Abstract
Smart system employed CO2 gas as new trigger has been attracting enormous attention in recent years, but few monomers that are capable of switching their hydrophobicity/hydrophility upon CO2 stimulation have been reported. A novel CO2 responsive monomer, 4-vinylbenzyl amidine, is designed and synthesized in this work with N,N-dimethylacetamide dimethyl acetal and 4-vinylbenzyl amine that is prepared through the Gabriel reaction. In bi-phase solvent of n-hexane and water, the monomer dissolves in n-hexane first and then transforms into water upon the CO2 treatment, indicating a hydrophobic to hydrophilic transition. This transformation is demonstrated as reversible by monitoring the conductivity variation of its wet dimethyl formamide solution during alternate bubbling/removing CO2. The protonation of 4-vinylbenzyl amidine upon CO2 treatment is demonstrated by 1H NMR which also accounts for the dissolubility change. The reversible addition-fragmentation chain-transfer polymerization of this monomer is also performed, finding the reaction only occurs in glacial acetic acid. The reason can be ascribed to the different radical structure produced in different solvent.
Introduction
CO2 as a new trigger for smart systems has various advantages over its traditional counterparts such as pH, temperature, redox, light and voltage [Citation1–4]. The first virtue is the good switchabilility, which means the responsive process can be repeated for much more times without significant attenuation or any contamination into the system [Citation5]. The easy-removing feature of the gas state of CO2 may account for this characteristic. Furthermore, the CO2 is a metabolite of living cells rendering it good biocompatibility [Citation6,7]. Last but not the least, CO2 is a waste gas with wide availability and low price. All these advantages push CO2-responsive compounds and polymers into the spotlight [Citation1].
Jessop team [Citation5] first reported a long-chain alkyl amidine compound which worked as a CO2-switchable surfactant that can stabilize emulsion under exposing of CO2 and break the emulsion by bubbling inert gas in higher temperature. Naturally, researchers want to figure out what would happen if the amidines groups are introduced into polymers. Some efforts have been made to achieve CO2-responsive polymers by post modification. Take our previous work as an example, we prepared poly(4-chloromethylstyrene) (PCMS) first by reversible addition-fragmentation chain transfer (RAFT) polymerization. Then the chloro groups were transferred into azido units in the second step. Finally, a homemade N′-propargyl-N,N-dimethylacetamidines (PDAA) were coupled with the azido units through a ‘click’ chemical process, yielding amidine-containing polymers [Citation8,9]. Lowe and coworkers [Citation10] synthesized polymers containing pentafluorophenyl acrylate (PFPA) and then substituted the pentafluorophenyl groups with amidine species histamine (HIS), resulting in amidine side chains. Both of these methods can target CO2-responsive polymers efficiently, but they seem tedious involving with functionalization after polymerization. Yuan and his coworkers [Citation11,12] first synthesized monomer (N-amidino)dodecyl acrylamide (AD) that can be switched by CO2 and developed ‘breathable’ vesicles with its block copolymer. Subsequently, similar acrylamide monomers containing amidine groups were synthesized with same route as well [Citation13,14]. Meanwhile, the CO2-sensitivity of commercial available monomers containing tertiary amine and acid groups were discovered by Zhao’s team [Citation15–18]. However, the CO2-responsive monomer is rarely reported to date, resulting in limited choice can be made to fabricate CO2-sensitive polymers.
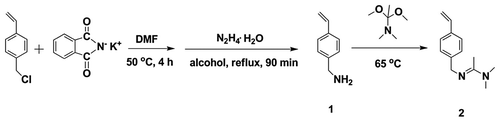
Here in this work, an amidine monomer that is responsive to CO2 is developed. 4-Vinylbenzyl chloride was first transfer into 4-Vinylbenzylamine through the Gabriel reaction. Then a CO2-responsive monomer, 4-vinylbenzyl amidine, is produced after a followed reaction of 4-Vinylbenzylamine with N,N-Dimethylacetamide dimethyl acetal (Scheme ). The CO2-responsive feature of this monomer is demonstrated by visualizing the hydrophobility to hydrophility transition and monitoring the conductivity variation as well as NMR shifts of its solution under CO2-stimulation. The discovery of this work paves the way to development of new CO2-sensitive polymer materials.
