ABSTRACT
Considering inherence optical properties of adjoint polyfluorenes and special functions of water-soluble conjugated glycopolymers, a triazole chain glycoconjugate via one-pot method were rapidly synthesized to prepare a lactate ligand polyfluorene with a clear fluorescent label by a nickel-catalyzed Yamamoto coupling polymerization. The water solubility and biocompatibility of the glycoconjugated polymer were ameliorated when the lactose group introduced as the side chain of the conjugated polymer. As a fluorescent multivalent system of glycoconjugates containing pyranogalactose groups, the interaction between pyranogalactose group and cholera toxin B subunit was studied by fluorescence spectrophotometric titration. PF-Lac has a broad application prospect in the check of cholera toxin and the study of glycoprotein interaction.
1. Introduction
Some important bioprocesses, such as cell recognition and divergence, cancer transfer, bacterial and virus infections, are closely related to carbohydrate-mediated biological interactions [Citation1–Citation3]. Multivalent interaction, so-called glycoside cluster effect plays a significant role in binding affinity, particularly in those specific biological interactions [Citation4,Citation5]. Glycopolymers are suitable to be the mimic system of glyco-biomacromolecules considering the polyvalence, molecular weight control and molecular structure control [Citation6–Citation8]. These properties have been shown to influence the biological interactions between carbohydrate-carrying chains and their combined targets [Citation9–Citation11].
Cholera is an acute purging infection caused by ingestion of eatables or water polluted by vibrio cholerae, characterized by sudden purging, followed by vomiting; decreased blood pressure, weak pulse, etc. Without appropriate treatment, it can lead to severe vomiting, massive loss of electrolytes such as sodium and potassium in the blood, systemic electrolyte disturbances and even death [Citation12]. In some developing countries, cholera still represents a major potential threat to the health of both human and animals. Cholera give rise to cholera toxin (CT), which has an AB5 hexamer structure. Five identical cholera toxin B subunits (CTB) selectively bind to glycolipid ganglioside GM1 [Citation13]. The CTB can attach to intestinal cells by binding with the pentasaccharide head group of GM1, and this mechanism initiates the disease. Some lactosyl-bearing multivalent systems, such as dendrimers [Citation14,Citation15], nanoparticles [Citation16], and the molecule with aggregation-induced emission feature [Citation17] have been used to study specific combine to CTB. CTB is known to link to lactose by distinguish the end glycolipid section, which is also the end sugar in the GM1 oligosaccharide head [Citation18].
Synthetic fluorescent glycoconjugates have been used as multivalent artificial models of carbohydrate systems and suitable well-defined tools for the study of carbohydrate-based biological events [Citation19–Citation26]. For example, studies on the interaction of carbohydrate-lectin [Citation27] and carbohydrate-bacteria [Citation21] by fluorescent conjugated sugar polymers have been reported. Water-soluble conjugated polymers provide an advantageous platform for the development of new electro-optical devices [Citation28,Citation29], chemical sensors and biosensors [Citation30,Citation31], taking the inherent optoelectronic properties and good water solubility into account. Fluorescent conjugated sugar polymers possessing fluorescent backbones and carbohydrate-binding moieties are used in the investigation of carbohydrate-mediated bio-interaction and biosensor applications because of their water solubility, their unique optical properties and their high sensitivity to external stimuli and good biocompatibility [Citation32,Citation33].
It is well known that polyfluorenes exhibit intrinsic optical properties [Citation34] and have been used as various sensors [Citation35]. Herein, a lactosyl-bearing polyfluorene was synthesized and characterized, the latent application of the CT fluorescence sensor is also investigated. The introduction of lactose part can improve the solubility of water and biocompatibility of the polyfluorene system. Moreover, lactose moieties can provide CTB-oriented binding sites via specific biological interactions, thus, enhance the binding affinity via multivalent interactions, which leads to aggregation of lactosyl-bearing polyfluorene and significant fluorescence quenching.
