Abstract
Objectives: The aim of the study was to investigate the dispensing patterns of prescription-only antiobesity preparations in South Africa (classified as Anatomical Therapeutic Chemical (ATC) group A08).
Design: Retrospective, cross-sectional drug utilisation study using electronic dispensing records.
Setting: Private sector community or retail pharmacies in South Africa.
Subjects: Patients who received one or more antiobesity medications in ATC group A08 in 2013.
Outcome measures: Number of patients by age and gender, prescribing frequency and cost of antiobesity prescriptions, and trends observed.
Results: A total of 27 703 patients were prescribed 52 555 products for antiobesity medication during 2013. The average age of patients was 41.71 (SD = 11.37) years, with male patients older than female patients (46.09 and 40.02 years, respectively). More females (72.19%) were dispensed antiobesity products, and females received their prescriptions at a younger average age than male patients. Five active ingredients were dispensed. Phentermine was prescribed the most, accounting for 92.44% of all the antiobesity prescriptions, followed by orlistat (6.08%), phendimetrazine (1.36%), D-norpseudoephedrine (0.06%) and diethylpropion (0.05%). Most patients (79.44%) received only short-term therapy (one or two prescriptions for an antiobesity product during the year). A small percentage (0.30%) of young patients (18 years and younger) received antiobesity products, despite the fact that the safety of these products in children has not been proven.
Conclusions: Most antiobesity preparations were prescribed to females. Phentermine was the most commonly dispensed active ingredient, followed by orlistat. Further studies on patient outcomes and the cost-effectiveness of these products should be conducted.
Introduction
Obesity is a major global public health problem.Citation1,2 It is one of the most serious and prevalent non-communicable diseases of the 21st century.Citation3 The obesity epidemic has been growing in developed nations for decades.Citation1 Growing affluence has been accompanied by the emergence of overweight and obesity, to the extent that the proportion of underweight and overweight persons has inverted.Citation4 The worldwide burden of obesity is estimated at 1.5 billion overweight persons and 500 million obese persons.Citation5 Obesity-associated comorbidities, including major diseases such as cardiovascular disease, diabetes (type 2) and various cancers, are well-known.Citation2 In June 2013, the American Medical Association officially recognised obesity as a chronic medical disease state.Citation3
A study in the LancetCitation6 reported that the worldwide proportion of adults with a body-mass index (BMI) of 25 kg/m2 or greater increased between 1980 and 2013 from 28.8% to 36.9% in men, and from 29.8% to 38.0% in women. Prevalence has also increased substantially in children and adolescents in developed countries, as well as in developing countries.Citation6 The result of this is an increase in the prevalence of chronic diseases associated with obesity in younger people.
Sub-Saharan Africa was minimally affected by the obesity epidemic due to under-nutrition and a major burden of HIV and tuberculosis until recently.Citation2,7 However, the obesity epidemic has also spread to developing countries, with obesity and its association with co-morbidities in Africa on the rise.Citation8 Obesity is now regarded as a major risk factor for emerging non-communicable diseases in middle income countries, including South Africa.Citation2
South Africa is undergoing a rapid epidemiological transition and has the highest prevalence of obesity in sub-Saharan Africa.Citation7 Although different studies and surveys report different numbers, the reported prevalence for overweight and obesity in South Africa is alarming. The Lancet study found that 70% of South African women are overweight and 42% are obese.Citation6 The study was conducted by the Institute for Health Metrics and Evaluation at the University of Washington, and was a first-of-its-kind analysis of data between 1980 and 2013 from 188 countries.Citation6 Socio-cultural, environmental and behavioural factors, including socio-economic status, are likely to explain the high prevalence of obesity in South Africa.Citation7,9
Lifestyle modifications are the first-line of treatment for obesity, followed by pharmacotherapy.Citation1 Several prescription medications are approved to treat obesity, yet little is known about their prescribing and use in South Africa. A studyCitation10 conducted on a small patient sample reported on the prescribing of appetite suppressants in 2010 and 2011, but no studies on larger South African patient populations could be found.
