Abstract
Nitrogen isotope (δ15N) has been employed to differentiate major sources of atmospheric N. However, it remains a challenge to quantify contributions of multiple sources based on δ15N values of the N mixture in atmospheric samples. This study measured δ15N of bulk N in PM2.5 at an urban site of Beijing during a severe haze episode of 22–30 January 2013 and a background site of Qinghai, north-western China from 6 September to 15 October 2013, then applied a Bayesian isotope mixing model (SIAR, Stable Isotope Analysis in R) to analyse the N sources. At Beijing site, δ15N values of PM2.5 (−4.1‰ to +13.5‰, +2.8 ± 6.4‰) were distributed within the range of major anthropogenic sources (including NH3 and NO2 from coal combustion, vehicle exhausts and domestic wastes/sewage). At Menyuan site, δ15N values of PM2.5 (+8.0‰ to +27.9‰, +18.5 ± 5.8‰) were significantly higher than that of potential sources (including NH3 and NO2 from biomass burning, animal wastes, soil N cycle, fertilizer application and dust N). High molar ratios of to
and/or
in PM2.5 at the background site suggested that the equilibrium of NH3 ↔
caused apparent 15N enrichments in ammonium. Results of the SIAR model showed that 39 and 32% of bulk N in PM2.5 of Beijing site were contributed from N emissions of coal combustion and vehicle exhausts, respectively, whereas N in PM2.5 at Menyuan site was derived mainly from N emissions of biomass burning (46%) and NH3 volatilization (34%). These results revealed that the stoichiometry between NH3 and acidic gases plays an important role in controlling the bulk δ15N signatures of PM2.5 and emissions of reactive N from coal combustion and urban transportation should be strictly controlled to advert the risk of haze episodes in Beijing.
1. Introduction
Urban air pollution is a globally challenging issue. Nitrogen (N) emissions play a key role in the formation of atmospheric particulates, especially secondary N-containing aerosols (Huang et al., Citation2014). Ammonia is the precursor of ammonium () and readily reacts with available acids formed by SO2 and NO2, and also it can be transformed to organic N or amines (Ge et al., Citation2011). Nitrogen oxides are major precursors of both inorganic (as nitrate ions (
)) and organic (as organic
) N aerosols (Berkemeier et al., Citation2016). Therefore, the source apportionment of N in PM2.5 is always of significance for better understanding origins of particulates and haze pollution (Guo et al., Citation2014).
Stable isotopes of N (i.e. δ15N values) have been used to trace major sources and processes of atmospheric N (Heaton, Citation1986; Michalski et al., Citation2004; Kendall et al., Citation2007; Pavuluri et al., Citation2010; Savarino et al., Citation2013). The analysis of bulk δ15N in PM2.5 is a quick method compared to δ15N measurements of inorganic and organic N components (Widory, Citation2007; Hegde et al., Citation2015; Bikkina et al., Citation2016), and it does also provide valuable information on δ15N of dry N deposition (Yeatman et al., Citation2001a; Heaton et al., Citation2004; Elliott et al., Citation2007, Citation2009). At a background site, the δ15N of PM2.5 allows us to examine the impacts of emissions from non-point sources’ agricultural N emissions on the N chemistry of regional atmosphere, whereas at an urban site, it can imprint the anthropogenic N emissions.
The bulk δ15N in atmospheric particulates is mainly determined by the δ15N of N precursors (Aggarwal et al., Citation2013; Hegde et al., Citation2015). Often, reported δ15N values of typical inorganic N sources (Table ) can be used in studies on sources and fates of atmospheric N (Elliott et al., Citation2007, Citation2009; Kendall et al., Citation2007; Kawashima and Kurahashi, Citation2011; Michalski et al., Citation2014). For PM2.5, dust is a primary N source (Zhang, Citation2010; Huang et al., Citation2014). At background site, NO2 and/or NH3 from microbial N cycle, fertilization application and animal wastes are strongly 15N-depleted (Elliott et al., Citation2007; Li and Wang, Citation2008; Felix et al., Citation2014), while N emissions from biomass burning (Kawashima and Kurahashi, Citation2011; Divers et al., Citation2014) are typically 15N-enriched. At urban site, most N sources of PM2.5 are anthropogenic. The NH3 from animal wastes (including sewages; Heaton, Citation1986), coal combustion and vehicle exhausts (Felix et al., Citation2013), as well as NO2 from vehicle exhausts (Walters et al., Citation2015) showed negative δ15N values, but NO2 from coal combustion had exclusively positive δ15N values (Felix et al., Citation2012).
Table 1. δ15N values (mean ± SD) reported for major NOx and NH3 emissions in the atmosphere.
Besides, the bulk δ15N in atmospheric particulates is influenced by the isotopic fractionations during gas (g) ↔ particle (p) exchange processes. However, isotope effects between N precursors and the aerosol N remain unclear, especially in field conditions. Isotope effects are assumed to be more significant between NH3 and , and much smaller in the case of NO2 and aerosol N (Yeatman et al., Citation2001a; Kawashima and Kurahashi, Citation2011). This assumption was supported by small differences in mean δ15N values between roadside NO2 (5.7‰) and local aerosol N (6.8‰) (Ammann et al., Citation1999; Pearson et al., Citation2000). Although the kinetic isotope effect of NH3 →
reaction is small at the beginning, it becomes significant when NH3 ↔
equilibrium attains and causes a preferential enrichment of 14N in NH3 and 15N in
of aerosols (Heaton et al., Citation1997; Fukuzaki and Hayasaka, Citation2009; Li et al., Citation2012). This explained generally higher δ15N values of
in aerosols than that in rain
and gaseous NH3 (Yeatman et al., Citation2001a, Citation2001b; Jia and Chen Citation2010; Felix et al., Citation2013). In a hypothetical model by Heaton et al. (Citation1997), the δ15N of particulate
stabilized at values of 33‰ (an enrichment coefficient) higher than that of NH3 when NH3 ↔
equilibrium was achieved at 25 °C. However, mechanisms for atmospheric NH3 ↔
equilibrium in the field circumstances are poorly understood, which is particularly important for interpreting the δ15N variations of PM2.5 at locations dominated by
-N.