Experimental part
Materials and characterization
4-Vinylbenzyl chloride (90%), phthalimide potassium salt (99%), hydrazine monohydrate (N2H4·H2O, 50%), KOH, K2CO3, N,N-dimethylacetamide dimethyl acetal (90%) 44-azobis(4-cyanovaleric acid) (ACVA; ≥98.0%), were all purchased from Sigma-Aldrich and used without further treatments. The organic solvents of A.R. grade were obtained from Guanghua Sci-Tech Co., Ltd. (Guangdong, China) and used as received. The CO2 and N2 gas with a purity of above 99.99% were obtained from the Jinnengda Gas Co. in Chengdu, China. The chain transfer agent (CTA), 4-cyano-4-thiothiopropylsulfanylpentanoic acid (CTPPA), was synthesized according to the previously reported procedures [Citation19,20].
1H NMR spectra were recorded at 25 °C on a Bruker AV300 NMR spectrometer at 300 MHz. The chemical shifts (δ) are reported in parts per million (ppm) with reference to the internal standard protons of tetramethylsilane (TMS). The conductivity was determined by an FE30 conductometer (Mettler Toledo, USA) at room temperature.
Synthesis of 4-Vinylbenzylamine (compound 1)
In a 250 mL round-bottom flask, 22.9 g 4-vinylbenzyl chloride (0.15 mol) and 27.8 g phthalimide potassium salt (0.15 mol) were dissolved into 100 mL dimethyl formamide (DMF) solution and stored at 50 °C for 4 h with stirring. The solvent was removed by reduced pressure distillation after reaction. Then the mixture was dissolved in CHCl3 and washed with NaOH solution (0.2 mol·L−1) and water successively. The raw product was concentrated and recrystallized in methanol for two times, yielding colorless transparent crystals.
In the following step, the colorless transparent crystals (~27.1 g) were dissolved into 50 mL ethanol with heating. Then 12.4 g N2H4·H2O (0.13 mol) was slowly added into the solution, producing white precipitations. After 90 min reaction, white solids were obtained by filtration. Then the solids were dissolved with 15 wt% KOH solution, followed by filtration. The filtrate was extracted for three times with diethyl ether and washed with 2 wt% K2CO3 aqueous solution. Colorless transparent liquid was obtained after drying with K2CO3, followed by removing solvent with rotary evaporation. 1H NMR (δ, ppm; solvent, CDCl3), 3.85 (–CH2NH2), 5.20–5.24, 5.70–5.74 (–CH=CH2), 6.66–6.75 (–CH=CH2), 7.2, 7.3 (–C6H4–CH=CH2).
Synthesis of 4-vinylbenzyl amidine (compound 2)
6.6 g N,N-Dimethylacetamide dimethyl acetal (45 mmol) was added into 150 mL round-bottom flask, followed by dropping 5.0 g colorless transparent liquid obtained from last step (compound 1) under N2 protection. The mixture was stirred for 15 min at room temperature and then heated to 65 °C. After 2 h reaction, the product was collected by removing side product methanol and excess N,N-Dimethylacetamide dimethyl acetal with rotary evaporation. 1H NMR (δ, ppm; solvent, CDCl3), 1.91 ((CH3)2 N– (CH3)C=N–), 2.95 ((CH3)2 N– (CH3)C=N–), 4.47–4.50 (–C6H4–CH2–N), 5.15–5.29 and 5.59–5.76 (CH2=CH–), 6.65–6.74 (CH2=CH–), 7.25–7.36 (–C6H4–).
RAFT polymerization of 4-vinylbenzyl amidine
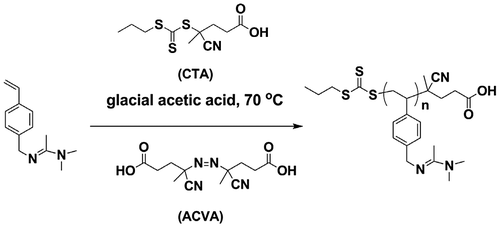
The RAFT polymerization is carried out as following typical procedure. 1.02 g 4-vinylbenzyl amidine (5.0 mmol), 14 mg CTPPA (0.05 mmol) and 3 mg initiator ACVA and 2 mL of solvent was added into a reaction tube. Then the system was deoxygenated with three freeze-thaw cycles or bubbling N2 for 30 min followed by keeping at 70 °C for 24 h. After the reaction, the product was checked with 1H NMR.
