Abstract
Context: Gastroenteritis (GE) remains the second major cause of death in the most vulnerable of the world’s populations. Potential treatments include the use of probiotics, with the yeast Saccharomyces boulardii being one such option.
Objectives: The primary objective was to assess the efficacy and safety of Saccharomyces boulardii in the treatment of acute GE in the paediatric population.
Method: Major electronic databases were searched from April 2014 to January 2015. Additional literature was obtained through hand-searching and reviewing of reference lists of articles and other systematic reviews. Randomised controlled trials (RCTs) in a hospital setting, involving participants < 16 years were used as the data source. Two reviewers independently screened studies for eligibility, assessed study quality and performed data extraction. Review Manager 5 was used to analyse data and a random-effects model of meta-analysis was applied owing to heterogeneity.
Results: Ten of 190 articles were selected for final inclusion. A meta-analysis of five of the included studies showed that Saccharomyces boulardii compared with the control significantly shortened the duration of diarrhoea (in days) (MD –0.57, 95% CI –0.83 to –0.30, p < 0.0001), but there was no difference between groups regarding time to achieving formed stools. No adverse effects were reported. The GRADE tool assessed overall methodological quality as moderate.
Conclusion: Saccharomyces boulardii showed a potential benefit in treating acute GE in the paediatric patient. A dose of 250 mg 1–2 times per day for up to 5 days showed some benefit and appears safe. Larger, rigorous RCTs are needed to investigate the efficacy and safety of Saccharomyces boulardii in order to offer specific treatment guidelines.
Trial registration: CRD42014009913.
Introduction
Despite being a symptom known to be preventable and treatable, gastroenteritis (GE) contributes 5–10% of the total deaths in the under-five age group.Citation1–3 The World Health Organization (WHO) defines diarrhoea/GE as ‘the passage of three or more loose/liquid stools per day, or more frequent passage than is normal for the individual’.Citation4 The consistency of stools, and not so much the number, is also important in diagnosing GE.Citation4–8 GE infections may be caused by one of three organisms, i.e. bacterial, viral or parasitic,Citation7 – Citation8 with rotavirus found to be responsible for 215 000 child deaths globally during 2013.Citation4 Approaches to curbing the impact of rotavirus-causing GE have been implemented and with some degree of success, i.e. increasing the number of vulnerable individuals who receive the rotavirus vaccine, increased protection against contracting GE-causing infections by encouraging mothers to breastfeed, improving accessibility to clean water supplies and educating populations about the importance of hygiene.Citation7 – Citation8
The bacteria that are found in the gastrointestinal tract are a complex ecosystem and able to coexist with the host, as long as a state of balance is maintained.Citation5,6,9–14 However, during disruptions in this balanced state, clinical disorders and disease can result. Gastrointestinal disorders, one of which being all forms of GE, can result in an imbalance, with the goal of treatment being reinstating balance to the gut bacteria’s ecosystem.Citation5,6,9–14
Probiotics have been identified as a possible treatment modality to restore beneficial gastrointestinal bacteria to their original balanced state.Citation9–13 The efficacy of these microorganisms is known to be strain-specific, making it important for them to be defined by their genus, species and strain.Citation6,9–11 Research has shown that the human gastrointestinal tract contains a heterogenous mix of 10Citation14 bacteria, of which < 0.1% is yeast.Citation6,9–11
Saccharomyces cerevisiae variety boulardii, more commonly referred to as Saccharomyces boulardii, is a non-pathogenic yeast that is suitable for human consumption, having been used in the treatment of inflammatory bowel disorders and several types of GE.Citation12,14–16 Saccharomyces boulardii’s action is threefold, i.e. luminal, trophic on intestinal mucosa and regulatory on the immune system.Citation5,14,16–19 The site of action for Saccharomyces boulardii is most commonly the colon and the yeast probiotic has been shown to survive passage to its target organ.Citation5,14,16–19 Most of the Saccharomyces strains have been shown to work optimally at temperatures between 22 °C and 30 °C; Saccharomyces boulardii, however, is able to survive temperatures of up to 37 °C, and therefore able to survive human body temperatures. Saccharomyces boulardii in a lyophilised form is able to survive gastric acid and bile.Citation5,14,16–19 Stool sampling tests have shown that levels of Saccharomyces boulardii can be 100 to 1 000 times lower than the oral dose offered, indicating that much of the oral dose is destroyed, but surviving doses have been found to be effective.Citation5,14,16–19 It is naturally resistant to antibiotics and proteolysis and able to survive in the competitive milieu of the intestinal tract. In human subjects, the concentration in the colon was found to be dose-dependent. When Saccharomyces boulardii was given to healthy subjects at doses used therapeutically (1 to 2 x 1010/d), colonic levels were found to be 2 x 108/gram stool. Furthermore, when offered orally, Saccharomyces boulardii was able to achieve steady-state concentrations within 3 days and was only cleared within 3–5 days after it had been discontinued. It has also demonstrated an ability to coexist and thrive in the presence of other agents, e.g. psyllium fibre increased Saccharomyces boulardii levels by 22%.Citation5,14,16–19
Probiotics (multiple single strains) with potentially multiple mechanisms of actionCitation14 were found to reduce the associated risk of acute GE (AGE) in children, with the effect dependent on the age of the host and the genera of the strain used.Citation20 Saccharomyces boulardii specifically was shown to result in quicker GE resolution than that displayed by control groups.Citation21 This yeast probiotic has the potential to be the sole or adjunct treatment in treating AGE, but, owing to research bias and confounding in documented studies, it remains difficult to develop guidelines on its role in managing AGE. As a result, our aim is to provide a systematic review of published studies, specifically assessing the efficacy and safety of Saccharomyces boulardii in the treatment of AGE in the paediatric population.