This study measured bulk δ15N of PM2.5 at an urban site (Chinese Research Academy of Environmental Sciences (CRAES), Beijing, northern China) and a national atmospheric background monitoring station (Menyuan, Qinghai province, northwestern China). Based on bulk δ15N of PM2.5 and major N sources, a Bayesian isotope mixing model (SIAR, Stable Isotope Analysis in R) (Parnell and Jackson, Citation2008) was used to estimate the proportions of different source contributions to N in PM2.5 and to evaluate anthropogenic N emissions during the haze events in Beijing. As inorganic N in the atmosphere of both sites was dominated by NH4-N, we hypothesized that 15N enrichments in PM2.5 relative to dominant sources were mainly derived from the NH3 ↔ equilibrium (assumed as 33‰) (Heaton et al., Citation1997; Li et al., Citation2012).
2. Materials and methods
2.1. Study sites
The sampling site in Beijing is located in the courtyard of CRAES (40°04′ N, 116°42′ E), at Lishuiqiao South of Beiyuan Road. Due to rapid urbanization and economic development, the vehicle exhausts and energy consumption are large in Beijing, resulting in deterioration of air quality. Atmospheric PM2.5 in Beijing was characterized by high contributions of secondary components from anthropogenic origins (Sun et al., Citation2006). Secondary inorganic ions (such as ,
and
) were the dominant contributors in PM2.5 of Beijing (Zhang et al., Citation2013). During the sampling period (January 2013), Beijing suffered from the worst PM2.5 pollutions in history (http://cleanairinitiative.org/portal/node/11599), registering the highest PM2.5 hourly concentration of 886 μg/m3 (http://www.nasa.gov/multimedia/imagegallery/imagefeature2425.html).
The background site is located on the Daban Mountain (37°36′ N, 101°15′ E) in Menyuan county, north-eastern of Qinghai province, which is one of the 14 National Background Stations established by the Chinese Ministry of Environmental Protection in 2012. It has a typical Plateau continental climate, with an altitude of 3295 m above sea level, lower than the average of the Tibetan Plateau (about 4000 m). The mean annual temperature and precipitation amount are 0.8 °C and 520 mm, respectively. Agricultural activity is not intensive locally, except in low-altitude areas far away from the Daban Mountain in Menyuan. The sampling period (6 September–15 October 2013) is within the harvesting period after an intensive fertilization and pronounced biomass burning. The mean hourly temperature was 6.5 °C (3–11 °C) during the study period. There is no fossil fuel emission locally, with limited road traffic on the national highway of G227.
2.2. Sample collection and chemical analyses
PM2.5 was collected using a pre-baked quartz filter (47 mm in diameter) and aerosol sampler (Leckel, MVS6, Germany) equipped with a size-segregating impactor. The operating air flow rate was 38.3 L/min. To collect sufficient PM2.5 sample for bulk δ15N analyses, sampling was conducted for every 47–71 h at Menyuan (n = 14) and for 23 h at Beijing (n = 14). Filter blanks were also collected following the same procedure. The PM2.5 mass on each filter was gravimetrically measured using microbalance (AWS-1, COMDE DERENDA, Germany, approved by European Standard) after being desiccated for at least 24 h under controlled temperature (20 ± 1 °C) and humidity (50 ± 5%). All filter samples were immediately stored at −20 °C prior to chemical analyses.
Concentrations of bulk N in PM2.5 (mainly including ,
and organic N) were measured using three punches (ca. 0.53 cm2 for each) of the filter in a vario MACRO cube (Elementar Analysensysteme GmbH, Germany) with an analytical precision of 0.02%. Based on N contents, bulk δ15N values of about 50 μg N in each PM2.5 sample were determined by a Thermo MAT 253 isotope ratio mass spectrometer (Thermo Scientific, Bremen, Germany) coupled with an elemental analyzer (Flash EA 2000). IAEA-N-1 (Ammonium sulphate; δ15N = 0.4‰), USGS25 (Ammonium sulphate, δ15N = −30.4‰) and IAEA-NO-3 (Potassium nitrate; δ15N = +4.7‰) were used as standards for the calibration of δ15N values. The average standard deviation for replicate analyses of an individual sample was ±0.1‰. The δ15N in PM2.5 was expressed in parts per thousand (per mille) by multiplying them by 1000:
where R = 15N/14N for samples and standard (atmospheric N2).
The concentrations of ,
and
in PM2.5 were measured during the sampling period at both sites by an ambient ion monitor (AIM-IC system: Model URG 9000B, URG Corporation, USA). It draws air in through a PM2.5 sharp-cut cyclone at a volumetric flow controlled rate of 3 L/min to remove the larger particles from the air stream. The real-time instruments installed at both the stations have a detection limit of 0.05 μg/m3. Gases such as SO2, NH3 and HNO3 are stripped from the air stream by passing through a liquid parallel plate denuder with continuously replenished solvent flowing across the surface. Then, the PM2.5 air stream is constrained into a supersaturated steam condensation coil and cyclone assembly and grown hygroscopically for collection. Enlarged particles are dissolved in water solutions for anion chromatographic analysis every hour following 60 min of ambient sampling. Concentrations of NO2 were measured using a NO–NO2–NOx chemiluminescence analyzer (Model 42i, Thermo-Fisher Scientific). The instruments were operated and maintained properly to ensure data integrity. Scheduled quality control procedures included daily zero and span checks, weekly precision checks and data validations.
