?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The kinetics of ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) initiated by 1.0, 1.5 and 2.0 mol% of stannous(II) octoate/n-hexanol (Sn(Oct)2/n-HexOH) wase successfully studied by non-isothermal differential scanning calorimetry (DSC) at heating rates of 5, 10, 15 and 20 °C/min. The DSC polymerization kinetic parameters of ε-CL were calculated using differential (Friedman) and integral isoconversional methods (Kissinger-Akahira-Sunose, KAS). The average activation energy (Ea) values obtained from Friedman and KAS methods were in the range of 64.9–70.5 kJ/mol and 64.9–80.4 kJ/mol, respectively. The values of frequency factor (A) were determined from model fitting method using Avrami-Erofeev reaction model. The average values of A for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) were 7.3x107, 2.8x106 and 1.2x106 min−1, respectively. From kinetics studied, the polymerization rate of ε-CL increased with increasing initiator concentration. The performance of Sn(Oct)2/n-HexOH in the synthesis of poly(ε-caprolactone) (PCL) was investigated by bulk polymerization at temperatures of 140, 160 and 180 °C. Sn(Oct)2/n-HexOH (1:2) could produce high number average molecular weight (= 9.0 × 104 g/mol) and %yield (89%) of PCL in a short period of time at Sn(Oct)2 concentration of 0.1 mol% and temperature of 160°C. The mechanism of the ROP of ε-CL with Sn(Oct)2/n-HexOH was proposed through the coordination-insertion mechanism.
Introduction
Poly(ε-caprolactone) (PCL) is very attractive polyester due to its versatile properties such as biodegradability, biocompatibility and capable blend with various polymers. PCL is widely interesting in industrial and academic research because of its important applications in packaging, agriculture and biomedical field [Citation1–3]. PCL is frequently used in medical and pharmaceutical applications such as drug delivery systems, suture and scaffold for tissue engineering due to the nontoxic products from degradation process can be eliminated or resorbable by the human body [Citation4–6].
Recently, the ring-opening polymerization (ROP) of ε-caprolactone (ε-CL) is considered as an potential route to obtain the biodegradable PCL with controlled properties. For this manner, effective metal-containing initiators are required to achieve the control ROP of ε-CL. Tin(II) bis(2-ethyl hexanoate) or tin(II) octoate (Sn(Oct)2), a well-known initiating system, is traditionally utilized in the ROP of cyclic esters [Citation6–11]. In ROP of cyclic esters, Sn(Oct)2 can be converted into tin alkoxide (Sn(OR)2), the actual initiator, by reaction with alcohol (R′OH) prior to initiate polymerization [Citation9]. After formation of Sn(OR)2, the ROP of cyclic esters with this Sn(OR)2 proceeds by the coordination-insertion mechanism [Citation10,Citation11]. The understanding of this mechanism has impacted the attention of many researchers until now.
Long time ago, the thermal stimulated reactions have been effectively studied by thermoanalytical techniques, especially differential scanning calorimetry (DSC) [Citation12–23]. In the recent years, isoconversional methods such as differential and integral methods, have been used to determine the values of effective activation energy (Ea) as a function of conversion (α) at which an equivalent stage of reaction occurs for various heating rates [Citation12,Citation13]. For the integral isoconversional methods such as Kissinger-Akahira-Sunnose (KAS) [Citation14,Citation15] and Ozawa-Flynn-Wall (OFW) [Citation22,Citation23], the more accurate methods, require the mathematics approximation of the temperature integral for calculation of Ea. Recently, an attempt to use DSC for investigating the kinetics of L-lactide (LL) polymerization with Sn(Oct)2 catalyst was reported in literature [Citation24]. The experiments were conducted under isothermal mode at constant temperature of 180–220°C. The Ea and pre-exponential factor (A) were calculated using model of reversible lactide polymerization. Li and co-workers [Citation19] also investigated the ROP of ε-CL initiated by titanium(IV) alkoxide (Ti[O(CH2)4OCH = CH2]4) by DSC technique. The obtained Ea values for this reaction were in the range of 75–86 kJ/mol. From our previous works, the non-isothermal DSC technique was successfully used to study the kinetics of the ROP of ε-CL initiated by different metal-containing initiators such as tributyltin(IV) alkoxides, tin(II) n-butyl L-lactate, tin(II) chloride, dibutyltin(IV) dichloride and tributyltin(IV) chloride [Citation16–18].