Methods
This project is registered with the Prospective Register of Ongoing Systematic Reviews (PROSPERO), trial registration number CRD42014009913.
Information sources and searches
A comprehensive literature search of the following electronic databases was conducted: Medline (accessed via PubMed); EBSCO host, including Academic Search Premier, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Africa Wide and CAB Abstracts; Cochrane Library, which includes the Cochrane Databases of Systematic Reviews (CDSR, Cochrane Reviews), Cochrane Central Register of Controlled Trials (CENTRAL; Clinical Trials), Databases of Abstracts of Reviews of Effects (DARE; Other Reviews); ISI Web of Knowledge – Web of Science; Scopus (abstract and citation database of peer-reviewed literature); ProQuest Medical Library; Science Direct; and SABINET (South African Bibliographic Information Network). Additional literature was obtained through hand-searching and reviewing of reference lists of articles and other systematic reviews.
The final search string used was: (probiotic OR Saccharomyces boulardii) AND (diarrh* OR gastroent*) AND (clinical trial* OR randomized control trial* OR random allocation OR placebo* OR random research OR comparative OR evaluation stud* OR follow up OR prospective* OR control* OR volunteer* OR single mask* OR double mask* OR treble mask* OR tripl* mask* OR double mask* OR treble mask* OR tripl* mask* OR single-blind OR double-blind OR treble blind OR tripl* blind). The only limits applied whilst using this search string were human and child (birth to 18 years), and therefore foreign-language articles were included. This search string was applied across all databases mentioned above, with all searches completed up to January 27, 2015.
Inclusion criteria
Only randomised controlled trials (RCTs) involving human participants and investigating the efficacy and safety of Saccharomyces boulardii were considered. Trials were included regardless of the lack of blinding or placebo treatment. All other study designs were excluded. Infants and paediatric patients, aged between 0 and 16 years, had to be admitted to a hospital setting with a diagnosis of AGE (≥ 3 unformed stools in the last 24 h and of ≤ 48 h duration). Studies including patients with the following characteristics were excluded: chronic illnesses, under-nutrition, severe dehydration, known allergies, recent history of use of one or a combination of probiotics, antibiotics and anti-diarrhoea medication. Only studies using Saccharomyces boulardii as the intervention were included. Any study in which the Saccharomyces boulardii intervention was confounded by another intervention and without a proper control was excluded. Use of other strains of Saccharomyces (as the intervention) was not included.
Outcomes
The interventions and outcome measures were identified by the authors based on clinical relevance (see Table ) with modifiers and confounders decided a priori.
Table 1: Outcome measures and modifiers/confounders
Data collection and extraction
Preliminary screening was conducted by one reviewer (MP) and articles that were clearly non-relevant to the current systematic review were filtered out of the search pool (e.g. non RCTs; multi-species trials, studies not related to AGE). Pre-piloted study eligibility forms were then used by each of the two identified reviewers (MP and EV), article titles and abstracts were screened, consensus was obtained for all articles and clearly non-relevant articles were removed. Thereafter, a pre-piloted standardised data extraction form was used by each of the two reviewers (MP and EV) to independently extract data from the full text articles used in this systematic review. Any disagreements were resolved by discussion between the two reviewers (MP and EV), with assistance from the rest of the author team as necessary. All excluded studies were listed, each with reasons for exclusion.
Risk-of-bias assessment
The domains of the methodology assessed were sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources to affect validity.Citation22–24 Assessment was done using the Cochrane risk-of-bias assessment tool, where the judgement of ‘yes’ was indicative of low risk of bias, ‘no’ was indicative of high risk of bias, and ‘unclear’ was indicative of uncertain risk of bias.Citation22–24 This was done by two independent reviewers (MP and EV) and disagreements between each of the reviewers’ judgements were resolved by discussion, with assistance from the rest of the author team as necessary.