3. Results
The PM2.5 levels at Beijing varied from 43.0 to 433.6 μg/m3 (mean = 264.3 ± 118.0 μg/m3) (Tables and S2). Volumetric concentrations of elements and ions in PM2.5 differed distinctly between the two study sites, thus N contents were presented in the unit of N mass in PM2.5 mass for comparison. The bulk N and δ15N values of PM2.5 at Beijing averaged 16.7 ± 4.6% (8.2% to 29.3%) and +2.8 ± 6.4‰ (−4.1‰ to +13.5‰), respectively (Tables and S2; Fig. ). The -N,
-N and
-S in PM2.5 at Beijing averaged 7.4 ± 3.4%, 5.0 ± 3.0% and 5.5 ± 2.4%, respectively. The mean molar ratio of
to (
) was 0.8 (Table ). Ambient concentrations of NO2 (this study), NH3 (during April of 2013) and SO2 (during January of 2013) averaged 89.2 ± 21.2 μg/m3, 14.1 μg/m3 and 22.9 μg/m3 (He et al., Citation2014; Wei et al., Citation2015), respectively, showing a mean molar ratio of ambient NH3 to (NO2 + 2 * SO2) of 0.3 (Table ).
Fig. 1. δ15N values of bulk N in PM2.5 and dominant N sources assigned for PM2.5 at the Beijing CRAES site (in red) and the Menyuan site (in blue). The box encompasses the 25th–75th percentiles, whiskers are SD values. The line and square in each box mark the median and arithmetic mean values, respectively. The number of jittered replicate δ15N data (dots around the boxes) is 1–34. Mean and SD values of source δ15N data were used in the SIAR model. δ15N values of N from dust were assumed as those of surface soils (Wang et al., Citation2014) according to the air mass backward trajectories (Fig. ).
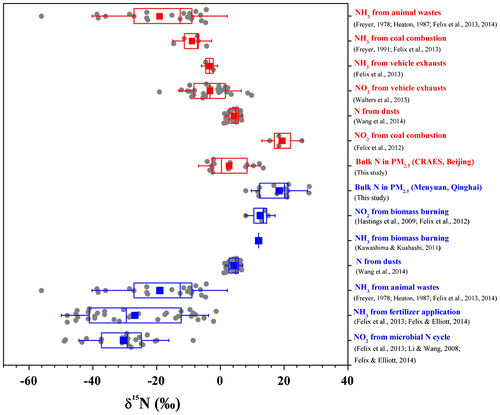
Table 2. Mass concentrations of inorganic N ( –N plus
–N plus  –N),
–N),  –S, bulk N, molecular ratios of
–S, bulk N, molecular ratios of  to
to  ,
,  to
to  ,
,  to (
to ( ) in PM2.5 at Beijing (CRAES site) and a background site (Menyuan, Qinghai province) of China. Data of ambient NH3 and SO2 at Beijing site were cited from Carmichael et al. (Citation2003), He et al. (Citation2014), Wei et al. (Citation2015). Data of NH3 and SO2 were cited from the background site of Waliguan in Qinghai Province (Carmichael et al., Citation2003).
) in PM2.5 at Beijing (CRAES site) and a background site (Menyuan, Qinghai province) of China. Data of ambient NH3 and SO2 at Beijing site were cited from Carmichael et al. (Citation2003), He et al. (Citation2014), Wei et al. (Citation2015). Data of NH3 and SO2 were cited from the background site of Waliguan in Qinghai Province (Carmichael et al., Citation2003).
The filter-based average concentrations of PM2.5 at the background site (Menyuan, Qinghai province) varied from 7.0 to 17.8 μg/m3 (mean = 13.0 ± 3.2 μg/m3) (Tables and S2), and (13.0 ± 4.8 μg/m3; 4.6–22.7 μg/m3) measured using an ambient monitor (AIM-IC system: Model URG 9000B, URG Corporation, USA). The bulk N concentrations and δ15N values of PM2.5 at the background site were 8.6 ± 5.6% and +18.5 ± 5.8‰ (+8.0‰ to +27.9‰), respectively (Tables and S1; Fig. ). Concentrations of -N,
-N and
-S in PM2.5 at Menyuan averaged 5.9 ± 1.8%, 1.9 ± 0.4% and 0.2 ± 0.0%, respectively (Table ), showing a mean molar ratio of
to (
) as 2.9 ± 1.0 (Table ). Ambient concentrations of NO2 averaged 4.3 ± 1.3 μg/m3 at the background site (Table ). Ambient NH3 and SO2 concentrations were not available at the Menyuan site (37°36′ N, 101°15′ E; 3295 m); however, these concentrations were reported as 4.8 μg/m3 and 0.31 μg/m3, respectively, at Waliguan (a global baseline station, 36°30′ N, 100°10′E, 3816 m), another background site in Qinghai (Carmichael et al., Citation2003). The estimated molar ratio of ambient NH3 to (NO2 + 2 * SO2) averaged 2.7 at the background site (Table ).
4. Discussion
4.1. Major sources of N in PM2.5 of Beijing
According to the source appointment of PM2.5 at Beijing during the severe haze episode of January 2013 (Huang et al., Citation2014; Zhang et al., Citation2015), the following six dominant sources can be assigned for bulk N of PM2.5.
S1: N from dust,
S2: NO2 from coal combustion,
S3: NH3 from coal combustion,
S4: NO2 from vehicle exhausts,
S5: NH3 from vehicle exhausts,
S6: NH3 from animal wastes (mainly domestic wastes and sewages).
It should be explained that NO is the initial precursor for NOx emission sources, but NO is quite reactive and readily oxidized to NO2 which is more often taken as the precursor of in the atmosphere. Thus, NO2 was used in this work uniformly and its δ15N values were assumed as those of corresponding NOx emissions.
In this study, agricultural and biogenic N emissions were not considered as the major sources of bulk N in PM2.5 of Beijing for two main reasons. First, the urban site is located in the centre of Beijing city cluster in CRAES. During the severe haze events occurring in Beijing, several studies have shown that aerosols have been mainly influenced by anthropogenic sources. Second, as the sampling of Beijing PM2.5 was conducted in the winter time, contributions of NO2 from microbial N cycle, NH3 emission from seawater (δ15N = −8‰ to −5‰ in Jickells et al., Citation2003) and lightening NOx (δ15N = −0.5‰ to +1.4‰; Hoering, Citation1957) were quite small, with relatively lower contribution than anthropogenic N sources to the formation of near-surface PM2.5, especially in urban circumstances.