Therefore, the objective of this work is to investigate the ROP of ε-CL with Sn(Oct)2/n-hexanol (n-HexOH) initiating system by non-isothermal DSC. The non-isothermal kinetics of this reaction is studied for the first time by isoconversional methods. The effect of Sn(Oct)2/n-HexOH concentration on the ROP of ε-CL is also discussed. The catalysis effect of this Sn(Oct)2/n-HexOH on the synthesis of high molecular weight PCL is investigated by bulk polymerization. Furthermore, the mechanistic aspect for the ROP of ε-CL will be studied and described.
Materials and methods
Materials
ε-Caprolactone (ε-CL) (Acros Organics, 99%) was purified by fractional distillation under reduced pressure. Tin(II) octoate (Sn(Oct2)) (Sigma-Aldrich, 95%) was purified by vacuum distillation before used. n-Hexanol (n-HexOH) (Scharlau, 99%) were dried over sodium metal and purified by distillation before used. Chloroform (Carlo Erba, 99%) and methanol (Carlo Erba, 99%) were used as received.
Methods
Non-isothermal DSC polymerization
Kinetics of the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) were investigated by Perkin-Elmer DSC-7 under non-isothermal condition. The 2 g of ε-CL with Sn(Oct)2/n-HexOH (1:2) was weighed in a dry vial and stirred vigorously for 10 min. For each experiment, about 5–10 mg of reaction mixture was accurately weighed into an aluminum DSC pan. The samples were heated from 20 to 260 °C at heating rates of 5, 10, 15, and 20 °C/min under a flowing nitrogen atmosphere of 20 mL/min without sample mass lost. The obtained data was analyzed and processed by Pyris DSC-7 software.
Theoretical consideration for non-isothermal DSC kinetics analysis
Normally, the ROP of cyclic ester monomer is exothermic reaction similar to other polymerization reaction. Therefore, the kinetic of ROP can be alternatively investigated by measuring the heat released from polymerization using non-isothermal differential scanning calorimetry (DSC) technique. Under non-isothermal condition, the overall rate of reaction can be determined from EquationEquation (1)(1)
(1) [Citation12].
where β is heating rate, dα/dT is polymerization rate, A is frequency factor, Ea is activation energy, R is universal gas constant, T is temperature, α is the fraction of monomer conversion determined from ratio of the released heat at any time and heat of polymerization [Citation17] and f(α) is the rection model or the conversion function. The values of Ea and A can be determined by the peak methods or the isoconversional methods [Citation18]. The dependency of Ea values with monomer conversion obtained from isoconversional methods can be used to investigate the complexity of polymerization such as the single or the multiple reactions [Citation12]. The differential isoconversional method of Friedman [Citation20] allows Ea to be determined at each given conversion degree as shown in EquationEquation (2)(2)
(2) .
where the subscript i denotes the ordinal number of a non-isothermal experiment conducted at the heating rate βi and the subscript α denotes the quantities evaluated at a specific conversion degree. For integral isoconversional method, Kissinger-Akahira-Sunose (KAS) is one of the accurate methods to determine the values of Ea under non-isothermal condition using Murray and White mathematic approximation [Citation13]. The KAS isoconversional method shows the linear relationship between ln(β/T2) and 1/T as shown in EquationEquation (3)(3)
(3) . From KAS plots, the values of Ea at each monomer conversion can be determined from the slope of these plots.
When replacing the Ea values obtained at different monomer conversions from Friedman isoconversional method into the compensation effect as shown in EquationEquation (4)(4)
(4) [Citation12], the value of A from EquationEquation (2)
(2)
(2) can be determined.
where a and b are compensation parameters that can be obtained from the linear least-squares best-fit technique with the knowledge of Ei and lnAi shown in EquationEquation (5)(5)
(5) . When replacement the reaction models (fi(α)) at each heating rate into EquationEquation (5)
(5)
(5) , The values of Ei and lnAi can be determined.