Grading the body of evidence
The Grades of Recommendations, Assessment, Development and Evaluation (GRADE) system for rating overall quality of evidence for the most relevant outcomes was applied.Citation22–24 The quality of evidence was further categorised as either high (confident that the true effect lies close to that of the estimated effect), moderate (moderately confident in the effect estimate), low (confidence in the effect estimate is limited) and very low (very little confidence in the effect estimate).Citation22–24
Statistical analysis
All dichotomous data resulted in the following information being extracted from each treatment group: the number of participants with the event and the total number of participants. Risk ratios (RRs) were calculated for all dichotomous data. All continuous data resulted in the following information being extracted from each treatment group: the arithmetic mean, standard deviation (SD) and number of participants. The SD was calculated using the 95% confidence interval (CI) and mean differences (MDs) were calculated for continuous data where applicable. Assessment of heterogeneity was achieved through the visual inspection of forest plots.Citation22,23 CIs were assessed and considered to have statistical heterogeneity if there was poor overlap of the results of individual studies. A chi-square test for heterogeneity (significance level p < 0.1) was conducted and the I2 statistic calculated.
Funnel plots are usually used to explore the possibility of small-study bias.Citation22,23 Tests for funnel plot asymmetry should only be used when there are a least 10 studies included in a meta-analysis, as fewer studies would result in the power of the tests being too low to identify chance versus real asymmetry.Citation22,23 Since a meta-analysis of 10 or more studies was not undertaken in this systematic review, funnel plots were not used to assess publication bias.
Results
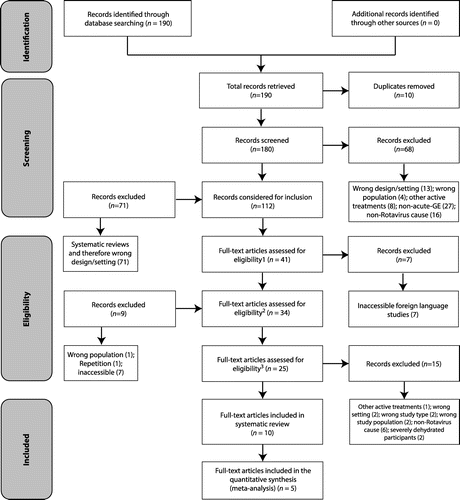
Ten studiesCitation25–34 met the inclusion criteria and were included in this systematic review (see Figure ). The 10 included studiesCitation25–34 were published between 2006 and 2013. Important information concerning these studies can be found in Table . A total of 1 401 participants were included from the combined 10 studies, with the smallest studyCitation30 involving 27 participants and the largest studyCitation31 involving 480 participants. Included studies were conducted in a hospital setting, but in multiple global locations, i.e. one in PakistanCitation,27 two in India,Citation28,32 one in Brazil,Citation29 one in MyanmarCitation34 and five in different hospitals within TurkeyCitation.25,26,30,31,33
Table 2: Characteristics of included studies
All 10 included studies adopted a study design that included both an intervention and control/placebo group, being monitored in parallel. The intervention arm consisted of ≥ 1 intervention, but with Saccharomyces boulardii always being used as an independent intervention. Across all 10 studies, Saccharomyces boulardii was used at a dose ranging from 200 mgCitation29 to 250 mgCitation25,27,28,30–34 with only one studyCitation26 offering the yeast probiotic at a slightly higher dose of 282.5 mg. In terms of frequency of treatment, 50% of studies offered the intervention dose once per dayCitation26,28,29,31,33 and 50% offered the intervention dose twice per dayCitation.25,27,30,32,34
Most studiesCitation25,28,29,31–34 considered the first five days as the ‘active’ treatment days, with one studyCitation27 using six days as the active treatment days. Only one studyCitation30 required the intervention to be implemented over a seven-day period. One studyCitation26 did not specify the minimum ‘active’ treatment phase but provided information on the mean duration time of GE in all study groups of (5.9 ± 2.0) days. Of all the included studies, only oneCitation27 followed participants for two months post discharge to assess incidence of GE episodes post intervention.
Not all included studies indicated or implemented the use of a placebo in their study designs, i.e. six studiesCitation25–28,31,34 did not describe or make use of a placebo, whilst the remaining four studiesCitation29,30,32,33 mentioned/described the placebo treatment used.
The four studies that described use of a placebo did so in different ways, i.e. one studyCitation33 offered both the intervention and an identical-looking placebo diluted in water or juice (as advised by the manufacturer); one studyCitation30 offered both the intervention and placebo dissolved in water; one studyCitation32 offered both the intervention and placebo in identical packets mixed with puffed rice powder; and one studyCitation29 offered both the intervention and placebo in capsule form, prepared by a faculty pharmacy.