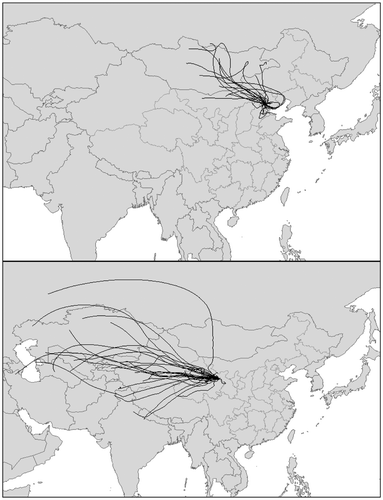
To date, δ15N values of various NO2 and NH3 emissions are unavailable in China. However, according to source δ15N data compiled from previous studies (Table , Fig. ), δ15N values were distinctive between most typical sources, which have been broadly used in isotopic tracing or partitioning of atmospheric N deposition (e.g. Elliott et al., Citation2007, Citation2009; Kawashima and Kurahashi, Citation2011). In this study, we did not use δ15N data of emissions influenced by post-emission processes and measured through controlled tests or simulation, e.g. the δ15N values of NH3 near highway (−5.0‰ to +0.4‰ in Smirnoff et al., Citation2012), NO2 near highway (+2‰ to +10‰ in Moore, Citation1977; Ammann et al., Citation1999; Pearson et al., Citation2000; −13.3‰ to +0.4‰ in Smirnoff et al., Citation2012), NO2 in tunnels (+15.0 ± 1.6‰ for NO2; +5.7 ± 2.8‰ for HNO3; Felix et al., Citation2014), NO2 from vehicle engine (−13.0‰ to +3.7‰; Moore, Citation1977; Freyer, Citation1978, Citation1991; Heaton, Citation1990), NO2 from controlled experiments of diesel combustion (+3.9‰ to +5.4‰; Widory, Citation2007) and coal combustion (−5.3‰; Widory, Citation2007). According to the air mass backward trajectories (Fig. ), the δ15N values of surface soils in northern China (+4.3 ± 1.8‰; Wang et al., Citation2014) were used as the value of N from dust in this study.
Fig. 2. Seventy-two-h air mass backward trajectories for all sampling dates at the Beijing CRAES site and the Menyuan site, based on NOAA HYSPLIT model back trajectories.
As bulk δ15N values of PM2.5 at Beijing were distributed within those of major sources (Fig. ), no substantial isotopic effect between N sources and bulk N of PM2.5 at Beijing was assumed. In particular, as inorganic N of PM2.5 was dominated by (with a mean molar ratio of
to
of 2.5; Table ), the isotope effect of NH3 ↔
equilibrium is considered quite low in the PM2.5 of Beijing. First, the low molar ratios of ambient NH3 to (NO2 + 2 * SO2) as 0.3 (Table ) reflected a relatively thorough neutralization of NH3 by acidic gases, producing relatively more stable ammonium salts of NH4NO3, NH4HSO4 and (NH4)2SO4. Second, the molar ratios of
to (
) were calculated as 0.8 (Table ), indicating a full fixation of
by existing
and
for PM2.5 of Beijing. In the calculation,
is the actual molar concentrations of
in PM2.5 while the (
) in PM2.5 represents the concentrations of
that can be fixed by
and
. More often, due to the high emissions of anthropogenic SO2 and NO2 in urban environments, NH3, after converting to
, reacts mainly with acids formed by SO2 and NO2, with little opportunity of NH3 losses from PM2.5; thus, no substantial 15N enrichment in
of PM2.5 (Yeatman et al., Citation2001a; Pavuluri et al., Citation2010; Kawashima and Kurahashi, Citation2011) is observed. Consequently, bulk δ15N values of PM2.5 at Beijing were mainly controlled by the mixing of N sources with inappreciable isotopic effects.
4.2. Major sources of N in PM2.5 of Menyuan
According to the molar ratios of ambient NH3 to NO2 (ca. 3.0) or to
(ca. 3.3) in PM2.5 at Menyuan (Table ), inorganic N in both ambient atmosphere and PM2.5 were dominated by NH3 and
, respectively. Moreover, δ15N values of PM2.5 did not assemble those of dust N and/or natural N (mainly NO2 from N cycle) emissions; instead, they were much higher than those of potential sources (Table , Fig. ). More likely, agricultural and biogenic NH3 sources should be important to bulk N of the background PM2.5. Hence, we assigned major N sources of PM2.5 at the background site as follows:
S7: N from dust,
S8: NO2 from biomass burning,
S9: NH3 from biomass burning,
S10: NH3 from animal wastes,
S11: NH3 from fertilizer application,
S12: NO2 from microbial N cycle.
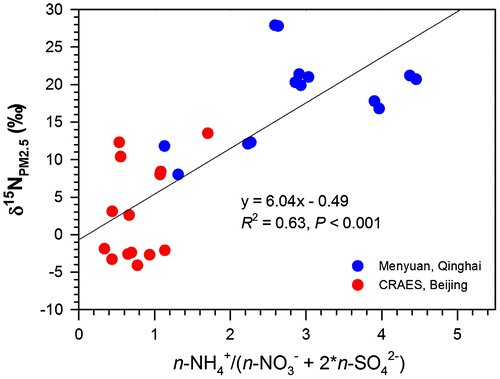
The stoichiometry between ambient NH3 and acidic gases (NO2 + 2 * SO2), and (
) in PM2.5 allowed us to further interpret different patterns of bulk δ15N values of PM2.5 against those major sources (Figs. and ). At Menyuan, the molar ratios of ambient NH3 to (NO2 + 2 * SO2) averaged 2.7 (Table ), illustrating an incomplete neutralization of ambient NH3 by NO2 and SO2. Molar ratios of
to (
) in PM2.5 (ca. 2.9; Table ) also suggested that part of
existed as relatively less stable ammonium salts (e.g. NH4Cl). The diffusion of NH3 back to the atmosphere during the reversible reaction and strong equilibrium between NH3 and
caused significant 15N enrichment in
of PM2.5, thus exhibiting much higher δ15N values of PM2.5 than potential sources (Fig. ). The regulation of acidic gases-to-NH3 stoichiometry on the reaction and isotopic effect between NH3 and
was supported by a positive correlation between δ15N values and
) ratios in PM2.5 (Fig. ). Accordingly, a net isotopic effect of NH3 (g) ↔
(p) at equilibrium (εeq) (33‰; Heaton et al., Citation1997) was considered in the SIAR model for the background PM2.5 (details down in Section 4.2). However, it should be noted that isotope effects for the atmospheric NH3(g) ↔
(p) equilibrium in the field circumstances remain unclear. The value of 33‰ is the only empirical one for 15N enrichment in particulate
(Heaton et al., Citation1997). Experimental studies have been conducted on the isotope fractionations of NH3 volatilization (e.g. Li et al., Citation2012), but it is uncertain what factors can be used to make corrections of the isotope effects for background PM2.5. Further studies are strongly needed to verify the relationships between the isotope effects and the ratio of NH3(g) to
(p), which may be a feasible factor to make a correction of the isotope effects.