There are two classes of antiobesity products available in South Africa, namely centrally-acting and peripherally-acting antiobesity preparations. Centrally-acting antiobesity products include diethylpropion (amfepramone), phentermine and phendimetrazine. These active ingredients are sympathomimetics with central nervous system (CNS) stimulant properties similar to dexamphetamine, and they have significant abuse potential.Citation11 Cathine (D-norpseudoephedrine) is also a sympathomimetic compound with CNS stimulant properties. It is used as an adjunct to lifestyle modification, and it also has significant abuse potential.Citation11 These compounds are Schedule 6 (except for phentermine which is a Schedule 5 medicine). Peripherally-acting antiobesity products include only one product, namely orlistat, which is a Schedule 3 product and it is therefore less strictly controlled. Orlistat is indicated for the management of obesity in conjunction with a hypocaloric diet in patients with BMI ≥ 30 kg/m2, or ≥ 27 kg/m2 with co-morbidities, only if 2.5 kg has been lost on diet alone over a 4-week period.Citation11 Orlistat is a gastrointestinal lipase inhibitor which lowers the absorption of dietary fat by approximately 30%.Citation11 However, severe gastrointestinal adverse events due to fat malabsorption may occur.Citation11 These antiobesity preparations should not be combined, and their long-term use is not advised.Citation11 Their duration of efficacy is limited, and differs for each individual agent.Citation11 There may be cross-tolerance to other agents.Citation11 There are limited studies showing safety and efficacy of the noradrenergics beyond 6 months.Citation11
Studies on the prescribing patterns of antiobesity products in South Africa are limited. In addition, there are no safety and efficacy data for the use of these agents in children. The aim of the study was therefore to investigate the dispensing patterns of antiobesity prescriptions in Anatomical Therapeutic Chemical (ATC) group A08 using electronic dispensing records of community pharmacies in South Africa.
Methodology
A retrospective, cross-sectional drug utilisation study was conducted on a private sector community (or retail) pharmacy dispensing database of approximately 54 million records dispensed by 327 community pharmacies in South Africa in 2013. A community pharmacy refers to pharmacies from which some, or all, of the services as prescribed in terms of regulation 18 of the Regulations Relating to the Practice of Pharmacy in the Pharmacy Act are provided to the general public or any defined group of the general public, but excludes an institutional pharmacy (for example, a hospital pharmacy). According to the South African Pharmacy Council, there are 3 082 registered community pharmacies in South Africa.Citation12 The data therefore included approximately 10% of all community pharmacy dispensing records in the country in 2013. The database consisted of dispensing information from pharmacies in all nine provinces of South Africa, and included dispensing records from all the participating pharmacies. Prescriptions included both medical aid claims and prescriptions that were paid for at the pharmacies’ dispensaries. Most of the prescriptions were classified under private purchases (63.89%) since not all medical schemes reimburse antiobesity preparations, while the rest (36.11%) were claimed under a variety of medical aid scheme options.
The ATC Classification SystemCitation13, MIMS (Monthly Index of Medical Specialities)Citation14 and the South African Medicines FormularyCitation11 were used to classify medicines. All products in ATC subgroup A08 (antiobesity preparations, excluding diet products) were extracted and analysed. The ATC Classification system is recommended by the World Health Organisation as the standard medicine classification system for drug utilisation studies in the world in order to enable comparative studies to be conducted.
Each medication record contained information on the age and gender of the patient, with a unique number to identify each patient, the date of the prescription, detailed information on the dispensed drug (name, package size, formulation, strength and quantity) and gross sales value. Dosage instructions were available in the database, but were not consistent in how they were recorded and were therefore not analysed in detail. At the time of the study, one Euro (€1.00) was equal to R12.86 (South African Rand), one US Dollar ($1.00) was equal to R9.88 and one British Pound (£1.00) was equal to R15.03 (30 June 2013).