Methodological quality
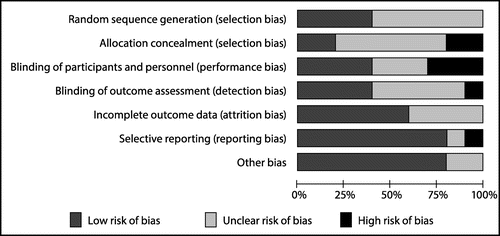
Random sequence generation was found to be adequate in 4 of the 10 studies.Citation25,28,31,34 No studies were found to be at high risk of bias in this domain. Adequate allocation concealment was achieved in 2 of the 10 studies,Citation29,32 with 2 studiesCitation25,27 classified as high risk of bias. The blinding of participants and personnel was found to be adequate in 4 of the 10 studies.Citation29,30,32,33 Three studiesCitation25,26,34 posed a high risk to blinding practices and the remaining three studiesCitation27,28,31 did not provide enough details to be totally clear about bias infringements in this domain.
In total, 50% of the studiesCitation26,27,30,31,34 did not clearly indicate how blinding of outcome assessment was guaranteed. The remaining studies consisted of only one studyCitation33 that did not provide for adequate blinding of this domain and four studiesCitation25,28,29,32 achieving adequate blinding. Six studiesCitation25,27–29,31,32 provided enough information to be considered to have adequate prevention of attrition bias. Only eight studiesCitation25–29,32–34 clearly reported on all outcomes initially mentioned.
Sources of funding could possibly play a role as a potential source of bias: two of the included studiesCitation27,30 were funded and supported by pharmaceutical companies, with one studyCitation30 declaring no conflict of interest in relation to the study. One studyCitation28 acknowledged receiving financial support from a university affiliated with the hospital where the study was conducted. Another studyCitation29 reported support from a government council involved with scientific and technological development. Some 50% of the included studiesCitation25,26,31–33 did not disclose any information about source of funding or financial support received. However, one of these studiesCitation32 made a simple declaration that no conflict of interest and no funding were received for the study. The one remaining studyCitation34 was the only study where authors commented that it was completed with a very limited budget owing to there being no involvement of the company commercialising the yeast probiotic that was used in the interventional arm. Other areas of bias did not appear to be a concern in eight of the included studiesCitation25–27,29,30,32–34 and was considered adequate (see Figure ).
GRADE assessment
GRADEpro software (http://www.gradepro.org) was used to assess overall methodological quality as follows: duration of diarrhoea (rated moderate); mean number of stools per day (only one study of low quality); frequency of diarrhoea (one study but of high quality); number having <3 stools per day (one study of moderate quality); and duration of hospital stay (two studies with evidence rated as low). A summary of findings table was generated (see Table ).
Table 3: Summary of findings table using GRADE: Saccharomyces boulardii compared with control or placebo for AGE
Summary of main results
Primary outcomes
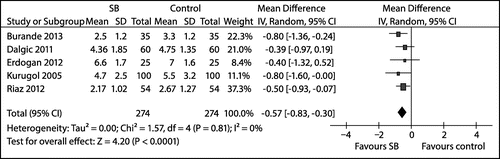
All of the included studies investigated the efficacy of Saccharomyces boulardii on GE caused by rotavirus but reported their findings in somewhat different ways. Seven studiesCitation26–28,31–34 reported duration of diarrhoea (in days), whilst one studyCitation28 reported the outcome as recovery from loose motions. Five studiesCitation26,28,31–33 were pooled in a random effects meta-analysis which showed that Saccharomyces boulardii significantly shortened the duration of diarrhoea (in days), compared with the control or placebo group (MD –0.57; 95% CI –0.83 to –0.30; n = 548 children; five studies). Furthermore, there was no significant heterogeneity detected between the trials (tau2 = 0.00; chi2 = 1.57; df = 4; p = 0.81; I2 = 0%) (see Figure ).
Figure 3: Forest plot of trials comparing Saccharomyces boulardii versus control in children with gastroenteritis: difference in duration of diarrhoea (in days).
Although two studiesCitation27,34 reported the mean duration of diarrhoea without the corresponding SDs, and therefore could not be included in the above meta-analysis, the study authors reported that Saccharomyces boulardii significantly shortened the duration of diarrhoea (in days), compared with the control group in both studies, i.e. one studyCitation27 (MD –1.2 (3.6 versus 4.8); n = 100 children; p = 0.001); and one studyCitation34 (MD –1.6 (3.08 versus 4.68); n = 100 children; p < 0.05).
Three studiesCitation27,30,32 could not be used in the above meta-analysis owing to limited information; one research groupCitation27 did report that use of Saccharomyces boulardii offered statistically significant effects on the number of stools per day compared with the control group for Day 3 (MD –1.6 (2.8 versus 4.4); p = 0.01) and Day 6 (MD –1.7 (1.6 versus 3.3); p = 0.001), but not for Day 0 (MD (9.5 versus 8.8); p = 0.37). Results from the remaining studyCitation30 showed a significant difference in the mean number of stools per day between the Saccharomyces boulardii group and the control group for Day 1 (MD –0.86; 95% CI –1.15 to –0.57), Day 2 (MD –1.21; 95% CI –1.49 to –0.93), Day 3 (MD –1.68; 95% CI –1.93 to –1.43) and Day 4 (MD –1.38; 95% CI –1.65 to –1.11), but there was no difference on Day 0 (MD 0.31, 95% CI –0.06 to 0.68). Overall, the pooled effect size for the duration of treatment of AGE in this study favoured the Saccharomyces boulardii group (p = 0.001).