4.3. Using the SIAR model to partition bulk N in PM2.5
The proportional contributions (F, %) of major sources to N in PM2.5 are estimated using the SIAR model. This model uses a Bayesian framework to establish a logical prior distribution based on Dirichlet distribution (Evans et al., Citation2000), and then to determine the probability distribution for the contribution of each source to the mixture (Parnell and Jackson, Citation2008). It can substantially incorporate the uncertainties associated with multiple sources, fractionations and isotope signatures (Moore and Semmens, Citation2008; Davis et al., Citation2015). In our estimations, uncertainties should be evaluated for the δ15N variabilities of bulk N in PM2.5 and N sources, isotopic effect of the NH3 (g) ↔ (p) equilibrium.
By defining a set of N mixture measurements on J isotopes with K source contributors, the mixing model can be expressed as follows (Parnell et al., Citation2010):
where all F values sum to 1 (unity), Xij is the isotope value j of the mixture i, in which i = 1, 2, 3, …, N and j = 1, 2, 3, …, J; Sjk is the source value k on isotope j (k = 1, 2, 3, …, K) and is normally distributed with mean μjk and standard deviation ωjk; Fk is the proportion of source k estimated by the SIAR model; cjk is the fractionation factor for isotope j on source k and is normally distributed with mean λjk and standard deviation τjk; and εij is the residual error representing the additional unquantified variation between individual mixtures and is normally distributed with mean 0 and standard deviation σj. A detailed description of this model can be found in Moore and Semmens (Citation2008), Jackson et al. (Citation2009) and Parnell et al. (Citation2010). To estimate the contributions of N sources in the PM2.5 samples at two study sites (n = 14 for each), one isotope (j = 1) (δ15N of bulk N) and six potential N sources (as discussed in Sections 4.1 and 4.2: S1–S6 for Beijing and S7–S12 for Menyuan) (Fig. ) are utilized. δ15N values of replicate PM2.5 samples at each study site were analysed in the SIAR model as one group.
Our estimation showed that the contribution of NO2 () reached 41 ± 11% in bulk N of PM2.5 in Beijing, which was much higher than
at the background site (22 ± 10%) (Table ). The mean ratios of FNH3 to
were about 1.6 and 4.4 for PM2.5 at Beijing and at the background site, respectively (Table ), which generally followed the molar ratios of
to
in PM2.5 (Table ). Aqueous phase reaction experiments have shown that atmospheric NO2 and NH3 potentially react with organic compounds to form organic N (Ge et al., Citation2011; Pavuluri et al., Citation2015), which might contribute to the high secondary organic aerosols during the study haze event in Beijing (Huang et al., Citation2014).
Table 3. Fractional contributions (F, %) of dominant N precursors and sources to bulk N in PM2.5 at Beijing (CRAES site) and a background site (Menyuan, Qinghai province) of China. Values (mean ± SD; n = 104) were calculated based on the output of the SIAR model.
In Beijing, anthropogenic N in PM2.5 averaged 81% of its bulk N and was mainly derived from N emissions of fossil fuel combustions, with the highest contribution (ca. 25%) from NO2 of coal combustion (Table ; Fig. ). The N emissions from coal combustion showed higher contributions (ca. 39%) than traffic emissions (ca. 32%), fossil-derived NO2 contributed more N (ca. 39%) than fossil-derived NH3 (ca. 30%) (Table ; Fig. ). Comparable contributions (ca. 14–16%) were observed between NH3 from coal combustion and NH3 from traffic emissions, between NH3 and NO2 from vehicle exhausts (Fig. ). Accordingly, fossil-derived NH3 emissions substantially contributed to urban PM2.5 pollution; regulatory controls of N emissions from coal combustion and urban transportation are important to advert the risk of severe haze episodes in Beijing.
Fig. 4. Fractional contributions of dominant N sources to bulk N in PM2.5 at the Beijing CRAES site and the Menyuan site. Dots around the boxes (n = 104) show the percentages estimated by the SIAR model. The box encompasses the 25th–75th percentiles, whiskers are the 5th and 95th percentiles. The line and cross in each box mark the median and arithmetic mean values, respectively.
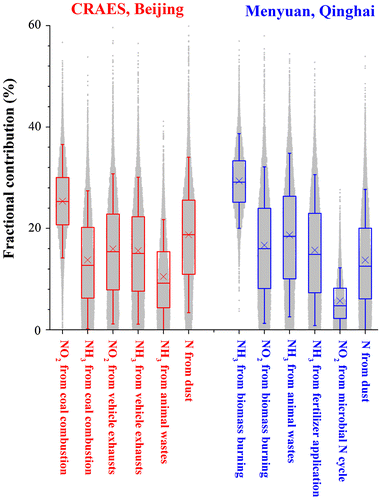
The N in PM2.5 at the background site was mainly contributed by N emissions from biomass burning (46 ± 10%) and NH3 volatilization (34 ± 12%) (Table ). The contribution of NH3 from biomass burning (29 ± 6%) was comparable with the total contributions of NH3 from animal wastes and fertilizer application (ca. 35%) (Table ). Biomass burning contributed less N as NO2 (17 ± 10%) than as NH3 (29 ± 6%) to N of PM2.5 at the background site (Table ). Higher production of NH3 than NO2 from biomass burning has been documented previously (Crutzen and Andreae, Citation1990). A burning experiment by Lobert et al. (Citation1990) showed higher emission ratios of NH3 (ca. 3.8%) than that of SO2 (ca. 0.3%) during biomass burning. The emission factors of NH3 were ca. 2–5 times higher than that of SO2 from various types of biomass burning (Andreae and Merlet, Citation2001).