2. Experiment
2.1 Materials
Peracetylated lactose (1) and HBr (33% solution in acetic acid) were commercially available from Acros Scientific Co. Ltd. Tetrabutylammonium hydrogen sulfate and sodium azide were commercially available from J&K Scientific Co. Ltd. 1,5-cyclooctadiene (1,5-COD), bis(1,5-cyclooctadiene)nickel(0) (1,5-COD-Ni)and 2,2ʹ-bipyridyl (2,2ʹ-Bpy) were bought from Aldrich Chemicals. Sodium methoxide and sodium ascorbate were purchased from Shanghai Chemical Technology Co. Ltd. CTB, bovine serum albumin (BSA) and phosphate buffer solution (PBS, 50 mM, pH 7.3) were bought from Sigma-Aldrich Co. Ltd. N, N-Dimethylformamide (DMF), dichloroethane (DCE), chloroform, methanol and tetrahydrofuran (THF) were dried before use. Unless otherwise stated, Other chemical reagents can be bought commercially and used as they are. The Millipore Purification System (Mily Q Water) was used to purify deionized water. 9,9-bis(4-propargyloxy phenyl)-2,7-dibromo fluorene (2) was prepared according to reported methods [Citation36].
2.2 Instrumental characterization and structural analysis
Measurement of Nuclear Magnetic Resonance (NMR) spectra (1H and 13C) were on Bruker DMX400 NMR spectrometer (Bruker Daltonics Inc., Germany). VG PLATFORM mass spectrometer was applied to record mass spectra by electrospray ionization (ESI (+)) (VG Elemental, England). The infrared (IR) spectra of small molecules and polymers were recorded with KBr pellets on a Perkin–Elmer Paragon 1000 Fourier transform infrared (FTIR) spectrometer (PerkinElmer Instruments Co. Ltd., USA). Perkin Elmer Lamda 900 UV-vis-NIR spectrophotometer (Perkin Elmer Instruments Co. Ltd.) was used to record UV-vis spectra by a quartz pool with a path length of 1 cm. Measurement of Fluorescence Spectra were used by Perkin Elmer LS55 Luminescence Spectrometer (PerkinElmer Instruments Co. Ltd., USA) at 1 cm Path Length. Screening molecular mass of polymers dissolved in THF was used by Agilent 1100 GPC-SEC Analysis System (Agilent Technologies, USA). The average molecular weight and molecular weight (Mn and Mw) of polystyrene were reckoned by calibration curve of polystyrene reference material.
2.3 Synthesis of monomer 3 via one-pot reactions
For the solution of acetylated lactose 1 (1.0 g, 1.5 mmol) in dry chloroform (15 mL), HBr (33% solution in acetic acid, 2.0 mL) was added dropwise in an ice water bath. Stirring the liquor at room temperature was for about 6 h under nitrogen protection until the reaction was accomplished. The volatile compounds are removed by decompression, and chloroform (10 mL), saturated baking soda solution (2.0 M, 5 mL), sodium azide (0.16 g, 4.6 mmol) and tetrabutylammonium bisulfate (0.5 g, 3.0 mmol) were added sequentially. Two hours later, 9,9-bis(4-propargyloxy phenyl)-2,7-dibromo fluorene 2 (292 mg, 1.0 mmol), CuSO4 · 5H2O (12.5 mg, 0.1 mmol), L-ascorbic acid Sodium Salt (30 mg, 0.30 mmol) and alcohol (5 mL) were added to the solution. The reaction mixture was kept stirring at room temperature for about 16 h. After removing solvent in vacuum, water (20 mL) was added to extract the product with ethyl acetate (170 mL). The merged organic layer was dehydrated with anhydrous Na2SO4 and evaporated in vacuum. Column chromatography (ethyl acetate-light petroleum, 2:1) was used for purification of the product 3 as foamed solid (0.9 g, 31%). 13C NMR (100 MHz, CDCl3): δ 19.2, 19.4, 19.5, 19.6, 19.8, 19.9, 20.1, 59.5, 59.5, 60.8, 61.0, 63.5, 65.6, 68.2, 69.6, 70.0, 71.7, 74.6, 75.0, 84.6, 100.2, 113.9, 120.3, 121.0, 128.3, 123.0, 136.2, 136.8, 143.9, 152.5, 156.3, 168.2, 168.5, 169.1, 169.2, 169.3, 169.5. 1H NMR (400 MHz, CDCl3): δ 1.77 (s, 6H), 1.95 (s, 6H), 2.01 (s, 6H), 2.03 (s, 6H), 2.05 (s, 6H), 2.06 (s, 6H), 2.14 (s, 6H), 3.95–3.87 (m, 6H), 4.15–4.06 (m, 6H), 4.46 (d, J = 10.9 Hz, 2H), 4.93 (dd, J = 2.9, 9.9 Hz, 2H), 5.08 (d, J = 8.2 Hz, 2H), 5.13 (s, 4H), 5.40–5.34 (m, 6H), 5.83 (d, J = 9.8 Hz, 2H), 6.83 (d, J = 9.0 Hz, 4H), 7.04 (d, J = 8.6 Hz, 4H), 7.45–7.43 (m, 4H), 7.55 (d, J = 9.1 Hz, 2H), 7.76 (s, 2H). ESI(+)-MS: calcd. for C83H90O36N6Br2: 1907.45 [M]; found 1946.86 [M + K]+.