Billoo et al.Citation27 reported on the mean number of episodes of diarrhoea after Month 1 and Month 2 but there were no SDs reported. Saccharomyces boulardii was found to significantly shorten the mean number of episodes of diarrhoea compared with the control group for both Month 1 (MD –0.44 (0.2 versus 0.64); n = 100 children; p = 0.001) and Month 2 (MD –0.24 (0.32 versus 0.56); n = 100 children; p = 0.04).
Another studyCitation29 reported the number of children having diarrhoea on each day after starting the intervention and the results show that Saccharomyces boulardii significantly reduced the risk of diarrhoea compared with the control group for Day 2 (RR 0.54; 95% CI 0.42 to 0.70; n = 176 children) and Day 3 (RR 0.54; 95% CI 0.38 to 0.77; n = 176 children) but not on Day 1 (RR 0.96; 95% CI 0.87 to 1.05; n = 176 children). Overall, the effect of Saccharomyces boulardii for the first three days of treatment did not demonstrate superiority over the control (p = 0.19).
The authors Htwe et al.Citation34 reported on the number of children having < 3 stools per day after starting the intervention and the results show that significantly more children were having < 3 stools per day in the Saccharomyces boulardii group (n = 50) compared with the control group (n = 50) on Day 2 (RR 1.80; 95% CI 1.10 to 2.95), Day 3 (RR 1.39; 95% CI 1.05 to 1.85), and Day 4 (RR 1.23; 95% CI 1.05 to 1.44). On Day 1, none of the children had < 3 stools per day in either group. On Day 6 and Day 7, all the children had < 3 stools per day. On Day 5, there was no difference in the number of children having < 3 stools per day in the two groups (RR 1.04; 95% CI 0.97 to 1.11). Although this analysis appeared to moderately favour the Saccharomyces boulardii group, it was not statistically significant (p = 0.11).
These same authorsCitation34 reported on the number of children having solid stools per day after starting intervention and the results show that significantly more children were having solid stools in the Saccharomyces boulardii group (n = 50) compared with the control group (n = 50) on Day 2 (RR 3.00; 95% CI 0.32 to 27.87), Day 3 (RR 3.17; 95% CI 1.89 to 5.31), Day 4 (RR 1.63; 95% CI 1.30 to 2.06) and Day 5 (RR 1.25; 95% CI 1.08 to 1.44). On Day 1, none of the children had solid stools in either group. On Day 7, all the children had solid stools. On Day 6, there was no difference in the number of children having solid stools between the two groups (RR 1.04; 95% CI 0.97 to 1.11). Although the results appeared to favour the Saccharomyces boulardii group, this was not statistically significant (p = 0.06).
In addition to the above, one other primary outcome of the current systematic review was to investigate the safety of use of this yeast probiotic in the paediatric hospitalised patient. None of the included studies reported on any significant side effects associated with Saccharomyces boulardii use.
Secondary outcomes
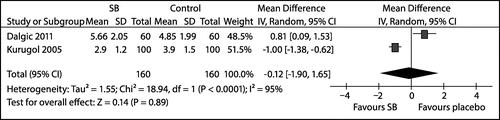
Two studiesCitation31,33 reported on the duration of hospital stay (in days) and their results were combined in a meta-analysis that resulted in significant statistical heterogeneity (tau2 = 1.55; chi2 = 18.94; df = 1; p < 0.0001; I2 = 95%) (see Figure ).
None of the 10 studies evaluated other outcomes (e.g. cost-effectiveness, optimal dosing and delivery method, frequency/duration of treatment, timing of delivery of Saccharomyces boulardii).
Discussion
In this systematic review, we set out to assess the effectiveness and safety of Saccharomyces boulardii in the management of AGE in the paediatric hospitalised population. Like the current systematic review, other researchers like Szajewska et al. (2007),Citation21 McFarland (2010),Citation16 Allen et al. (2010)Citation14 and Pan et al. (2012)Citation35 have attempted to review and possibly put forward treatment guidelines for the use of Saccharomyces boulardii in the management of GE, but often in a mixed population of both adult and paediatric participants, whilst this review focused exclusively on the latter.
