[Citation30,31] Hysteresis can be observed apparently in the whole range of relative pressure based on the isotherms, which might be attributed to the swelling in a flexible polymer framework induced by adsorbate molecules dissolved in nominally nonporous parts of the polymer matrix after filling of open and accessible voids or the restricted access of adsorbate to the pores blocked by narrow openings.[Citation32–34] The BET specific surface area value of HCP-TCPP is 1050 m2 g−1, which is calculated in the relative pressure (P/P0) range from 0.01 to 0.1 according to the previous reports.[Citation35] The specific surface area value can be comparable to reported porphyrin-based porous polymers obtained by different coupling polymerization methods such as oxidative coupling reaction [Citation22] and Sonogashira–Hagihara coupling reaction.[Citation15] The PSD of the porous polymer was calculated from the adsorption branch of the isotherm with the NLDFT approach, the dominant pore size of polymer is centered at 0.63 nm.
Figure 5. Nitrogen adsorption–desorption isotherms of HCP-TCPP measured at 77 K and related PSD profile (inset) calculated by NLDFT.
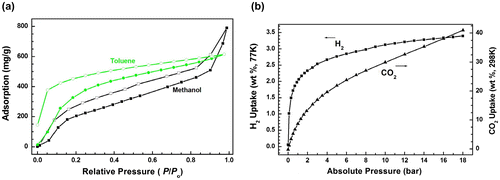
As we know, the work related to vapor adsorption performance of hypercrosslinked porous polymers is very limited compared with gas adsorption. The porous polymers with high porosity allow potential access by a variety of small molecules.[Citation36] Herein, we investigated the capability of the obtained material for adsorption of solvent vapors such as toluene and methanol. The related toluene and methanol adsorption–desorption isotherms at 298 K are shown in Figure (a). The adsorption amount of methanol by HCP-TCPP is high up to 800 mg g−1 (about 25.0 mmol g−1) at its saturated vapor pressure, which is higher than that of toluene (600 mg g−1, 6.5 mmol g−1) probably owing to the smaller molecular size for methanol. The high uptake capacity of HCP-TCPP for methanol could be ascribed to its high porosity and strong affinity to the absorbent molecules, which would have potential use to eliminate harmful small organic molecules in the environment. Further test also displays that polymer HCP-TCPP, possessing the high BET specific surface area and total pore volume, exhibits good hydrogen uptake of 3.44 wt % (77 K) and high carbon dioxide uptake of 41.1 wt % (298 K) at 18.0 bar (Figure (b)).
Porous polyporphyrin networks were reported to be the effective heterogeneous catalyst for a Knoevenagel reaction, which is a base-catalyzed condensation between aromatic aldehydes and acidic methylene derivatives.[Citation37,38] Here, the Knoevenagel reaction between various aromatic aldehydes and malononitrile in the presence of HCP-TCPP was performed to study the catalytic activities of prepared porous polymer using reported optimal reaction conditions.[Citation17] As shown in Table , aromatic aldehydes with different electron-withdrawing or electron-donating groups were all smoothly transformed to the corresponding desired products with good to excellent yields. Meanwhile, we also investigated the possibility of recycling HCP-TCPP in the model reaction using benzaldehyde and malononitrile as substrates. The catalyst was reused in the next round of condensation after being separated from the reaction mixtures by centrifugation, washed, and dried. It’s found that HCP-TCPP can also be reused in subsequent reactions without significant decrease in activity even up to 6 runs. No significant decrease in amount of catalyst after several times of reuse was observed.
Table 1. The Knoevenagel reaction between various aromatic aldehydes and malononitrile catalyzed by HCP-TCPP.
4. Conclusions
To summary, hypercrosslinked porous polyporphyrin was obatined via the Friedel–Crafts alkylation of tetracarbazolylporphyrin using formaldehyde dimethyl acetal as an external cross-linker through metal-free protocol. The BET specific surface area value of HCP-TCPP is 1050 m2 g−1, which can be comparable to reported porphyrin-based porous polymers obtained by different coupling polymerization methods such as Suzuki coupling reaction and Sonogashira–Hagihara coupling reaction. The related gas adsorption capacities under high pressure for hydrogen and carbon dioxide have been investigated and the capabilities for adsorption of solvent vapors such as toluene and methanol have also been explored. The adsorption amount of methanol by HCP-TCPP is high up to 800 mg g−1 (about 25.0 mmol g−1) at its saturated vapor pressure, which is higher than that of toluene (600 mg g−1, 6.5 mmol g−1). Further test also displays that polymer HCP-TCPP, possessing the high BET specific surface area and total pore volume, exhibits good hydrogen uptake of 3.44 wt % (77 K) and high carbon dioxide uptake of 41.1 wt % (298 K) at 18.0 bar. Owing to the basic pyrrole containing macrocyclic cavities, the obtained porous polymer can also be used as a heterogeneous catalyst for the Knoevenagel condensation between various aldehydes and malononitrile. Aromatic aldehydes with different electron-withdrawing or electron-donating groups were all smoothly transformed to the corresponding desired products with good to excellent yields. It’s found that HCP-TCPP can also be reused in subsequent reactions without significant decrease in activity even up to 6 runs. No significant decrease in amount of catalyst after several times of reuse was observed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Science and Technology Foundation of Guizhou Province [grant number QKHJZ-2014–2176]; the Science and Technology Cooperation Project of Guizhou Province [grant number QKHLHZ-2015–7564]; the PhD Start-up Foundation of Zunyi Medical College [grant number F-710].