Results and discussion
CO2-responsiveness of 4-vinylbenzyl amidine
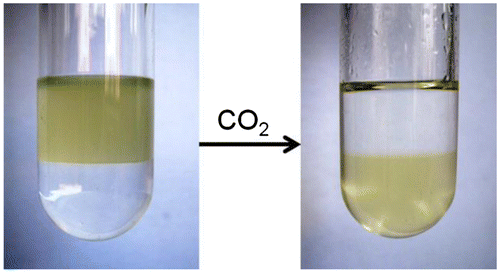
As above-mentioned, we previously developed CO2-responsive polymers by combing RAFT polymerization and ‘click’ chemistry, which is not suitable for preparation of complex copolymers, such as multi-block and star structures [Citation8,9]. Therefore, we try to synthesize a CO2-responsive monomer in this work. After we confirm the chemical structure of this monomer by NMR (see experimental part), its solubility is tested, finding it can be dissolved in common organic solvent including n-hexane, CH2Cl2, CHCl3, THF, DMF, DMSO. To check out the CO2-sensitivity, we then investigate whether it can transform from organic solvent into water phase, meaning whether it can transition from hydrophobicity to hydrophility. As shown in Figure , in a bi-phase solvent of n-hexane and water (v/v = 1:1), several drops monomer is added into the system. The monomer is dissolved into n-hexane in upper phase without CO2 treatment, appearing as a yellow solution above water. Then CO2 gas is bubbled into the solution, resulting in a yellow aqueous solution in the bottom. Meanwhile, the upper n-hexane becomes colorless and transparent. The n-hexane is a non-polar solvent, this transition implies the monomer can transform from hydrophobicity into hydrophilicity upon CO2 stimulation.
Figure 1. The hydrophobic to hydrophilic transformation of 4-vinylbenzyl amidine upon stimulation of CO2. The solvent is n-hexane and water (v/v = 1:1).
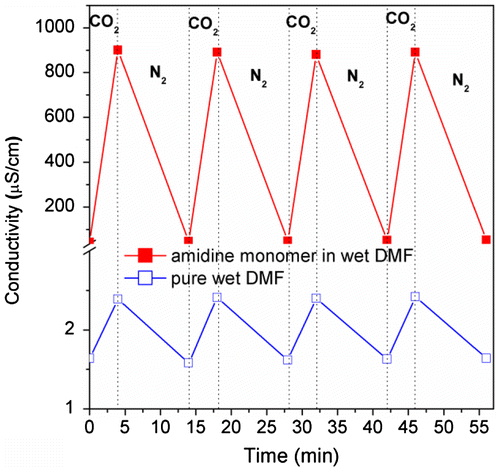
To confirm the switchability of this monomer, we monitor the conductivity variation of its wet DMF solution during CO2 is bubbling and removing alternately. As shown in Figure , the conductivity of the monomer solution jumps from 43.0 to 900.1 μS cm−1 after bubbling CO2 for 4 min. Subsequently, it drops back to around 45.6 μS cm−1 after removing CO2 by N2 treatment. Furthermore, this jumping-dropping cycle can be repeated for four times without significant attenuation, indicating the CO2-responsiveness of this monomer is reversible. As a comparison, the conductivity of wet DMF solution without monomer inside only increases from 1.6 to 2.4 μS cm−1 under the stimulation of CO2, which further proves the switchable transition of the monomer under stimulation of CO2.
Figure 2. Conductivity variation of 4-vinylbenzyl amidine in wet DMF solution (~1 v% water) when CO2 and N2 is alternatively bubbling.
Beside the conductivity, we further investigate the reaction of the monomer with CO2 by 1H NMR characterization (Figure ). The monomers were dissolved into wet DMSO-d6 and characterized by 1H NMR before and after the treatment of CO2. By comparing the main proton peak around the amidine group, one can easily indentify the significant chemical shift, i.e. f[–C6H4–CH2–N=C(CH3)–], g[N=C(CH3)–N(CH3)2], h[N=(CH3)C–N(CH3)2] increases from 4.32, 2.84, 1.85 to 4.46, 3.01, 1.95 ppm, respectively. This result proves the protonation of the monomer after reaction with CO2. The protonation reaction is shown in Figure , i.e. the amidine react with CO2 and water producing bicarbonate. Actually, similar reaction of amidine and CO2 in the presence of water has been recognized by other researchers [Citation21]. This protonation produces a compound with positive charge [Citation5] which also accounts for why the monomer can transform from hydrophobic to hydrophilic state under stimulation of CO2.