5. Remarks
This study attempted to quantify major sources of N in PM2.5 based on bulk δ15N analysis using a Bayesian isotope mixing model. The isotopic effect of NH3 ↔ equilibrium was recognized under the condition of lower acid gases relative to ambient NH3, which was a main reason for higher bulk δ15N of PM2.5 than potential sources at the background site. Based on the estimations of SIAR model, PM2.5 of Beijing derived N mainly from coal combustion and vehicle exhausts, while background PM2.5 derived N mainly from biomass burning and NH3 volatilization. Regulatory controls of N emissions from coal burning and urban transportation are important and effective steps to reduce the risk of severe haze episodes in Beijing. However, emissions of N from non-fossil emissions (particularly biomass burning) in broad rural areas should be stressed to meet a rigorous reduction of reactive N emissions in China.
Although δ15N interpretation using the SIAR model provided proportional contributions of major sources to bulk N in PM2.5, further investigations are needed to validate the assumptions and boundary conditions in this work. Particularly, δ15N analyses of gaseous N emissions should be implemented for reducing the uncertainties of source δ15N values. So far, isotopic studies on gaseous N emissions from typical anthropogenic and natural emissions are still sparse globally, especially in China. Isotope effects revealed in conversions between NO and NO2, NOx and (Monse et al., Citation1969; Walters and Michalski, Citation2015), NH3 and
(Heaton et al., Citation1997) and the regulatory mechanisms behind the kinetic and equilibrium isotope effects should be explored and properly considered in future studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the State Environmental Protection Commonweal Trade Scientific Research, Ministry of Environmental Protection of China [grant number 2013467010] and the National Natural Science Foundation of China [grant number 41273026], [grant number 41473081], [grant number 41522301], [grant number 41603007]. Xue-Yan Liu was also supported by the 11st Recruitment Program of Global Experts (the Thousand Talents Plan) for Young Professionals granted by the central budget of China, and Youth Innovation Promotion Association of Chinese Academy of Sciences [grant number 2015327].
Acknowledgements
All the financial support from fund and research support from the staff of CRAES are gratefully acknowledged.
References
- Aggarwal, S. G., Kawamura, K., Umarji, G. S., Tachibana, E., Patil, R. S. and co-authors. 2013. Organic and inorganic markers and stable C-, N-isotopic compositions of tropical coastal aerosols from megacity Mumbai: sources of organic aerosols and atmospheric processing. Atmos. Chem. Phys. 13, 4667–4680.10.5194/acp-13-4667-2013
- Ammann, M., Siegwolf, R., Pichlmayer, F., Suter, M., Saurer, M. and co-authors. 1999. Estimating the uptake of traffic-derived NO2 from 15N abundance in Norway spruce needles. Oecologia 118, 124–131.10.1007/s004420050710
- Andreae, M. O. and Merlet, P. 2001. Emission of trace gases and aerosols from biomass burning. Glob. Biogeochem. Cy. 15, 955–966.10.1029/2000GB001382
- Berkemeier, T., Ammann, M., Mentel, T. F., Pöschl, U. and Shiraiwa, M. 2016. Organic nitrate contribution to new particle formation and growth in secondary organic aerosols from α-pinene ozonolysis. Environ. Sci. Technol. 50, 6334–6342.
- Bikkina, S., Kawamura, K. and Sarin, M. 2016. Stable carbon and nitrogen isotopic composition of fine mode aerosols (PM2.5) over the Bay of Bengal: impact of continental sources. Tellus B. 68, 31518.10.3402/tellusb.v68.31518
- Carmichael, G. R., Ferm, M., Thongboonchoo, N., Woo, J. H., Chan, L. Y. and co-authors. 2003. Measurements of sulfur dioxide, ozone and ammonia concentrations in Asia, Africa, and South America using passive samplers. Atmos. Environ. 37, 1293–1308.10.1016/S1352-2310(02)01009-9
- Crutzen, P. J. and Andreae, M. O. 1990. Biomass burning in the tropics: impact on atmospheric chemistry and biogeochemical cycles. Science. 250, 1669–1678.10.1126/science.250.4988.1669
- Davis, P., Syme, J., Heikoop, J., Fessenden-Rahn, J., Perkins, G. and co-authors. 2015. Quantifying uncertainty in stable isotope mixing models. J. Geophys. Res.: Biogeosci. 120, 903–923.10.1002/2014JG002839
- Divers, M. T., Elliott, E. M. and Bain, D. J. 2014. Quantification of nitrate sources to an urban stream using dual nitrate isotopes. Environ. Sci. Technol. 48, 10580–10587.10.1021/es404880j
- Elliott, E. M., Kendall, C., Boyer, E. W., Burns, D. A., Lear, G. and co-authors. 2009. Dual nitrate isotopes in actively and passively collected dry deposition: utility for partitioning NOx sources contributing to landscape nitrogen deposition. J. Geophys. Res. Biogeosci. 114, G04020.
- Elliott, E. M., Kendall, C., Wankel, S. D., Burns, D. A., Boyer, E. W. and co-authors. 2007. Nitrogen isotopes as indicators of NOx source contributions to atmospheric nitrate deposition across the Midwestern and Northeastern United States. Environ. Sci. Technol. 41, 7661–7667.