The values of Ei and lnAi can be extracted from slope and intercept of the linear plots between ln[dα/dT)/fi(α)] and 1/T under suitable fi(α) (highest R2 values in the α = 0.2–0.8). When the Ei value shown in EquationEquation (6)(6)
(6) is replaced by E0 which is the average value of Ea obtained from Friedman method combination with a and b values, lnA0 can be determined [Citation12,Citation18]. Thus, the reaction model (f(α)) can be determined by substituting the E0 and A0 into EquationEquation (1)
(1)
(1) resulting in EquationEquation (6)
(6)
(6) .
Synthesis of poly(ε-caprolactone) by bulk polymerization
The ROP of ε-CL with Sn(Oct)2/n-HexOH (1:2) initiating system were carried out in round-bottomed flask with ground-glass joints and magnetic stirring. To investigate the effectiveness of the Sn(Oct)2/n-HexOH, ε-CL (4 g) with 0.1, 0.2, 0.3, 0.4 and 0.5 mol% of Sn(Oct)2/n-HexOH (1:2) were weighted accurately into the reaction flask in a controlled atmosphere glove box under nitrogen gas at room temperature. The flasks were immersed in a pre-heated silicone oil bath at a constant temperatures 140, 160 and 180 °C for 1 h. At the end of polymerization, the flasks were cooled down to room temperature. The crude PCLs were purified by dissolving in chloroform and re-precipitating in cold methanol before drying in a vacuum oven at 45 °C until the constant weight. The chemical structure and the %conversion of the obtained PCLs were characterized by proton nuclear magnetic resonance spectroscopy (1H-NMR) (Bruker Avance 400). The molecular weight averages and polydispersity (PDI) of the purified PCLs were determined by Water e2695 gel permeation chromatography (GPC) at 40°C with refractive index and viscosity detector. Tetrahydrofuran (THF) was used as an eluent with a flow rate of 1.0 mL/min.
Results and discussion
Non-isothermal DSC kinetic analysis for the ROP of ε-caprolactone initiated by tin(II) octoate/n-hexanol
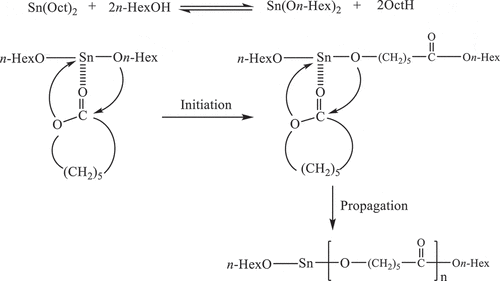
The polymerization measurement under non-isothermal DSC condition provides good kinetic information under different heating rates in a short period of time. For non-isothermal DSC polymerization, the reaction mixtures of ε-CL with 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) are heated from 20 to 300°C at heating rates of 5, 10, 15 and 20°C/min. Non-isothermal DSC curves for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) in the temperature ranges between 100 and 260°C at various heating rates are displayed in . From , it is found that the polymerization exotherms shift to higher temperature range as heating rate increases similar to literatures [Citation17,Citation24,Citation25]. At high heating rate, the curves showed wider and sharper exotherms which are related to the high polymerization rate [Citation16]. From ), the polymerization exotherms obtained from ROP of ε-CL with 1.0 mol% of Sn(Oct)2/n-HexOH (1:2) system are observed at higher temperature range than 1.5 and 2.0 mol%, respectively. This indicates that the initiator concentration affects the polymerization temperature.
Figure 1. Non-isothermal DSC curves for the ROP of ε-CL initiated by SnOct2/n-HexOH (1:2): (a) 1.0, (b) 1.5, (c) 2.0 mol% at heating rates of 5, 10, 15 and 20°C/min and (d) non-isothermal DSC curve for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% Sn(Oct2)/n-HexOH (1:2) at a heating rate of 10°C/min.