Figure 3. 1H NMR spectra of 4-vinylbenzyl amidine before and after reaction with CO2 and the reaction equation of amidine and CO2. The solvent is wet DMSO-d6.
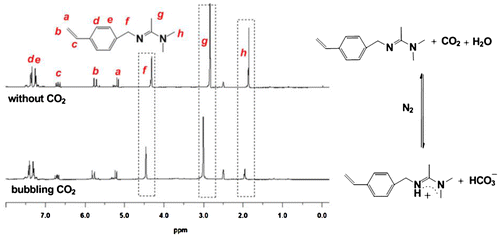
Figure 4. Snapshots of the RAFT polymerization product of 4-vinylbenzyl amidine. (a) product after reaction; (b) product in watch-glass; (c) product after drying.

Figure 5. Illustration of possible radical structures of 4-vinylbenzyl amidine in radical polymerization (structure 3 and 4) and reported amandine monomers by the groups of Yuan (compound 5, Ref. [Citation11]) and Yung (compound 6, Ref. [Citation14]).
![Figure 5. Illustration of possible radical structures of 4-vinylbenzyl amidine in radical polymerization (structure 3 and 4) and reported amandine monomers by the groups of Yuan (compound 5, Ref. [Citation11]) and Yung (compound 6, Ref. [Citation14]).](/cms/asset/044d405b-a2df-4e7e-854c-c38c102814f5/tdmp_a_1270027_f0005_b.gif)
Polymerization of 4-vinylbenzyl amidine
After confirm the chemical structure and CO2-responsive feature of the monomer, we try to carry out the RAFT polymerization (Scheme ). However, we get nothing when the reaction is done in organic solvent, neither in THF nor DMF. The reason, in our mind, might be the basicity of the amidine group (will discuss further). Long and his coworkers [Citation22] reported RAFT polymerization of 4-vinylimidazole in glacial acetic acid (HAc), which is another basic monomer that is difficult to conduct controlled polymerization. Inspired by this discovery, we perform RAFT polymerization of 4-vinylbenzyl amidine using HAc as solvent (Scheme ). The reaction conditions are illustrated in Table .
Table 1. Reaction conditions of RAFT polymerization of 4-vinylbenzyl amidine.
At first, we tried to polymerize 4-vinylbenzyl amidine in mixed solvent of DMF and HAc, but failed. Then various efforts were made, including utilization of pure HAc, increasing of the initiator ratio and optimization of de-oxygenization method (Table ). Finally, we find this monomer can be polymerized in conditions: (1) pure HAc, (2) initiator/CTA = 1, (3) bubbling N2 for 30 min to remove oxygen (entry 5, Table ). Nevertheless, the product appears as cross-linked gel with yellow color rather than polymer dissolved in solution (Figure ). This gel cannot be dissolved into any solvents. Further improvements of this polymerization are still progressing in our group. Interestingly, the de-oxygenization method really affects the success of this reaction, if comparing entry 3 and 5 in Table . The reason may be ascribed to the highly volatile feature of HAc, which make it firstly filling the reaction tube in vacuum that restrains the discharge of oxygen in solution.
Then we come back to the problem, why the 4-vinylbenzyl amidine cannot be polymerized in neutral state without acetic acid? In the presentence of initiator, two kinds of radicals are possible produced; structure 3 and 4 (Figure ). The radical 3 is more stable then 4, because it forms a huge conjugated structure combining benzene ring and the donor amidine group. That means the 4-vinylbenzyl amidine transforms into radical 3 in organic reaction system, hindering the polymerization we desired. When the amidine groups are protonated by acetic acid, the possible huge conjugated structure 3 cannot form anymore and the polymerization occurs through the same mechanism of the radical polymerization of polystyrene. It should be point out that the reported amidine monomers (compounds 5 and 6 in Figure ) can be polymerized through a radical mechanism in their natural state because they have no concerning to form stable structure like radical 3.
Conclusions
In conclusion, a CO2-responsive monomer, 4-vinylbenzyl amidine, was designed and synthesized in this work. It transforms from hydrophobic to hydrophilic state under the stimulation of CO2, and this transition is reversible. This monomer can be polymerized by RAFT technique in glacial acetic acid, yielding a gel-like homo-polymer. The results of this work provide another choice for preparing new CO2 responsive polymers and pave a way to the development of novel CO2-sensitive ‘smart’ system.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was financially supported by the National Natural Science Foundation of China [grant number: 21273223]; and the open funding of State Key Laboratory of Polymer Materials Engineering [grant number: sklpme2014-2-06].