- Evans, J. S. B. T., Handley, S. J., Perham, N., Over, D. E. and Thompson, V. A. 2000. Frequency versus probability formats in statistical word problems. Cognition. 77, 197–213.10.1016/S0010-0277(00)00098-6
- Felix, J. D. and Elliott, E. M. 2014. The isotopic composition of passively collected nitrogen dioxide emissions: vehicle, soil and livestock source signatures. Atmos. Environ. 92, 359–366.10.1016/j.atmosenv.2014.04.005
- Felix, J. D., Elliott, E. M., Gish, T., Maghirang, R., Cambal, L. and co-authors. 2014. Examining the transport of ammonia emissions across landscapes using nitrogen isotope ratios. Atmos. Environ. 95, 563–570.10.1016/j.atmosenv.2014.06.061
- Felix, J. D., Elliott, E. M., Gish, T., McConnell, L. and Shaw, S. 2013. Characterizing the isotopic composition of atmospheric ammonia emission sources using passive samplers and a combined oxidation-bacterial denitrifier isotope ratio mass spectrometer method. Rapid. Commun. Mass. Sp. 27, 2239–2246.10.1002/rcm.6679
- Felix, J. D., Elliott, E. M. and Shaw, S. L. 2012. The isotopic composition of coal-fired power plant NOx: the influence of emission controls and implications for global emission inventories. Environ. Sci. Technol. 46, 3528–3535.10.1021/es203355v
- Freyer, H. D. 1978. Preliminary 15N studies on atmospheric nitrogenous trace gases. Pure Appl. Geophys. 116, 393–404.10.1007/BF01636894
- Freyer, H. 1991. Seasonal variation of 15N/14N ratios in atmospheric nitrate species. Tellus B. 43, 30–44.10.3402/tellusb.v43i1.15244
- Fukuzaki, N. and Hayasaka, H. 2009. Seasonal variations of nitrogen isotopic ratios of ammonium and nitrate in precipitations collected in the Yahiko-Kakuda Mountains Area in Niigata Prefecture, Japan. Water Air. Soil Pollut. 203, 391–397.10.1007/s11270-009-0026-8
- Ge, X. L., Wexler, A. S. and Clegg, S. L. 2011. Atmospheric amines Part I. A review. Atmos. Environ. 45, 524–546.10.1016/j.atmosenv.2010.10.012
- Guo, S., Hu, M., Zamora, M. L., Peng, J., Shang, D. and co-authors. 2014. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA. 111, 17373–17378.10.1073/pnas.1419604111
- Hastings, M. G., Jarvis, J. C. and Steig, E. J. 2009. Anthropogenic impacts on nitrogen isotopes of ice-core nitrate. Science. 324, 1288.10.1126/science.1170510
- Heaton, T. H. E. 1986. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review. Chem. Geol. 59, 87–102.10.1016/0168-9622(86)90059-X
- Heaton, T. H. E. 1987. 15N/14N ratio of nitrate and ammonium in rain of Pretoria, south Africa. Atmos. Environ. 21, 843–852.10.1016/0004-6981(87)90080-1
- Heaton, T. H. E. 1990. 15N/14N ratios of NOx from vehicle engines and coal-fired power stations. Tellus. 42, 304–307.
- Heaton, T. H. E., Spiro, B. and Robertson, S. M. C. 1997. Potential canopy influences on the isotopic composition of nitrogen and sulphur in atmospheric deposition. Oecologia. 109, 600–607.10.1007/s004420050122
- Heaton, T. H. E., Wynn, P. and Tye, A. M. 2004. Low 15N/14N ratios for nitrate in snow in the High Arctic (79°N). Atmos. Environ. 38, 5611–5621.10.1016/j.atmosenv.2004.06.028
- Hegde, P., Kawamura, K., Joshi, H. and Naja, M. 2015. Organic and inorganic components of aerosols over the central Himalayas: winter and summer variations in stable carbon and nitrogen isotopic composition. Environ. Sci. Pollut. Res. 23, 5997–6001.
- He, H., Wang, Y., Ma, Q., Ma, J., Chu, B. and co-authors., 2014. Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days. Sci. Rep. 4, 4172–4176.
- Hoering, T. 1957. The isotopic composition of ammonia and the nitrate ion in rain. Geochim. Cosmochim. Acta. 12, 97–102.10.1016/0016-7037(57)90021-2
- Huang, R. J., Zhang, Y. L., Bozzetti, C., Ho, K. F., Cao, J. J. and co-authors. 2014. High secondary aerosol contribution to particulate pollution during haze events in China. Nature. 514, 218–222.
- Jackson, A. L., Inger, R., Bearhop, S. and Parnell, A. 2009. Erroneous behaviour of MixSIR, a recently published Bayesian isotope mixing model: a discussion of Moore & Semmens (2008). Ecol. Lett. 12, E1–E5.10.1111/ele.2009.12.issue-3
- Jia, G. and Chen, F. 2010. Monthly variations in nitrogen isotopes of ammonium and nitrate in wet deposition at Guangzhou, south China. Atmos. Environ. 44, 2309–2315.10.1016/j.atmosenv.2010.03.041
- Jickells, T. D., Kelly, S. D., Baker, A. R., Biswas, K., Dennis, P. F. and co-authors. 2003. Isotopic evidence for a marine ammonia source. Geo. Res. Lett. 30, 359–376.