Microsoft Access® and Excel® were used to analyse the data. Descriptive statistics were calculated. Results were described according to means and standard deviations for numerical data, and frequencies and percentages for categorical data. The Pearson’s chi-squared test was used to determine statistical significant differences between sub-groups. A p-value < 0.05 was considered statistically significant.
Ethical approval to conduct studies on prescription databases was obtained from the Research Ethics Committee (Human) of the university (ethics clearance number: H08-HEA-PHA-005). The dispensing records in the database were de-identified, meaning that no patient or prescriber could be identified. A patient identifier was added to enable the researchers to uniquely identify each patient in terms of age and gender, and to calculate the number and spectrum of products that a specific patient received, but it was a neutral code and not an identity or medical aid number.
Results
Demographic information of patients
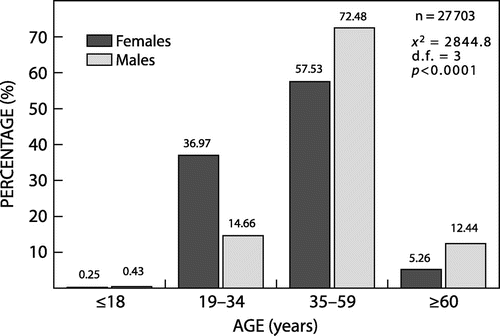
A total of 27 703 patients were dispensed one or more prescriptions in ATC group A08. The majority of the patients (72.19%; n = 20 000) were females. The average age of patients was 41.71 (SD = 11.37) years. The average age of female patients was 40.02 (11.01) years, and male patients was 46.09 (11.13) years. The percentage gender distribution of the 27 703 patients according to age groups is given in Figure . The chi-square test was used to detect differences between female and male patients in the different age groups (χ2 = 2844.8; d.f. = 3; p < 0.0001). Differences were observed, with proportionally more female patients in the younger adult age group (19 to 34 years), and more male patients in the older age groups (35 years and older).
General dispensing trends
The 27 703 patients were dispensed a total of 52 555 prescriptions for antiobesity products at a total cost of R12 887 891.55 during 2013. A prescription is, for the purposes of this study, defined as one issue of a specific medicine to a patient and can consist of a variable number of tablets or capsules. For chronic conditions, prescriptions are issued for a month’s supply. In this study, 93.66% of prescriptions were for 30 tablets or capsules or less (92.86% of prescriptions contained exactly 30 tablets or capsules). Most patients (79.44%) received only one or two prescriptions for an antiobesity product during the year. Patients were dispensed an average of 1.90 (SD = 1.69) prescriptions during the year. Female patients received an average of 1.83 (SD = 1.57) prescriptions and male patients an average of 2.08 (SD = 1.96) prescriptions during the 12 months.
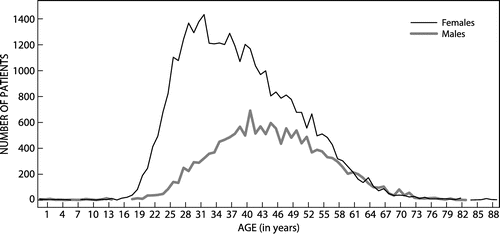
The maximum number of prescriptions dispensed per patient during the year was 26. A patient (a 56-year-old male) received multiple prescriptions of 14 capsules with dosage instructions to take either 1 or 2 capsules per day. It could therefore not be considered as irrational prescribing, since only a 7 or 14 day supply of medication was issued at a time, possibly to prevent abuse of the medication. The number of prescriptions dispensed according to age and gender is given in Figure . There were more female patients, and they were generally prescribed antiobesity preparations at a younger age compared to male patients.
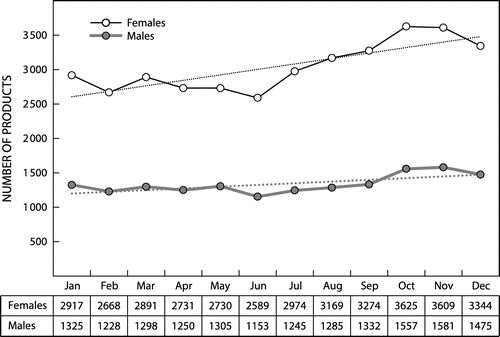
Seasonal variations in the dispensing patterns were investigated (see Figure ). For both females and males, there was generally an increase in the number of prescriptions dispensed towards the end of the year, and a slight decrease during the winter (June).