References
- Martín CF, Stöckel E, Clowes R, et al. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J Mater Chem. 2011;21:5475–5483.10.1039/c0jm03534c
- Xu S, Luo Y, Tan B. Recent development of hypercrosslinked microporous organic polymers. Macromol Rapid Commun. 2013;34:471–484.10.1002/marc.v34.6
- Luo Y, Li B, Wang W, et al. Hypercrosslinked aromatic heterocyclic microporous polymers: a new class of highly selective CO2 capturing materials. Adv Mater. 2012;24:5703–5707.10.1002/adma.v24.42
- Luo Y, Zhang S, Ma Y, et al. Microporous organic polymers synthesized by self-condensation of aromatic hydroxymethyl monomers. Polym Chem. 2013;4:1126–1131.10.1039/C2PY20914D
- Li B, Gong R, Wang W, et al. A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker. Macromolecules. 2011;44:2410–2414.10.1021/ma200630s
- Sprick RS, Thomas A, Scherf U. Acid catalyzed synthesis of carbonyl-functionalized microporous ladder polymers with high surface area. Polym Chem. 2010;1:283–285.10.1039/b9py00375d
- Preis E, Widling C, Brunklaus G, et al. Microporous polymer networks (MPNs) made in metal-free regimes: systematic optimization of a synthetic protocol toward N-arylcarbazole-based MPNs. ACS Macro Lett. 2013;2:380–383.10.1021/mz400126f
- Mackintosh HJ, Budd PM, McKeown NB. Catalysis by microporous phthalocyanine and porphyrin network polymers. J Mater Chem. 2008;18:573–578.10.1039/B715660J
- Chen L, Yang Y, Jiang D. CMPs as scaffolds for constructing porous catalytic frameworks: a built-in heterogeneous catalyst with high activity and selectivity based on nanoporous metalloporphyrin polymers. J Am Chem Soc. 2010;132:9138–9143.10.1021/ja1028556
- Xia J, Yuan S, Wang Z, et al. Nanoporous polyporphyrin as adsorbent for hydrogen storage. Macromolecules. 2010;43:3325–3330.10.1021/ma100026f
- Shultz AM, Farha OK, Hupp JT, et al. Synthesis of catalytically active porous organic polymers from metalloporphyrin building blocks. Chem Sci. 2011;2:686–689.10.1039/c0sc00339e
- Chen L, Yang Y, Guo Z, et al. Highly efficient activation of molecular oxygen with nanoporous metalloporphyrin frameworks in heterogeneous systems. Adv Mater. 2011;23:3149–3154.10.1002/adma.v23.28
- Feng X, Chen L, Dong Y, et al. Porphyrin-based two-dimensional covalent organic frameworks: synchronized synthetic control of macroscopic structures and pore parameters. Chem Commun. 2011;47:1979–1981.10.1039/c0cc04386a
- Modak A, Nandi M, Mondal J, et al. Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem Commun. 2012;48:248–250.10.1039/C1CC14275E
- Wang Z, Yuan S, Mason A, et al. Nanoporous porphyrin polymers for gas storage and separation. Macromolecules. 2012;45:7413–7419.10.1021/ma301426e
- Wang XS, Liu J, Bonefont JM, et al. A porous covalent porphyrin framework with exceptional uptake capacity of saturated hydrocarbons for oil spill cleanup. Chem Commun. 2013;49:1533–1535.10.1039/c2cc38067f
- Liu X, Sigen A, Zhang Y, et al. A porphyrin-linked conjugated microporous polymer with selective carbon dioxide adsorption and heterogeneous organocatalytic performances. RSC Adv. 2014;4:6447–6453.10.1039/c3ra46988c
- Dawson R, Cooper AI, Adams DJ. Nanoporous organic polymer networks. Prog Polym Sci. 2012;37:530–563.10.1016/j.progpolymsci.2011.09.002
- Wu D, Xu F, Sun B, et al. Design and preparation of porous polymers. Chem Rev. 2012;112:3959–4015.10.1021/cr200440z
- Feng L-J, Guo JW, Zhong X, et al. Fluorinated porous poly(spirobifluorene) via direct C-H arylation: characterization, porosity, and gas uptake. J Macromol Sci, A Pure Appl Chem. 2014;51:604–609.