- Kawashima, H. and Kurahashi, T. 2011. Inorganic ion and nitrogen isotopic compositions of atmospheric aerosols at Yurihonjo, Japan: implications for nitrogen sources. Atmos. Environ. 45, 6309–6316.10.1016/j.atmosenv.2011.08.057
- Kendall, C., Elliott, E. M. and Wankel, S. D. 2007. Tracing anthropogenic inputs of nitrogen to ecosystems. In: Stable Isotopes in Ecology and Environmental Science (eds. R.M. Michener and K.E. Lajtha). Blackwell, Oxford, pp. 375–449.10.1002/9780470691854
- Li, L., Lollar, B. S., Li, H., Wortmann, U. G. and Lacrampe-Couloume, G. 2012. Ammonium stability and nitrogen isotope fractionations for NH4+-NH3(aq)-NH3 (gas) systems at 20–70 °C and pH of 2–13: applications to habitability and nitrogen cycling in low-temperature hydrothermal systems. Geochim. Cosmochi. Acta. 84, 280–296.10.1016/j.gca.2012.01.040
- Li, D. J. and Wang, X. M. 2008. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application. Atmos. Environ. 42, 4747–4754.10.1016/j.atmosenv.2008.01.042
- Lobert, J. M., Scharffe, D. H., Hao, W. M. and Crutzen, P. J. 1990. Importance of biomass burning in the atmospheric budgets of nitrogen-containing gases. Nature. 346, 552–554.10.1038/346552a0
- Michalski, G., Bhattacharya, S. and Girsch, G. 2014. NOx cycle and the tropospheric ozone isotope anomaly: an experimental investigation. Atm. Chem. Phys. 14, 4935–4953.10.5194/acp-14-4935-2014
- Michalski, G., Meixner, T., Fenn, M., Hernandez, L., Sirulnik, A., Allen, E. and Thiemens, M. 2004. Tracing atmospheric nitrate deposition in a complex semiarid ecosystem using Δ17O. Environ. Sci. Technol. 38, 2175–2181.10.1021/es034980+
- Monse, E. U., Spindel, W. and Stern, M. J. 1969. Analysis of isotope-effect calculations illustrated with exchange equilibria among oxynitrogen compounds, ACS Adv. Chem. Ser. 89, 148–184.
- Moore, H. 1977. The isotopic composition of ammonia, nitrogen dioxide, and nitrate in the atmosphere. Atmos. Environ. 11, 1239–1243.10.1016/0004-6981(77)90102-0
- Moore, J. W. and Semmens, B. X. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480.10.1111/j.1461-0248.2008.01163.x
- Parnell, A. C., Inger, R., Bearhop, S. and Jackson, A. L. 2010. Source partioning using stable isotopes: coping with too much variation. PLoS ONE. 5, e9672.10.1371/journal.pone.0009672
- Parnell, A. C. and Jackson, A. 2008. SIAR: stable isotope analysis in R. Online at: http://cran.r-project.org/web/packages/siar/index.html (accessed 10 December 2012).
- Pavuluri, C. M., Kawamura, K. and Fu, P. Q. 2015. Atmospheric chemistry of nitrogenous aerosols in northeastern Asia: biological sources and secondary formation. Atmos. Chem. Phys. 15, 9883–9896.10.5194/acp-15-9883-2015
- Pavuluri, C. M., Kawamura, K., Tachibana, E. and Swaminathan, T. 2010. Elevated nitrogen isotope ratios of tropical Indian aerosols from Chennai: implication for the origins of aerosol nitrogen in South and Southeast Asia. Atmos. Environ. 44, 3597–3604.10.1016/j.atmosenv.2010.05.039
- Pearson, J., Wells, D., Seller, K. J., Bennett, A., Soares, A. and co-authors. 2000. Traffic exposure increases natural 15N and heavy metal concentrations in mosses. New Phytol. 147, 317–326.10.1046/j.1469-8137.2000.00702.x
- Savarino, J., Morin, S., Erbland, J., Grannec, F., Patey, M. D. and co-authors. 2013. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl. Acad. Sci. USA. 110, 17668–17673.10.1073/pnas.1216639110
- Smirnoff, A., Savard, M. M., Vet, R. and Simard, M. C. 2012. Nitrogen and triple oxygen isotopes in near-road air samples using chemical conversion and thermal decomposition. Rapid. Commun. Mass. SP. 26, 2791–2804.10.1002/rcm.6406
- Sun, Y. L., Zhuang, G. S., Tang, A. H., Wang, Y. and An, Z. S. 2006. Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environ. Sci. Technol. 40, 3148–3155.10.1021/es051533g
- Walters, W. W., Goodwin, S. R. and Michalski, G. 2015. Nitrogen stable isotope composition (15N) of vehicle emitted NOx. Environ. Sci. Technol. 49, 2278–2285.10.1021/es505580v
- Walters, W. W. and Michalski, G. 2015. Theoretical calculation of nitrogen isotope equilibrium exchange fractionation factors for various NOy molecules. Geochim. Cosmochim. Acta 164, 284–297.10.1016/j.gca.2015.05.029
- Wang, C., Wang, X. B., Liu, D. W., Wu, H. H., Lü, X. T. and co-authors. 2014. Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat. Commun. 5, 4799.10.1038/ncomms5799
- Wei, L. F., Duan, J. C., Tan, J. H., Yongliang, M. A., Kebin, H. E. and co-authors. 2015. Gas-to-particle conversion of atmospheric ammonia and sampling artifacts of ammonium in spring of Beijing. Sci. China. Earth. Sci. 63, 186–187.
- Widory, D. 2007. Nitrogen isotopes: tracers of origin and processes affecting PM10 in the atmosphere of Paris. Atmos. Environ. 41, 2382–2390.10.1016/j.atmosenv.2006.11.009
- Yeatman, S. G., Spokes, L. J., Dennis, P. F. and Jickells, T. D. 2001a. Comparisons of aerosol nitrogen isotopic composition at two polluted coastal sites. Atmos. Environ. 35, 1307–1320.10.1016/S1352-2310(00)00408-8
- Yeatman, S. G., Spokes, L. J. and Jickells, T. D. 2001b. Comparisons of coarse-mode aerosol nitrate and ammonium at two polluted coastal sites. Atmos. Environ. 35, 1321–1335.10.1016/S1352-2310(00)00452-0
- Zhang, R. Y. 2010. Getting to the critical nucleus of aerosol formation. Science. 328, 1366–1367.10.1126/science.1189732
- Zhang, R. J., Jing, J., Tao, J., Hsu, S.-C., Wang, G. and co-authors. 2013. Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmos. Chem. Phys. 13, 7053–7074.10.5194/acp-13-7053-2013
- Zhang, L., Wang, T., Lv, M. Y. and Zhang, Q. 2015. On the severe haze in Beijing during January 2013: unraveling the effects of meteorological anomalies with WRF-Chem. Atmos. Environ. 104, 11–21.10.1016/j.atmosenv.2015.01.001