2.4 Synthesis of polymer PF-AcLac by the Yamamoto coupling polymerization
Dry 1,5-cyclooctadiene (0.052 mL, 0.42 mmol) was put in anhydrous DMF (15 mL) solutions of 1,5-COD-Ni (112 mg, 0.41 mmol) and 2,2ʹ-Bpy (64 mg, 0.41 mmol). The mixture was heated and stirred at 80°C for 1 h, and sugar-bearing monomer 3 (286 mg, 0.15 mmol) was added to the purple solution. The mixture was heated and stirred at 80°C under the nitrogen atmosphere for 24 h. The resulting polymer was precipitated by pouring the reaction mixture into a methanol-water solution. PF-AcLac was isolated as grey powder (200 mg, 78%). 1H NMR (400 MHz, CDCl3): δ 1.77 (bs, 6H), 1.99 (bs, 6H), 2.05 (bs, 24H), 2.14 (bs, 6H), 3.92 (br, 6H), 4.10 (br, 6H), 4.44–4.53 (m, 4H),4.95–4.98 (m, 2H), 5.01–5.14 (m, 6H), 5.34–5.39 (m, 6H), 5.81 (br, 2H), 6.82–6.88 (m, 4H), 7.04–7.20 (m, 3H),7.45–7.51 (m, 3H),7.56–7.63 (m, 4H), 7.75 (bs, 2H). 13C NMR (100 MHz, CDCl3): δ 19.0, 19.5, 19.7, 19.8, 59.8, 60.6, 60.8, 65.6, 68.0, 69.5, 69.7, 69.9, 71.6, 74.7, 74.8, 76.1, 84.6, 100.1, 113.4, 113.8, 120.1, 126.0, 126.4, 128.4, 130.7, 136.8, 137.7, 143.9, 152.5, 156.4, 168.1, 168.3, 168.5, 169.0, 169.1, 169.2, 169.3. IR: ν (cm–1): 2938 (w), 1752 (s), 1593 (m), 1513 (m), 1482 (s), 1372 (m), 1321 (m), 1235 (s), 1111 (s), 809 (m). GPC (THF, polystyrene standard): Mn = 28, 600 g/mol; polydispersity = 1.74.
2.5 Synthesis of polymer PF-Lac
CH3ONa (1.0 M methanol solution) was added to the protected sugar polymer PF-AcLac (150 mg) in CH2Cl2/CH3OH (v/v = 1:3, 20 mL) solution drop by drop until the solution pH reached 11. The reaction mixture was stirred at room temperature overnight. The solid was gathered by centrifugation at 8000 rpm and washed twice with CH2Cl2. After the solvent is removed under diminished pressure, the residue was diluted with 10 ml water. The obtained solution was placed in a cellulose dialysis tube (with a trapped molecular weight of 3,500), dialyzed with water for 3 days, and freeze-dried to obtain the required solid polymer PF-Lac (95.5 mg, 94%). IR: ν (cm–1): 3421 (bs), 2934 (w), 1590 (m), 1513 (m), 1479 (s), 1325 (m), 1103 (s), 804 (m). 1H NMR (400 MHz, DMSO-d6): δ 3.31–3.44 (m, 6H), 3.51–3.73 (m, 14H), 3.75–3.88 (m, 4H), 4.28 (br, 2H), 5.11 (s, 4H), 5.68 (br, 2H), 6.95–7.04 (m, 4H), 7.15–7.24 (m, 2H), 7.25–7.70 (m, 4H), 7.75–8.10 (m, 4H), 8.44 (bs, 2H), 13C NMR (100 MHz, DMSO-d6): δ 60.0, 60.9, 68.2, 70.6, 71.9, 73.3, 75.2, 75.6, 77.7, 79.2, 79.7, 86.9, 103.5, 114.7, 123.7, 125.1, 126.5, 127.3, 128.7, 131.7, 134.3, 136.4, 139.2, 142.5, 157.2. GPC (THF, polystyrene standard): Mn = 19, 900 g/mol; polydispersity = 1.71.