References
- Darabi A, Jessop PG, Cunningham MF. CO2-responsive polymeric materials: synthesis, self-assembly, and functional applications. Chem Soc Rev. 2016;45:4391–4436.10.1039/C5CS00873E
- Yan Q, Zhao Y. Block copolymer self-assembly controlled by the “green” gas stimulus of carbon dioxide. Chem Commun. 2014;50:11631–11641.10.1039/C4CC03412K
- Lin S, Theato P. CO2-responsive polymers. Macromol Rapid Commun. 2013;34:1118–1133.10.1002/marc.201300288
- Liu H, Lin S, Feng Y, et al. CO2-responsive polymer materials. Polym Chem. 2017. doi:10.1039/C6PY01101B.
- Liu YX, Jessop PG, Cunningham M, et al. Switchable surfactants. Science. 2006;313:958–960.10.1126/science.1128142
- Yan Q, Zhao Y. CO2-stimulated diversiform deformations of polymer assemblies. J Am Chem Soc. 2013;135:16300–16303.10.1021/ja408655n
- Che N, Yang S, Kang H, et al. Synthesis and properties of CO2-switchable Dex-g-PAHMA copolymers. Polym Chem. 2014;5:7109–7120.10.1039/C4PY00987H
- Guo ZR, Feng YJ, Wang Y, et al. A novel smart polymer responsive to CO2. Chem Commun. 2011;47:9348–9350.10.1039/c1cc12388b
- Guo ZR, Feng YJ, He S, et al. CO2-responsive “smart” single-walled carbon nanotubes. Adv Mater. 2013;25:584–590.10.1002/adma.201202991
- Quek JY, Roth PJ, Evans RA, et al. Reversible additio-fragmentation chain transfer synthesis of amidine-based, CO2-responsive homo and AB diblock (Co) polymers comprised of histamine and their gas-triggered self-assembly in water. J Polym Sci Part A Polym Chem. 2013;51:394–404.10.1002/pola.26397
- Yan Q, Zhou R, Fu C, et al. CO2-responsive polymeric vesicles that breathe. Angew Chem Int Ed. 2011;50:4923–4927.10.1002/anie.v50.21
- Yan Q, Wang J, Yin Y, et al. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew Chem Int Ed. 2013;52:5070–5073.10.1002/anie.201300397
- Zhang Q, Yu G, Wang W-J, et al. Preparation of N2/CO2 triggered reversibly coagulatable and redispersible latexes by emulsion polymerization of styrene with a reactive switchable surfactant. Langmuir. 2012;28:5940–5946.10.1021/la300051w
- Ma Y, Yung L-YL. Detection of dissolved CO2 based on the aggregation of gold nanoparticles. Anal. Chem. 2014;86:2429–2435.10.1021/ac403256s
- Han D, Tong X, Boissière O, et al. General strategy for making CO2-switchable polymers. ACS Macro Lett. 2011;1:57–61.
- Zhang JM, Han DH, Zhang HJ, et al. In situ recyclable gold nanoparticles using CO2-switchable polymers for catalytic reduction of 4-nitrophenol. Chem Commun. 2012;48:11510–11512.10.1039/c2cc35784d
- Kumar S, Tong X, Dory YL, et al. A CO2-switchable polymer brush for reversible capture and release of proteins. Chem Commun. 2013;49:90–92.10.1039/C2CC36284H
- Han D, Boissiere O, Kumar S, et al. Two-way CO2-switchable triblock copolymer hydrogels. Macromolecules. 2012;45:7440–7445.10.1021/ma3015189
- Convertine AJ, Benoit DSW, Duvall CL, et al. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control Release. 2009;133:221–229.10.1016/j.jconrel.2008.10.004
- Xu XW, Smith AE, Kirkland SE, et al. Aqueous RAFT synthesis of pH-responsive triblock copolymer mPEO-PAPMA-PDPAEMA and formation of shell cross-linked Micelles. Macromolecules. 2008;41:8429–8435.10.1021/ma801725w
- Jessop PG, Heldebrant DJ, Li X, et al. Green chemistry: reversible nonpolar-to-polar solvent. Nature. 2005;436:1102-1102.10.1038/4361102a
- Allen MH Jr, Hemp ST, Smith AE, et al. Controlled radical polymerization of 4-vinylimidazole. Macromolecules. 2012;45:3669–3676.10.1021/ma300543h