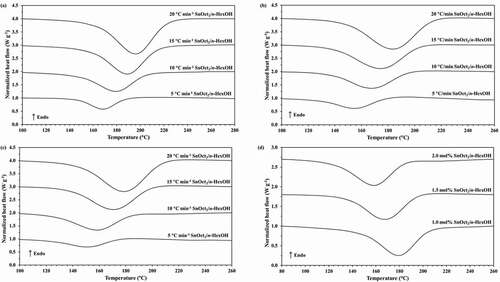
From the obtained results in , the values of monomer conversion (α) and polymerization rate (dα/dt) can be determined and the plots of monomer conversion and polymerization rate for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% Sn(Oct)2/n-HexOH (1:2) against temperature are depicted in . From , it is found that as heating rate increases, the monomer conversion approaches 1 at higher temperature. The monomer conversion for the ROP of ε-CL initiated by 2.0 mol% Sn(Oct)2/n-HexOH (1:2) reaches 1.0 at lower temperature range than 1.5 and 1.0 mol% Sn(Oct)2/n-HexOH, respectively. From , it is found that the polymerization rate of ε-CL with 2.0 mol% of Sn(Oct)2/n-HexOH increases to maximum at lower temperature range than 1.5 and 1.0 mol%, respectively. This indicates that the polymerization rate of ε-CL increases with increasing initiator concentration.
Figure 2. Plots of monomer conversion against temperature for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at heating rates of 5, 10, 15, and 20°C min−1.
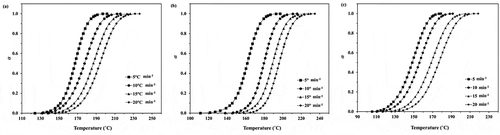
Figure 3. Plots of polymerization rate against temperature for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at heating rates of 5, 10, 15, and 20°C min−1.
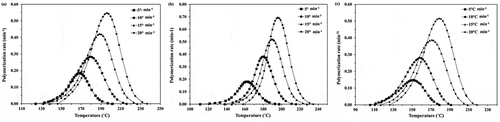
From the obtained results, the values of activation energy (Ea) can be effectively determined by the isoconverisonal methods. The assumption in the isoconversional method is that the effective activation energy solely depends on conversion and not on temperature. In this work, the values of Ea for the whole stage of ε-CL polymerization are investigated from Friedman (EquationEquation (2)(2)
(2) ) and KAS isoconversional method (EquationEquation (3)
(3)
(3) ). The example of KAS plots for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) are presented in and the plots of Ea against monomer conversion are illustrated in .
Figure 4. The KAS plots for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2).
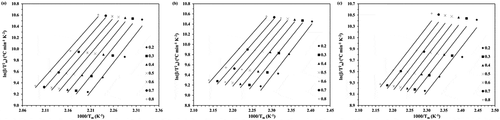
Figure 5. Plots of Ea against monomer conversion obtained from the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2): (a) Friedman and (b) KAS isoconversional method.
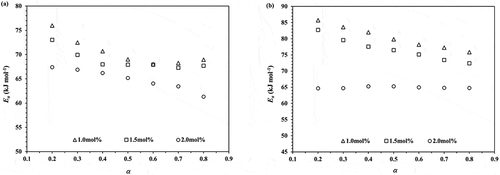
From , the average Ea values for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) obtained from the Friedman isoconversional method are 70.49 ± 2.89, 68.83 ± 2.04 and 64.93 ± 2.15 kJ/mol, respectively. From KAS isoconversional method, the average Ea values are found to be 80.63 ± 3.57, 76.77 ± 3.59 and 64.94 ± 0.27 kJ/mol, respectively. The KAS isoconversional method is more accurate method in the determination of Ea and can be used to evaluate a single or a multiple step reaction process [Citation9,Citation10]. The values of Ea obtained from 2.0 mol% Sn(Oct)2/n-HexOH seem to be more constant for the whole stage of polymerization and are lower than 1.5 and 1.0 mol% Sn(Oct)2/n-HexOH, respectively. The higher rate of polymerization obtained from 2.0 mol% of Sn(Oct)2/n-HexOH is related to the higher amount of true initiator presented in reaction mixture. As described in literature [Citation10], Sn(Oct)2 is not a true active specie. Sn(Oct)2 will react with n-HexOH and convert to tin(II) n-hexoxide (Sn(OnHex)2) before initiates polymerization of ε-CL. This will be further described in the study of polymer mechanism. To support these findings, the values of frequency factor (A) are also calculated by EquationEquation (2)(2)
(2) . It is important that the f(α) is required before calculating the A values for the ROP of ε-CL. Thus, EquationEquation (4)
(4)
(4) can be used to determine the compensation parameter (a and b) with the learning of Ei and lnAi. From the obtained f(α) at different monomer conversions and heating rates, the best-fitting model can be investigated by comparing the obtained values of f(α) with the theoretical f(α) values shown in [Citation12].