2.6 Studies of the interactions between PF-Lac and CTB by fluorometric titration analysis
PF-Lac stock solution was prepared in PBS aqueous solution (50 mM, pH 7.3). Equivalent CTB (2 μL) was added to the same PBS buffer and PF-Lac solution (1.5 × 10–6 M, 2.0 mL). The final concentration of CTB was ranged from 1.0 × 10−8 to 8.0 × 10−8 M. After each addition, the samples were balanced overnight and the spectra were recorded. The excitation wavelength was 320 nm and the emission scan ranged from 310 to 630 nm.
2.7 Studies on the selectivity of fluorometric detection for CTB
Preparation of PF-Lac stock solution was in PBS (50 mM, pH 7.3). BSA (2 μL) and CTB (2 μL) fractions in alike PBS buffer were injected in PF-Lac solution (1.5 × 10–6 M, 2.0 mL) subsequently. After each addition, the sample was balanced overnight and the spectra were recorded. The final concentrations of BSA and CTB were 8.0 × 10−8 M, respectively. The excitation wavelength is 317 nm and the radiate scanning range is 330–630 nm.
3. Results and discussion
3.1 Synthesis and characterization of monomer and polymers
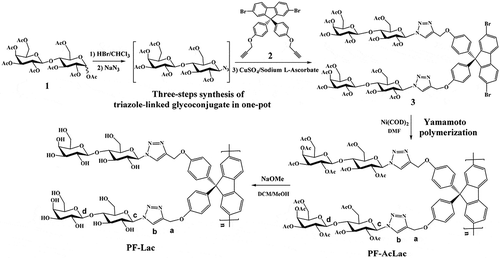
The synthetic routes of monomers and sugar polymers are outlined in . For monomer preparation, a rapid one-pot synthetic method to triazole-linked glycoconjugate from commercially available sugar acetate was used [Citation37,Citation38], which includes bromination, azidation and Cu(I)-catalyzed Huisgen 1,3-dipole cyclic addition reaction. As shown in , peracetylated lactose was used as the starting material for this method. After bromination with HBr in acetic acid and removal of all volatiles of solvents and acids, subsequent azide conversion can furnish intermediate acetylated lactosyl azide. 1,3-Dipolar selective ligation between propargyl fluorene derivative 2 and sugar azide intermediate catalyzed by in situ copper (I) are easy to produce lactose-containing monomer 3. A signal peak at 7.76 ppm (2H) in the 1H NMR spectrum as well as two peaks at 120.6 ppm and 144.0 ppm in the 13C NMR spectrum confirmed the formation of the triazole ring.
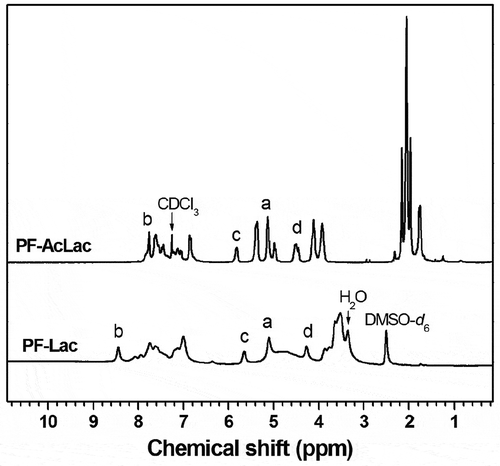
The Ni-catalyzed Yamamoto coupling polymerization of monomer 3 can afford a well-defined and protected glycopolymer, PF-AcLac, which is a poly(fluorene) containing the side-chain lactosyl derivatives. The PF-AcLac was treated with CH3ONa/CH3OH to deprotect all acetyl groups. The desired water-soluble conjugated glycopolymers (PF-Lac) were obtained in excellent yield (94%). The 1H and 13C NMR spectra of polymers PF-AcLac and PF-Lac are shown in and the supporting information, which can prove the successful preparation of the desired polymers. There are two reasons for HNMR signal of proton b down-field shifted significantly from PF-AcLac to PF-Lac. One is that the chemical environment of proton b is changed due to fully de-acetylation from PF-AcLac to PF-Lac. The more important is that the solvent for HNMR study is different from PF-AcLac to PF-Lac. For PF-AcLac, solvent is CDCl3. While for PF-Lac, solvent is DMSO-d6. The potential hydrogen bond acceptor would lead to shift the proton signal downfield [Citation39]. Selection of 9,9-bis (4-hydroxyphenyl) fluorene as polymeric monomers is beneficial to provide more effective shielding effect on the polyfluorenyl skeleton, because there is a non-flexible phenylene spacer between the main chain of the polymer and the side chain of the sugar, which can inhibit the formation of aggregates or receptors [Citation40].