The antiobesity products in this study were only available on prescription. The majority of the products were Schedule 5 (92.44%), followed by Schedule 3 (6.08%) and Schedule 6 (1.48%). Both Schedule 5 and Schedule 6 products are strictly controlled. Schedule 6 products cannot be repeated, and a patient needs a new prescription every time they are dispensed.
Diagnoses codes (ICD-10 codes)Citation15 were included in the database, but they were not specific. The majority (92.37%) of prescriptions had a “Z” code (factors influencing health status and contact with health services), 3.40% had a “U” code (codes for special purposes), 1.66% had an “E” code (endocrine, nutritional and metabolic diseases) and 1.30% an “R” code (symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified).
Prescribing frequency and cost of the different antiobesity prescriptions
The prescribing frequency of the different antiobesity active ingredients is given in Table . Phentermine was prescribed the most, accounting for 92.44% of all the antiobesity prescriptions, followed by orlistat (6.08%).
Table 1: Prescribing frequency of the different antiobesity prescriptions
The average cost per antiobesity prescription in this study was R245.23. The highest average cost was for the branded phendimetrazine, at an average cost of R615.27 per prescription. Phendimetrazine was also the only active ingredient with a generic product that was prescribed (528 prescriptions were dispensed of the generic, compared to 189 of the branded product).
Dispensing patterns of antiobesity preparations to patients 18 years and younger
A small percentage of patients (0.30%; n = 82) were 18 years or younger. Of these patients, 49 were female and 33 were male. A total of 179 prescriptions were dispensed to them, with 130 prescriptions for phentermine and 17 prescriptions for orlistat. They received an average of 1.79 (SD = 1.61) prescriptions over the year, with 53 of the 82 patients only receiving one product during the year. The youngest patient was 2 years of age.
Discussion
The majority of patients (72.19%) who were dispensed antiobesity medication in this study were female. The average age of patients was 41.71 years, with female patients younger than male patients. These findings were similar to those of a previous South African study that was conducted on a smaller database.Citation10
Female patients received antiobesity prescriptions at a younger age compared to male patients. In a study conducted in Northern IrelandCitation16, multi-level logistic regression analysis was used to investigate factors associated with the prescription of antiobesity medication. They used a population primary care prescribing database covering approximately 1.5 million people aged 16 years and older during 2009 and 2010. They reported that although 25.0% of people were obese, only 1.3% (2.1% of females, 0.6% of males) received antiobesity medication.Citation16 The relationship between medication rates and age differed by gender with prescriptions higher in younger females and older males.Citation16 These findings are generally in agreement with the results of this study.