Table 1. Ideal reaction mechanism and kinetics model for solid state reaction [Citation12,Citation18,Citation26]
From model fitting method, the Ei and lnAi values can be determined from the slope and the intercept of the plots between ln[(dα/dt)/fi(α)] and 1/T at different heating rates using apparent model that gives the best linearity for α ranges of 0.2–0.8. The apparent reaction model, namely Avrami-Erofeev nucleation model (f(α) = m(1-α)m[-ln(1-α)]m−1/m; m = 1.5 and 2.0) is used to determine compensation parameter [Citation10,Citation21]. From the results, it was found that the Avrami-Erofeev nucleation and growth model (A2) with m = 1.5 yields the best linearity (R2 > 0.99) of plots of ln[dα/dt)/fi(α)] against 1/T for the ROP of ε-CL with Sn(Oct)2/n-HexOH (1:2) as depicted in . The average values of Ei and lnAi for the ROP of ε-CL with 1.0 mol% Sn(Oct)2/n-HexOH are 118.2 kJ/mol and 30.8. For 1.5 mol% of Sn(Oct)2/n-HexOH, the average values of Ei and lnAi are 105.5 kJ/mol and 28.4. For 2.0 mol% of Sn(Oct)2/n-HexOH, the average values of Ei and lnAi are 103.7 kJ/mol and 28.2. The determined compensation parameters for the ROP of ε-CL with Sn(Oct)2/n-HexOH (1:2) are summarized in .
Table 2. Compensation parameters for f(α) = m(1-α)m[-ln(1-α)]m−1/m (m = 1.5 and 2.0) for the ROP of ε-CL with Sn(Oct)2/n-HexOH (1:2) at different heating rates
Figure 6. Plots of f(α) against α for the reaction models shown in (line) and the experimental plots of f(α) against α for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at a heating rate of 5 °C min−1 (dot).
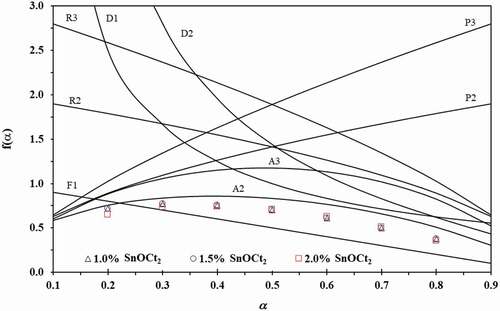
From , the reaction model can be reconstructed based on EquationEquation (6)(6)
(6) by using E0 and A0 obtained from all Sn(Oct)2/n-HexOH. The example plots of f(α) against α for the ROP of ε-CL with all Sn(Oct)2/n-HexOH initiators at a heating rate of 5 °C/min compare to theoretical models are shown in . From the results, it is found that the reconstructed model for the ROP of ε-CL initiated by Sn(Oct)2/n-HexOH approach to A2 model. Therefore, the A2 model will be used in EquationEquation (5)
(5)
(5) to determine the values of A. The average values of A for the ROP of ε-CL with 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH are 7.3x107, 2.8x106 and 1.2x106 min−1, respectively. From the obtained kinetics results, it clearly demonstrates that the concentration of Sn(Oct)2/n-HexOH affects the kinetics of polymerization. The reactivity of 2.0 mol% Sn(Oct)2/n-HexOH in the ROP of ε-CL is higher than 1.5 and 1.0 mol% Sn(Oct)2/n-HexOH, respectively.