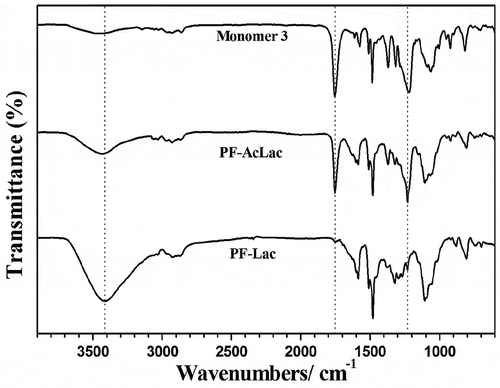
FT-IR spectra demonstrated the successful polymerization and deacetylation of conjugated glycopolymers. As shown in , absorption bands at 2850–2950 cm–1 in the FT-IR spectra of the monomer, polymers PF-AcLac and PF-Lac represent CH stretching from alkyl chains of monomers. After treating PF-AcLac with CH3ONa/CH3OH, the bands at 1750 cm–1 (C = O) and 1200 cm–1 (C–O stretching in ester) of PF-AcLac disappeared and confirmed perdeacetylation. Moreover, in the FT-IR spectra, there is a wide absorption band at 3400 cm−1 of polymer PF-Lac showed the extension of –OH, which are the free hydroxyl groups on the sugar part. This information also proves clearly that the polymerization of sugar-containing aromatic monomer is a good way to prepare well-defined conjugated glycopolymers.
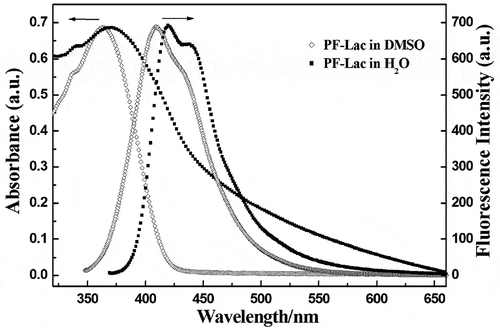
PF-AcLac is soluble in general solvents, such as CHCl3, CH2Cl2, acetyl acetate and THF, but not soluble in methanol and water. Gel permeation chromatography (GPC) analysis of polystyrene standard materials indicate that the number average molecular weight (Mn) of PF-AcLac is 28,600 g/mol with polydispersity of 1.74. After deprotection, the solubility of glycopolymer PF-Lac was different from its precursor. PF-Lac is soluble in water medium and even better in DMF and DMSO. Moreover, it exhibits intrinsic optical properties. For the PF-Lac in DMSO solution, the maximum absorption and emission peaks were found at 366 nm and 415 nm, and the vibration peak at 433 nm. These peaks are due to the π-π* transition of the main chain of polyfluorene. In aqueous solution, the maximum absorption peak of PF-Lac is at 378 nm, the maximum emission peak is at 424 nm and the vibration peak is at 442 nm. As shown in , we can see that the absorption and emission spectra of conjugated glycopolymer PF-Lac in water exhibit a significant red shift compared with the polymer in DMSO solution, because the planar conformation or aggregation of the polymer backbone is enhanced due to increased solvent polarity [Citation41].