Of the five active ingredients dispensed in this study, phentermine dominated prescribing (accounting for 92.44% of all prescriptions in ATC group A08). In the previous South African studyCitation10 conducted on 2010 and 2011 data, most prescriptions (80.95%) were also for phentermine, followed by D-norpseudoephedrine (14.29%) and diethylpropion (4.76%). Phentermine has been described as an old but understudied medicationCitation17,18, and as a non-addictive medication that is likely to be helpful for many who are already overweight.Citation17 Phentermine has been used in the United States of America since 1959 for the short-term management of obesity.Citation19 Its mechanism of action is believed to be dependent on modulation of catecholamines in the satiety centers of the hypothalamus, thus reducing appetite.Citation1 Despite the widespread use of phentermine in South Africa, there are no pharmacoeconomic studies reporting on its cost-effectiveness in South Africa. Orlistat was the second most dispensed agent (6.08%). Orlistat is the only weight-loss agent approved for long-term clinical use in Europe.Citation20
Most patients received only one prescription for an antiobesity medication during the year. This was positive, since these agents are indicated for short-term use. These findings are generally in agreement with a study by Hampp and colleaguesCitation21 who reported that in 2011, approximately 2.74 million patients in the United States of America used antiobesity drugs, and predominantly used phentermine (2.43 million patients). Eighty-five percent of antiobesity medicine users in their study were female, 62% were aged 17 to 44 years, and 4.5% had a body mass index of ≤ 24.9 kg/m2.Citation21 Duration of use was generally short.Citation21
Various other classes of medicine can also be used to achieve weight loss, for example antidepressants and anti-epileptic medicines (such as fluoxetine, sertraline, bupropion and topiramate). In most cases, these medicines will be prescribed off label if used for weight loss and not for their primary indication. Topiramate is one such example where patients who received topiramate lost weight, and this generated interest in evaluating this active ingredient as a potential antiobesity drug. Topiramate has been approved by the Food and Drug Administration (FDA) for the treatment of obesity, although it is primarily used to treat convulsive disorders and migraines.Citation1 The FDA had approved the combination of phentermine/topiramate in 2012, largely on the strength of evidence from three clinical trials, known as the CONQUER, EQUIP, and SEQUEL trials.Citation1 Data from these three trials showed phentermine/topiramate to be efficacious in inducing and maintaining weight loss. Across the three trials, approximately 75% of treated subjects exhibited a 5% weight loss, and approximately 50% exhibited a 10% weight loss.Citation1 A pharmacoeconomic study in the United States investigated the cost-effectiveness of the combination of phentermine and topiramate extended-release, and found that although base-case results suggested that the combination was cost-effective, the result hinged on the duration of use of the product and the extent to which benefits were maintained post-medication cessation.Citation22 The combination of phentermine and topiramate is not available in South Africa.
A small percentage of patients that were 18 years and younger received antiobesity preparations in this study. Because of the lack of long-term paediatric treatment trials showing safety and efficacy, these drugs are not recommended as weight loss medications in young patients. The only exception is orlistat, which is approved by the FDA for the treatment of obesity in adolescents 12 years and older. It reduces the body mass index (BMI) by 0.5 to 4 kg/m2, but gastrointestinal adverse effects may limit its use.Citation23
South Africa is currently experiencing two epidemics. Firstly, the HIV/AIDS and tuberculosis epidemic; and, secondly, an increase in non-communicable diseases. Overweight and obesity play a significant role in this increase in the incidence of non-communicable diseases, especially under the younger population. Weight loss of 5% to 10% of initial weight, achieved through intensive lifestyle intervention, reduces cardiovascular disease risk factors, prevents or delays the development of type-2 diabetes, and improves other health consequences of obesity.Citation24 If lifestyle modifications alone are not effective, there are antiobesity preparations available. These agents are not without side effects and potential risks. However, if used rationally, antiobesity preparations have an important supplementary role to play in combatting the emerging obesity epidemic in South Africa. More research is needed on the effectiveness of antiobesity preparations in South Africa. These studies should ideally be conducted by dietitians in conjunction with pharmacists.
Limitations of the study
The study had several limitations. Only prescriptions that were dispensed by a group of community pharmacies were included in the study, and the study covered only a period of one year. No clinical information was available, such as the BMI of patients, and diagnoses were also not specific. Appetite suppressants that patients can buy over-the-counter and the various herbal products that can be used to control weight were also not included in the study.
Conclusion
Lifestyle modification is considered to be the first-line therapy for obese adult and paediatric patients. Second-line therapy for severe obesity is pharmacotherapy. This study investigated the dispensing patterns of prescription-only antiobesity preparations by community pharmacies. A follow-up study is currently being conducted to determine which medication classes were co-prescribed with antiobesity preparations, especially whether patients using antiobesity preparations are also on antidiabetic, hypolipidaemic and/or antihypertensive medication. In addition, limited information is available on the cost-effectiveness of these products in South Africa and also on the long-term outcomes of patients who have used these products. These are aspects that warrant further investigation.