Except for the study setting not being a hospital, the systematic review conducted by Szajewska et al. (2007)Citation21 is the closest match to the inclusion criteria of the current systematic review. These authorsCitation21 conducted a systematic review of only RCTs to test the effectiveness of Saccharomyces boulardii in treating AGE in children. Five RCTs involving 619 participants were included. The combined data showed that Saccharomyces boulardii significantly reduced the duration of diarrhoea when compared with the control arm. Using a fixed and random effects model, this yeast probiotic still produced a mean difference of –1.1 days (95% CI –1.2 to –0.8). Although a smaller study sample (n = 548) and a smaller mean difference of –0.57 days (95% CI: –0.83 to –0.30), the current systematic review also produced results in favour of use of Saccharomyces boulardii to treat AGE, but specifically in the paediatric patient. Significant changes in GE experienced by the Saccharomyces boulardii group versus the control group were noted on Day 3, in addition to Day 6 and Day 7, similar to results reported by McFarland (2010).Citation16 Szajewska et al.Citation21 also reported than in one RCT study (n = 88) the risk of diarrhoea lasting > 7 days was significantly reduced in the Saccharomyces boulardii versus the control group (RR 0.25; 95% CI 0.08 to 0.83; number needed to treat = 5, 95% CI 3 to 20). Such results pointed in the direction of Saccharomyces boulardii displaying moderate clinical benefit in otherwise healthy infants and children with AGE.
The Cochrane Review carried out by Allen et al. (2010)Citation14 was another systematic review aimed at assessing the effect and – like the current systematic review – safety of probiotics, including Saccharomyces boulardii, in treating GE. This review was much larger than the systematic reviews mentioned earlier as it included 63 studies with a combined 8 014 participants. Within this large pool of studies, 56 included infants and young children. The included studies took the form of either RCTs or quasi-RCTS that compared the effect of a specified probiotic with either a placebo/no probiotic in people with AGE. The overall result was indicative of probiotics (including Saccharomyces boulardii) having the ability to reduce the duration of GE. But similar to McFarland (2010)Citation,16 these authorsCitation14 also acknowledged challenges faced in conducting their systematic review. Included studies in their systematic review varied in their definitions of both AGE and AGE resolution, the studies were all undertaken in a wide range of different settings and there was variation in terms of the organism tested, dosage offered and participant characteristics. The authorsCitation14 concluded that if used alongside oral rehydration solution, probiotics (including Saccharomyces boulardii) appeared to be safe and have the potential to reduce AGE duration and reduce stool frequency.
The systematic review conducted by Pan et al. (2012)Citation35 was similar to the current systematic review in that three of the studiesCitation27,33,34 included in the current systematic review also featured in theirCitation35 list of included studies. Similar to challenges experienced with the current systematic review, these authors also had difficulty retrieving suitable RCTs for inclusion, i.e. only 8 included studies from a total pool of 678. These 8 studies included participants that ranged between the ages of 1 month up to 12 years, were all described as being randomised into either the Saccharomyces boulardii or the control (commercialised oral rehydration solution) group, received about the same dosage of Saccharomyces boulardii (500 mg/d) but with only two studies indicating smaller doses of Saccharomyces boulardii (250 mg/d) for participants < 12 months. All participants received the intervention for a period of 5–7 days, with only one study continuing to follow the participants up to Day 14. Although only 25% of the included studies reported on the cause of the GE, and not all studies were carried out in a hospital setting, the authors reported that the results of their meta-analysis showed that the Saccharomyces boulardii group was more effective than the control group in decreasing the following: duration of diarrhoea (MD –0.92, 95% CI –1.32 to –0.52), stool frequency on Day 3 (MD –1.92, 95% CI –1.63 to –0.95), Day 4 (MD –0.51, 95% CI –0.89 to –0.33), and Day 7 (MD –0.44, 95% CI –0.72 to –0.16), respectively. Despite only 25% of included studies indicating the cause of the diarrhoea in each of their studies, the authors concluded that Saccharomyces boulardii displayed therapeutic effects in treating children with AGE.
Safety of use of the yeast probiotic was the other primary outcome under investigation, and of the 10 included studies, only 1 studyCitation33 reported on a single participant complaining of ‘meteorism’, which is defined as excess gas accumulating in the gastrointestinal system and causing abdominal distension.Citation36 No additional information was provided by the authors and neither was there mention of the participant needing to be removed from the trial. Other than this reporting, no serious adverse reaction in the Saccharomyces boulardii group was registered during any of the included studies.
Overall, offering this unique yeast probiotic at a dose of 250 mg once to twice per day for up to 5–7 days has shown some statistically significant benefit in decreasing the duration of AGE. Although no statistical difference was noted between the groups with the number of days in hospital, the days to appearance of the first semi-formed stool were found to be fewer in the Saccharomyces boulardii group as compared with the control group.Citation9–12,14,19,28–32,34
Overall completeness and applicability of evidence
Any factor that disrupts the bowel’s multifaceted ecosystem can result in the development of gastrointestinal disease, with GE being one of the most documented symptoms. The difficulty this presents is that GE can be categorised in various ways, i.e. according to cause (e.g. bacterial, viral, parasitic)Citation,3,4,6 or by severity (e.g. mild, moderate and severe).Citation3,4,6 This systematic review was very specific as it aimed to include only those studies addressing mild–moderate GE caused by the rotavirus. In addition, subjects needed to be between 0 and 16 years, be in a generally healthy condition with no other co-morbidities and qualify for hospitalisation. The addition of studies investigating the effects of Saccharomyces boulardii only made this a very challenging search for supporting studies. Reviewers identified 10 RCTs involving a combined 1 401 participants between the ages of 0 and 16 years for inclusion in this systematic review.