3.2 Fluorescence ‘turn-off’ detection of CT
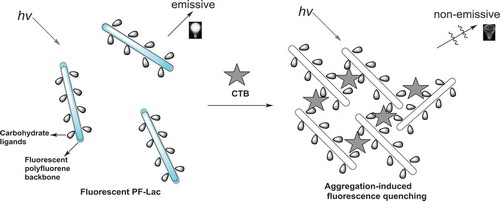
As mentioned above, CT consists of the five same monomers with a galactose binding site of GM1 ganglioside. Therefore, various types of molecules containing identical terminal galactose groups can be designed to bind to CTB. Lactose composition of amylaceum and a terminus-galatosum unit are usually used to design CT transducer. Water-soluble PF-Lac, possessing both a polyfluorene backbone and many lactosyl units, shows high photoluminescence in water. We can assume that when CTB was added into the dilute water solution of PF-Lac, the binding of CTB with polylactose-attached polyfluorene occurred, leading to the aggregation of PF-Lac. Therefore, the fluorescence of PF-Lac is quenched significantly due to aggregation, which can be used for CTB sensing. Schematic representation of fluorescence ‘turn-off’ assay for CTB by PF-Lac was shown in .
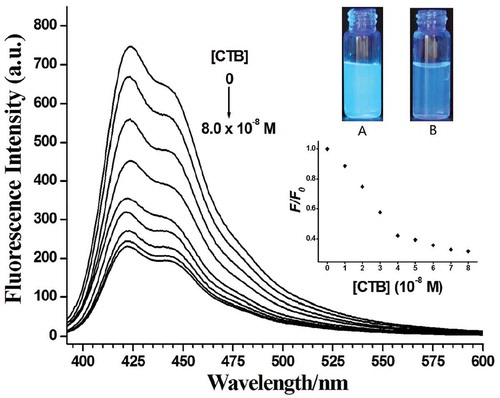
The interaction between PF-Lac and CTB was studied by detecting the fluorescence intensity of PF-Lac in the presence of CTB in PBS. As shown in , when CTB (0–8.0 × 10–8 M) was gradually added to the solution of PF-Lac (1.5 × 10–6 M) in the PBS (50 mM, pH = 7.3), most of fluorescence was quenched. The possible mechanism of fluorescence quenching of PF-Lac is attributed to the aggregation of PF-Lac/CTB binding, resulting in self-quenching. In static quenching, the quencher forms a ground-state complex with the fluorophore, and a linear Stern–Volmer relationship (F0/F = 1+ KSV[Q]) occurs [Citation25,Citation42]. KSV, as the Stern–Volmer constant, indicates the efficiency of quenching. The Stern–Volmer quenching constant of polymer PF-Lac by CTB was calculated as 1.6 × 107 M−1 based on the linear part of the curve (Figure S1).
Figure 4. Fluorescence spectra of polymer PF-Lac (1.5 × 10–6 M) in the absence and presence of various concentrations of CTB in PBS solution at room temperature. CTB concentrations from top to bottom are 0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0 × 10−8 M (Inset displays the photos of the corresponding solutions of PF-Lac in the absence (A) and presence (B) of CTB (8.0 × 10−8 M) under UV light (365 nm) illumination).
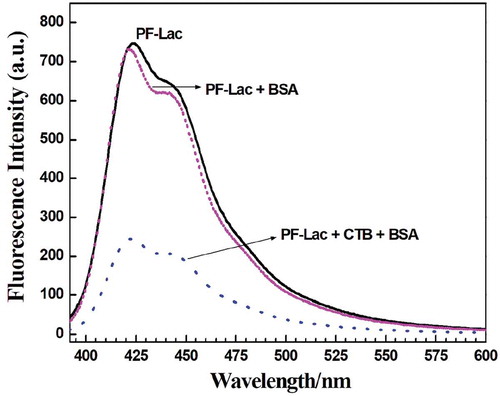
To study the selectivity of fluorescence check for CTB and the specificity of carbohydrate–protein interaction, the PF-Lac assay was conducted with other bio-macromolecules. We can see that the photoluminescence intensity of PF-Lac did not change significantly at presence of the BSA protein under the same conditions (). In addition, when PF-Lac was presented with the same amount of CTB and BSA in PBS solution, the intensity of fluorescence quenching was almost identical as that observed in the solution of CTB. These results revealed that PF-Lac shows great potential use in the selective detection of biomacromolecules.