Acknowledgements
| • | This work is based upon research supported by the National Research Foundation (NRF). Any opinion, findings and conclusions or recommendations expressed are those of the author and therefore the NRF does not accept any liability in regard thereto. | ||||
| • | The pharmacy group for providing the data for the study. | ||||
References
- Kim GW, Lin JE, Biomain ES, et al. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014 Jan;95(1):53–66.
- Sartorius B, Veerman LJ, Manyema M, et al. Determinants of obesity and associated population attributability, South Africa: empirical evidence from a national panel survey, 2008-2012. Zeeb H, editor. PLOS ONE. 2015;10(6):e0130218.10.1371/journal.pone.0130218
- Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014 Jan–Feb;56(4):465–72.10.1016/j.pcad.2013.09.005
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrin. 2013;9:13–27.
- Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25.10.1016/S0140-6736(11)60814-3
- Ng M, Fleming T, Robinson M., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;384(9945):766–81.
- Micklesfield LK, Lambert EV, Hume DJ, et al. Socio-cultural, environmental and behavioural determinants of obesity in black South African women. Cardiovasc J Afr. 2013;24:1–7.
- Adeboye B, Bermano G, Rolland C. Obesity and its health impact in Africa: a systematic review. Cardiovasc J Afr. 2012 Oct;23(9):512–21.10.5830/CVJA-2012-040
- Alaba OA, Chola L. Prevalence of age-adjusted obesity in South Africa and the influence of social determinants: an ecological analysis. Lancet. 2013;381:S6.10.1016/S0140-6736(13)61260-X
- Truter I. Prescription appetite suppressants: a drug utilisation study using a claims database. JAPS. 2014 Aug;4(8):32–5.
- Rossiter D, editor. South African Medicines Formulary (SAMF). 11th ed. Cape Town: Health and Medical Publishing Group of the South African Medical Association; 2014.
- South African Pharmacy Council. Statistics for registered persons and organisations [homepage on the Internet]. 2015. c2015. Available from: http://www.pharmcouncil.co.za/B_Statistics.asp.
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2015 [homepage on the Internet]. Oslo. 2015. c2015. Available from: http://www.whocc.no/atc_ddd_index/.
- Snyman J, editor. MIMS Monthly Index of Medical Specialities (MIMS). Saxonwold: MIMS. 2011;51(6).
- ICD-10 Version: 2016. ICD-10 - World Health Organization. 2015. c2015. Available from: http://apps.who.int/classifications/icd10/browse/2016/en.
- Patterson L, Kee F, Hughes C, et al. The relationship between BMI and the prescription of antiobesity medication according to social factors: a population cross sectional study. BMC Public Health. 2014;14:815.10.1186/1471-2458-14-87
- McGill A-T. Past and future corollaries of theories on causes of metabolic syndrome and obesity related co-morbidities part 2: a composite unifying theory review of human-specific co-adaptations to brain energy consumption. Arch Pub Health. 2014;72:30.10.1186/2049-3258-72-31
- Pugh R. New E: obesity drug phentermine is not addictive 20th European congress on obesity. Abstract T5:OS2.3, presented May 15, 2013. Obes Facts. 2013;6:22–48.
- Fleming JW, McClendon KS, Riche DM. New obesity agents: lorcaserin and phentermine/topiramate. Ann Pharmacother. 2013;47:1007–16.10.1345/aph.1R779
- Rodgers RJ, Tschop MH, Wilding JPH. Antiobesity drugs: past, present and future. Dis Model Mech. 2012;5(5):621–6.10.1242/dmm.009621
- Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacother. 2013 Dec;33(12):1299–307.10.1002/phar.1342
- Finkelstein EA, Kruger E, Karnawat S. Cost-Effectiveness Analysis of Qsymia for Weight Loss. PharmacoEconomics 2015 Jul;33(7):699–706.10.1007/s40273-014-0182-6
- Boland CL, Harris JB, Harris KB. Pharmacological management of obesity in pediatric patients. Ann Pharmacother. 2015 Feb;49(2):220–32.10.1177/1060028014557859
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity. JAMA. 2014;311(1):74–86.10.1001/jama.2013.281361