The study settings within which each of the included studies took place were in many different countries across the globe (i.e. Pakistan, India, Brazil, Myanmar and Turkey). Aside from varied geographical settings, the included studies also included participants that were from varied backgrounds, of differing socioeconomic status, with different research resources, different research teams and therefore varied methodological quality standards. One of the secondary outcomes of the current systematic review was to investigate the effect of Saccharomyces boulardii on the days of hospitalisation. Of the 10 included studies, only 3 studiesCitation25,31,33 reported on this outcome and each with a different result.
Quality of evidence
The 10 studies included in this review lacked meticulousness when it came to methodological quality. All 10 trials met the prerequisite of being RCTs. When judgement concerning each methodological quality item for each included study was undertaken, shortcomings across some of the domains for some of the studies were noted. Selection bias was clearly prevented in four studiesCitation25,28,31,34 as simple randomisation methods were described, i.e. computer-generated random numbersCitation,28,31 according to identification numbersCitation25 and simple alternated allocation to treatment and control groups.Citation34 The remaining six studiesCitation26,27,29,30,32,33 reported carrying out randomisation but details on how this was achieved were unclear.
The quality of the individual included studies ranged between low and moderate, with unclear risk of bias displayed for especially the first four domains, i.e. random sequence generation, allocation concealment, blinding of participants/personnel and blinding of outcome assessment (see ). Some of the results for some outcomes showed clear differences between groups within single studies. However, the manner in which outcomes were reported (i.e. number of stools per day, days to < 3 stools per day, mean number of stools) resulted in only one meta-analysis being done.
Potential biases in overview process
One of the biggest ‘threats’ to systematic reviews is publication bias, defined as ‘the publication or non-publication of research findings, depending on the nature and direction of results’.Citation22,23 As a result, this would impact on the ‘true’ nature of the research topic under investigation. Although an exhaustive electronic search strategy was employed in this systematic review, there is always the possibility that applicable research papers could have been missed or overlooked during various stages of the search and selection process. The use of two reviewers (MP and EV) independently assessing studies for inclusion in this systematic review was an additional measure to address this form of bias.
Strengths and limitations of this review
Although only 10 studies successfully met the predetermined inclusion criteria, this led to a more appropriate comparison, and therefore pooling of results between the intervention and control groups of each individual study. Although foreign-language studies were excluded from this review, all potentially eligible studies reported in languages other than English were documented for future assessment. Of the latter only one study appeared to be possibly relevant to this investigation.
Lastly, the authors of this systematic review were able to identify and use RCTs that met the predefined inclusion criteria but could not control for the different geographical settings in which each of these trials was conducted and their influence on the results of this review.
Conclusions
Overall, the results indicate that Saccharomyces boulardii shortened the duration of AGE caused by rotavirus (in days) when compared with the control/placebo group, with the included studies displaying little/no heterogeneity. In addition, no adverse effects were associated with the use of this yeast probiotic in treating AGE in otherwise healthy children. Therefore, the results of the current systematic review indicate that there is a potential benefit associated with the use of Saccharomyces boulardii to treat AGE in the paediatric patient.
However, owing to factors such as small sample sizes, unclear and inconsistent quality of methodology, and reporting bias owing to source of funding and support, a definitive conclusion and recommendation for the use of a specific probiotic like Saccharomyces boulardii to be used as treatment or treatment adjunct for AGE in the paediatric hospitalised patient cannot yet be made. Future research initiatives investigating the subject of the benefits/harm associated with the use of Saccharomyces boulardii must therefore endeavour to consist of larger RCTs which: minimise heterogeneity associated with study participants enrolled, clearly predefine aetiologies, e.g. GE or AGE, minimise methodological variability (e.g. blinding), standardise the presentation in which the intervention is offered, and conduct single-strain probiotic investigations. In addition, secondary outcomes like length of hospital stay and cost-effectiveness can also be investigated.
| Abbreviations | ||
| AGE | = | acute gastroenteritis |
| CI | = | confidence interval |
| GE | = | gastroenteritis |
| GRADE | = | Grades of Recommendations, Assessment, Development and Evaluation |
| MD | = | mean difference |
| PROSPERO | = | Prospective Register of Ongoing Systematic Reviews |
| RCTs | = | randomised controlled trials |
| RRs | = | risk ratios |
| SDs | = | standard deviations |
| WHO | = | World Health Organization |
Author contributions
All authors contributed to the preparation of this systematic review and approved the final manuscript.