4. Conclusion
Fluorescent conjugated polyfluorene with side-chain lactopyranosyl ligand (PF-Lac) was synthesized by one-pot formation of triazole-linked glycoconjugates and Yamamoto C-C coupling polymerization. Using fluorescent fluorene scaffolds and sugar-binding ligands, this well-defined fluorescent glycoconjugate polymer is used to study the interaction of CTB subunits by fluorescence spectrophotometric titration. When CTB is presented in deliquate PF-Lac aqueous solution, the binding of CTB with polyfluorene containing multi-lactose groups occurred, which cause the accumulation of PF-Lac. Therefore, the fluorescence of PF-Lac is quenched due to self-aggregation. We believe that these methods for insight into carbohydrate-mediated biological interactions have shown potential applications in the sensing of biomacromolecules and the glycobiology study.
Supplemental Material
Download PDF (320.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Krasnova L, Wong CH. Understanding the chemistry and biology of glycosylation with glycan synthesis. Annu Rev Biochem. 2016;85:599–630.
- Sun X, James TD. Glucose sensing in supramolecular chemistry. Chem Rev. 2015;115:8001–8037.
- Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555.
- Mueller C, Despras G, Lindhorst TK. Organizing multivalency in carbohydrate recognition. Chem Soc Rev. 2016;45:3275–3302.
- Mooibroek TJ, Casas-Solvas JM, Harniman RL, et al. A threading receptor for polysaccharides. Nat Chem. 2016;8:69–74.
- Appelhans D, Klajnert-Maculewicz B, Janaszewska A, et al. Dendritic glycopolymers based on dendritic polyamine scaffolds: view on their synthetic approaches, characteristics and potential for biomedical applications. Chem Soc Rev. 2015;44:3968–3996.
- Chen Y, Lord MS, Piloni A, et al. Correlation between molecular weight and branch structure of glycopolymers stars and their binding to lectins. Macromolecules. 2015;48:346–357.
- Park H, Rosencrantz RR, Elling L, et al. Glycopolymer brushes for specific lectin binding by controlled multivalent presentation of N-acetyllactosamine glycan oligomers. Macromol Rapid Comm. 2015;36:45–54.
- Lin K, Kasko AM. Carbohydrate-based polymers for immune modulation. ACS Macro Lett. 2014;3:652–657.
- Yilmaz G, Becer CR. Glyconanoparticles and their interactions with lectins. Polym Chem. 2015;6:5503–5514.
- Wibowo A, Peters EC, Hsieh-Wilson LC. Photoactivatable glycopolymers for the proteome-wide identification of fucose-α(1-2)-galactose binding proteins. J Am Chem Soc. 2014;136:9528–9531.
- Burki T. End of a cholera epidemic in South Sudan declared. Lancet. 2018;391:647.
- Mertz JA, McCann JA, Picking WD. Fluorescence analysis of galactose, lactose, and fucose interaction with the cholera toxin B subunit. Biochem Biophys Res Commun. 1996;226:140–144.
- Delbianco M, Bharate P, Varela-Aramburu S, et al. Carbohydrates in supramolecular chemistry. Chem Rev. 2016;116:1693–1752.
- Dohi H, Kanazawa T, Saito A, et al. Bis(β-lactosyl)-[60]fullerene as novel class of glycolipids useful for the detection and the decontamination of biological toxins of the Ricinus communis family. Beilstein J Org Chem. 2014;10:1504–1512.
- Calavia PG, Chambrier I, Cook MJ, et al. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J Colloid Interf Sci. 2018;512:249–259.
- Hu X-M, Chen Q, Wang J-X, et al. Tetraphenylethylene-based glycoconjugate as a novel fluorescence “turn-on” sensor for cholera toxin. Chem-Asian J. 2011;6:2376–2381.
- Turnbull WB, Precious BL, Homans SW. Dissecting the cholera toxin-ganglioside GM1 interaction by isothermal titration calorimetry. J Am Chem Soc. 2004;126:1047–1054.
- Zeng X, Qu K, Rehman A. Glycosylated conductive polymer: a multimodal biointerface for studying carbohydrate–protein interactions. Acc Chem Res. 2016;49:1624–1633.
- Martos-Maldonado MC, Casas-Solvas JM, Quesada-Soriano I, et al. Poly(amido amine)-based mannose-glycodendrimers as multielectron redox probes for improving lectin sensing. Langmuir. 2013;29:1318–1326.