Polymerization mechanism and synthesis of poly(ε-caprolactone) via bulk polymerization
The polymerization of ε-CL with Sn(Oct)2/n-HexOH is proposed through coordination mechanism as depicted in Scheme 1. In the first step, tin(II) alkoxide as Sn(On-Hex)2 is formed by the exchange reactions between Sn(Oct)2 and n-HexOH [Citation9–11]. After formation of true Sn(On-Hex)2 initiator, the initiation step is started by the coordination of carbonyl oxygen of ε-CL with tin atom. After that, the nucleophilic attack of – On-Hex group to carbonyl carbon of ε-CL resulting in the acyl-oxygen bond cleavage of ε-CL ring. From this process, the propagating species (-SnO-) is formed and the propagation step proceeds by the insertion of ε-CL into reactive Sn-O bond presented in these active species.
To confirmed the efficiency of Sn(Oct)2/n-HexOH in the synthesis of PCLs, the bulk polymerizations of ε-CL with Sn(Oct)2/n-HexOH are conducted at 140, 160 and 180°C for 1 h. After complete polymerization time, the obtained crude PCLs are dissolved in CHCl3 and re-precipitation in cold methanol. The molecular weight averages and polydispersity index (PDI) are determined by GPC technique and the results are summarized in . From , it is found that Sn(Oct)2/n-HexOH can control the molecular weight of PCL by adjusting their concentration. The molecular weight of PCL increases with decreasing of Sn(Oct)2/n-HexOH concentration. The highest molecular weight of PCL (of 9.0 × 104 g/mol) is obtained at 0.1 mol% of Sn(Oct)2/n-HexOH and temperature of 160°C. Furthermore, the %yield of all synthesized PCLs are higher than 78%. Based on the obtained results, Sn(Oct)2/n-HexOH acts as highly efficient initiator in the synthesis of high molecular weight PCL.
Table 3. Number average molecular weight (), weight average molecular weight (
), polydispersity index (PDI) and %yield of PCLs from bulk polymerization of ε-CL initiated by Sn(Oct)2/n-HexOH at 140, 160 and 180 °C for 1 h
Conclusions
The kinetics of the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) was successfully studied by non-isothermal DSC technique. The isoconversional and model fitting methods were applied to determine the Ea and A values for the ROP of ε-CL with Sn(Oct)2/n-HexOH. From Friedman and KAS isoconversional kinetics analysis, the Ea values for the ROP of ε-CL with 2.0 mol% of Sn(Oct)2/n-HexOH were lower than 1.5 and 1.0 mol%, respectively. The average values of A for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH are 7.3x107, 2.8x106 and 1.2x106min−1, respectively. The reactivity of Sn(Oct)2/n-HexOH in the ROP of ε-CL was increased with increasing their concentration. From polymer synthesis via bulk polymerization, the molecular weight of PCL was controlled by Sn(Oct)2/n-HexOH concentration. Sn(Oct)2/n-HexOH could produce high molecular weight PCL with high %yield in 1 h. The mechanism of the ROP of ε-CL with Sn(Oct)2/n-HexOH was proposed through the coordination-insertion mechanism. The results from this work may be applied to study the catalytic performance of others initiator in the ROP of cyclic esters.
Acknowledgments
The authors wish to thank the financial support from the University of Phayao, Chiang Mai University and the Thailand Research Fund. The Unit of Excellence on Advanced Nanomaterials, School of Science, University of Phayao, the Department of Chemistry and Materials Science Research Center, Faculty of Science, Chiang Mai University and Faculty of Sciences and Agricultural Technology, Rajamangala University of Technology Lanna are also acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem Soc Rev. 2009;38:3484–3504.
- Sharma S, Aiswarya TT, Mirza I, et al. Biocompatible polymers and its applications. Ref Module Mater Sci Mater Eng. 2020. DOI:https://doi.org/10.1016/B978-0-12-820352-1.00044-4.
- Luckachan GE, Pillai CKS. Biodegradable polymers- A review on recent trends and emerging perspectives. J Polym Environ. 2011;19:637–676.
- Potrc T, Baumgartner S, Roskar R, et al. Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur J Pharm Sci. 2015;75:101–113.