- Feng L-J, Guo JW, Zhong X, et al. Fluorescent microporous polymeric microsphere: porosity, adsorption performance, and TNT sensing. J Macromol Sci, A Pure Appl Chem. 2014;51:706–711.
- Feng L-J, Chen Q, Zhu J-H, et al. Adsorption performance and catalytic activity of porous conjugated polyporphyrins via carbazole-based oxidative coupling polymerization. Polym Chem. 2014;5:3081–3088.10.1039/c3py01430d
- Lindsey JS, Schreiman IC, Hsu HC, et al. Rothemund and Adler-Longo reactions revisited: synthesis of tetraphenylporphyrins under equilibrium conditions. J Org Chem. 1987;52:827–836.10.1021/jo00381a022
- Loiseau F, Campagna S, Hameurlaine A, et al. Dendrimers made of porphyrin cores and carbazole chromophores as peripheral units. Absorption spectra, luminescence properties, and oxidation behavior. J Am Chem Soc. 2005;127:11352–11363.10.1021/ja0514444
- Chen Q, Luo M, Hammershøj P, et al. Microporous polycarbazole with high specific surface area for gas storage and separation. J Am Chem Soc. 2012;134:6084–6087.10.1021/ja300438w
- Chen Q, Liu D-P, Luo M, et al. Nitrogen-containing microporous conjugated polymers via carbazole-based oxidative coupling polymerization: preparation, porosity, and gas uptake. Small. 2014;10:308–315.10.1002/smll.v10.2
- Chen Q, Liu D-P, Zhu J-H, et al. Mesoporous conjugated polycarbazole with high porosity via structure tuning. Macromolecules. 2014;47:5926–5931.10.1021/ma501330v
- Zhu J-H, Chen Q, Sui Z-Y, et al. Preparation and adsorption performance of cross-linked porous polycarbazoles. J Mater Chem A. 2014;2:16181–16189.10.1039/C4TA01537A
- Sing KSW, Everett DH, Haul RAW, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem. 1985;57:603–619.
- Rose M, Klein N, Böhlmann W, et al. New element organic frameworks viaSuzuki coupling with high adsorption capacity for hydrophobic molecules. Soft Matter. 2010;6:3918–3923.10.1039/c003130e
- Chen Q, Luo M, Wang T, et al. Porous organic polymers based on propeller-like hexaphenylbenzene building units. Macromolecules. 2011;44:5573–5577.10.1021/ma200915f
- Weber J, Schmidt J, Thomas A, et al. Micropore analysis of polymer networks by gas sorption and 129Xe NMR spectroscopy: toward a better understanding of Intrinsic microporosity. Langmuir. 2010;26:15650–15656.10.1021/la1028806
- Chen Q, Wang Q, Luo M, et al. Microporous polymeric microsphere via surfactant-free Suzuki coupling polymerization in a single-phase: porosity and gas uptake. Polymer. 2012;53:2032–2037.10.1016/j.polymer.2012.03.014
- Jeromenok J, Weber J. Restricted access: on the nature of adsorption/desorption hysteresis in amorphous, microporous polymeric materials. Langmuir. 2013;29:12982–12989.10.1021/la402630s
- Walton KS, Snurr RQ. Applicability of the BET method for determining surface areas of microporous metal−organic frameworks. J Am Chem Soc. 2007;129:8552–8556.10.1021/ja071174k
- Pan L, Chen Q, Zhu J-H, et al. Hypercrosslinked porous polycarbazoles via one-step oxidative coupling reaction and Friedel–Crafts alkylation. Polym Chem. 2015;6:2478–2487.10.1039/C4PY01797H
- Makowski P, Weber J, Thomas A, et al. A mesoporous poly(benzimidazole) network as a purely organic heterogeneous catalyst for the Knoevenagel condensation. Catal Commun. 2008;10:243–247.10.1016/j.catcom.2008.08.028
- Zhang X-J, Bian N, Mao L-J, et al. Porous polybenzimidazoles via template-free suzuki coupling polymerization: preparation, porosity, and heterogeneous catalytic activity in Knoevenagel condensation reactions. Macromol Chem Phys. 2012;213:1575–1581.10.1002/macp.201200074