- Seto H, Kamba S, Kondo T, et al. Metal mesh device sensor immobilized with a trimethoxysilane-containing glycopolymer for label-free detection of proteins and bacteria. ACS Appl Mater Interfaces. 2014;6:13234–13241.
- Ma F, Rehman A, Liu H, et al. Glycosylation of quinone-fused polythiophene for reagentless and label-free detection of E. coli. Anal Chem. 2015;87:1560–1568.
- Chen Q, Bian N, Cao C, et al. Glucosamine hydrochloride functionalized tetraphenylethylene: a novel fluorescent probe for alkaline phosphatase based on the aggregation-induced emission. Chem Commun. 2010;46:4067–4069.
- Chen Q, Han B-H. Prepolymerization and postpolymerization functionalization approaches to fluorescent conjugated carbazole-based glycopolymers via “click chemistry”. J Polym Sci Part A: Polym Chem. 2009;47:2947–2958.
- Chen Q, Xu Y, Du Y, et al. Triphenylamine-based fluorescent conjugated glycopolymers: synthesis, characterization and interactions with lectins. Polymer. 2009;50:2830–2835.
- Shie J, Liu Y, Hsiao J, et al. A cell-permeable and triazole-forming fluorescent probe for glycoconjugate imaging in live cells. Chem Commun. 2017;53:1490–1493.
- Sun P, He Y, Lin M, et al. Glyco-regioisomerism effect on lectin-binding and cell-uptake pathway of glycopolymer-containing nanoparticles. ACS Macro Lett. 2014;3:96–101.
- Okamoto K, Zhang J, Housekeeper JB, et al. C–H arylation reaction: atom efficient and greener syntheses of π-conjugated small molecules and macromolecules for organic electronic materials. Macromolecules. 2013;46:8059–8078.
- Chen X, Liu H, Xu Z, et al. Highly regiosymmetric homopolymer based on dioxythiophene for realizing water-processable blue-to-transmissive electrochrome. ACS Appl Mater Interfaces. 2015;7:11387–11392.
- Gorityala BK, Lu Z, Leow ML, et al. Design of a “turn-off/turn-on” biosensor: understanding carbohydrate-lectin interactions for use in noncovalent drug delivery. J Am Chem Soc. 2012;134:15229–15232.
- Cecioni S, Imberty A, Vidal S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem Rev. 2015;115:525–561.
- Wang K, Zhang XY, Zhang XQ, et al. Fluorescent glycopolymer nanoparticles based on aggregation-induced emission dyes: preparation and bioimaging applications. Macromol Chem Phys. 2015;216:678–684.
- Chen Q, Cui Y, Zhang T-L, et al. Fluorescent conjugated polyfluorene with pendant lactopyranosyl ligands for studies of Ca2+-mediated carbohydrate- carbohydrate interaction. Biomacromolecules. 2010;11:13–19.
- Liang J, Cui J, Guo T, et al. Highly efficient blue polyfluorenes using blending materials as hole transport layer. Org Electron. 2017;51:111–118.
- Cingil HE, Storm IM, Yorulmaz Y, et al. Monitoring protein capsid assembly with a conjugated polymer strain sensor. J Am Chem Soc. 2015;137:9800–9803.
- Chen Q, Cheng Q-Y, Zhao Y-C, et al. Glucosamine hydrochloride functionalized water-soluble conjugated polyfluorene: synthesis, characterization, and interactions with DNA. Macromol Rapid Commun. 2009;30:1651–1655.
- Chittaboina S, Xie F, Wang Q. One-pot synthesis of triazole-linked glycoconjugates. Tetrahedron Lett. 2005;46:2331–2336.
- Lim D, Brimble MA, Kowalczyk R, et al. Protecting-group-free one-pot synthesis of glycoconjugates directly from reducing sugars. Angew Chem Int Edit. 2014;53:11907–11911.
- Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–7515.
- Marsitzky D, Vestberg R, Blainey P, et al. Self-encapsulation of poly-2,7-fluorenes in a dendrimer matrix. J Am Chem Soc. 2001;123:6965–6972.
- Xue C, Donuru VR, Liu H. Versatile prepolymerization and postpolymerization functionalization approaches for well-defined fluorescent conjugated fluorene-based glycopolymers. Macromolecules. 2006;39:5747–5752.
- Lakowicz JR. Principles of fluorescence spectroscopy. 3rd ed. New York: Springer; 2006.