- Kleawla A, Suksomran W, Charuchinda A, et al. Kinetic studies if the ring-opening bulk polymerization of ε-caprolactone using a novel Tin(II) alkoxide initiator. J Solid Mech Mater Eng. 2007;1(4):613–623.
- Stjerndahl A, Wistrand AF, Albertsson AC. Industrial utilization of tin-initiated resorbable polymers: synthesis on a large scale with a low amount of initiator. Biomacromolecules. 2007;8:937–940.
- Albertsson A-C, Gruvegård M. Degradable high-molecular-weight random copolymers, based on ε-caprolactone and 1,5-dioxepan-2-one, with non-crystallizable units inserted in the crystalline structure. Polymer. 1995;36:1009–1016.
- Kricheldof HR, Kreiser-Saunder I, Sticker A. Polylactones 48. SnOct2-initiated polymerizations of lactide: a mechanistic study. Macromolecules. 2000;33:702–709.
- Kowalski A, Duda A, Penczek S. Mechanism of cyclic ester polymerization initiated with tin(II) octoate. 2. Macromolecules fitted with tin(II) alkoxide species observed directly in MALDI-TOF spectra. Macromolecules. 2000;33(3):689–695.
- Kowalski A, Duda A, Penczek S. Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate, 1. Polymerization of ε-caprolactone. Macromol Rapid Commun. 1998;19:567–572.
- Duda A, Penczek S, Kowalski A, et al. Polymerizations of ε-caprolactone and L,L-dilactide initiated with tin octoate and tin butoxide a comparison. Macromol Symp. 2000;153:41–53.
- Vyazovkin S, Burnham AK, Criado JM, et al. ICTAC Kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
- Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–176.
- Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Std. 1956;57:217–221.
- Akahira A, Sunose T. Trans joint convention of four electrical institutes. Res Report Chiba Inst Technol (Sci Technol). 1971;16:22–31.
- Limwanich W, Khunmanee S, Kungwan N, et al. Effect of tributyltin alkoxides chain length on the ring-opening polymerization of ε-caprolactone: kinetics studies by non-isothermal DSC. Thermochim Acta. 2015;599:1–7.
- Punyodom W, Limwanich W, Meepowpan P. Tin(II) n-butyl L-lactate as novel initiator for the ring-opening polymerization of epsilon-caprolactone: kinetics and aggregation equilibrium analysis by non-isothermal DSC. Thermochim Acta. 2017;655:337–343.
- Limwanich W, Meepowpan P, Kungwan N, et al. Influence of butyl group of tin chloride initiators on the non-isothermal DSC ring-opening polymerization of ε-caprolactone: the studies of kinetics, mechanism and polymer synthesis. Themochim Acta. 2020;638:178458.
- Li P, Zerroukhi A, Chen J, et al. Kinetics study of Ti[O(CH2)4OCH=CH2]4 initiated ring‐opening polymerization of ε‐caprolactone by differential scanning calorimetry. J Appl Polym Sci. 2008;110:3990–3998.
- Friedman H. Kinetics of thermal degradation of char forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Polym Symp. 1964;6(1):183–195.
- Sbirrazzuoli N. Is the Friedman method applicable to transformations with temperature dependent reaction heat? Chem Phys. 2007;208:1592–1597.
- Ozawa TA. New method of analyzing thermogravimetric data. Bull Chem Soc Japan. 1965;38:1881–1886.
- Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Standards. 1966;70A:487–523.
- Sedush NG, Chvalun SN. Kinetics and thermodynamics of l-lactide polymerization studied by differential scanning calorimetry. Eur Polym J. 2015;62:198–203.
- Meelua W, Molloy R, Meepowpan P, et al. Isoconversionalkinetic analysis of ring-opening polymerization of ε-caprolactone: steric influence of titanium(IV) alkoxides as initiators. J Polym Res. 2012;19:1–11.
- Pham QT, Zhan YX, Wang FM, et al. Mechanisms and kinetics of non-isothermal polymerization of N, N ‘-bismaleimide-4,4 ‘-diphenylmethane with barbituric acid in dimethyl sulfoxide. Thermochim Acta. 2019;676:139–144.