Data sharing
If requested, the authors are prepared to provide the data on which the manuscript is based.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
None to declare.
Acknowledgements
The authors would like to acknowledge Mrs Wilhelmine Pool for her excellent work conducting a thorough electronic search of studies on the subject matter.
References
- United Nations International Children’s Fund (UNICEF) . Millennium project commissioned by the United Nations Secretary General and supported by the United Nations development group. MDGs. 2009-2015 [cited 2016]. Available from: http://www.data.unicef.org
- United Nations Children’s Fund (UNICEF) . Pneumonia and diarrhea: tackling the deadliest disease’s for the world’s poorest children. Statistics and Monitoring Section – Division of Policy and Strategy . June 2012 [cited 2016]. Available from: www.data.unicef.org
- United Nations Children’s Fund (UNICEF) . Committing to child survival: a promise renewed. Progress report 2015. Division of Data, Research and Policy. September 2015 [cited 2016]. Available from: www.unicef.org
- World Health Organization . WHO: health topics, diarrhea [Internet]. 2013 [cited 2014 Mar 12]. Available from http://www.who.int/topics/diarrhoea/en/
- Kelesidis T , Pothoulakis C . Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Ther Adv Gastroenterol. 2012;5(2):111–25.10.1177/1756283X11428502
- Jach ME , Sajnaga E , Kozak E , et al. Use of yeasts for prevention and therapy. Current Issues in Pharmacy and Medical Sciences. 2013;26(2):198–202.10.12923/j.2084-980X
- World Gastroenterology Organisation Global Guidelines . Review team. Acute diarrhea in adults and children: a global perspective. February 2012. [cited 2016]. Available from: http://www.worldgastroenterology.org/guidelines/global-guidelines
- Ciccarelli S , Stolfi I , Caramia G . Management strategies in the treatment of neonatal and pediatric gastroenteritis. Infection and Drug Resistance. 2013;6:133–61.
- Canani RB , Cirillo P , Terrin G , et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007;335(7615):340. Available from www.bmj.com.
- Johnston BC , Supina AL , Vohra S . Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials. CMAJ. 2007;175(4):377–83.
- Johnston BC , Goldenberg JZ , Vandvik PO , et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database of Systematic Reviews. 2011;(11). Art. No.:CD004827. doi:10.1002/14651858.CD004827.pub3.
- Buts JP . Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci. 2009;54:15–8.10.1007/s10620-008-0322-y
- Guandalini S . Probiotics and treatment of diarrhea. J Clin Gastroenterol. 2011;45: S149–S153.10.1097/MCG.0b013e3182257e98
- Allen SJ , Martinez EG , Gregorio GV , et al. Probiotics for treating acute infectious diarrhea. Cochrane Database of Systematic Reviews. 2010. Available from: http://onlinelibrary.wiley.com/doi/10.1002/psb.701/pdf.
- Hatoum R , Labrie S , Fliss I . Antimicrobial and probiotic properties of yeast: from fundamental to novel applications. Frontiers in Microbiology. 2012;3(421):1–12.
- McFarland LV . Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16(18):2202–22.10.3748/wjg.v16.i18.2202
- Czerucka D , Piche T , Rampal P . Review article: yeast as probiotics - Saccharomyces boulardii . Aliment Pharmacol Ther. 2007;26:767–78.10.1111/j.1365-2036.2007.03442.x
- Dalmasso G , Loubat A , Dahan S , et al. Saccharomyces boulardii prevents TNF-alpha-induced apoptosis in EHEC-infected T84 cells. Res Microbiol. 2006;157(5):456–65.10.1016/j.resmic.2005.11.007
- Szajewska H , Mrukowicz J. Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in prevention of antibiotic-associated diarrhea. Aliment Pharmacol Ther. 2005; 1: 22(5):365–72.10.1111/apt.2005.22.issue-5
- Sazawal S , Hiremath G , Dhingra U , et al. Efficacy of probiotics in prevention of acute diarrhea: a meta-analysis of masked, randomized, placebo-controlled trials. Lancet Infect Dis. 2006;6(6):374–82.10.1016/S1473-3099(06)70495-9
- Szajewska H , Skorka A , Dylag M . Meta-analysis: Saccharomyces boulardii for treating acute diarrhoea in children. Aliment Pharmacol Ther. 2007;25(3):257–64.
- Higgins JPT , Green S , editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org
- Kugley S , Wade A , Thomas J , et al. Searching the studies: a guide to information retrieval for Campbell systematic reviews. Campbell Methods Guides. 2016:1. Available from http://www.campbellcollaboration.org
- Howick J , Chalmers I , Glasziou P , et al. The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine. Available from: http://www.cebm.net/index.aspx?o=5653.
- Eren M , Dinleyici EC , Vandenplas Y . Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non-bloody Diarrhea in children: a randomized controlled, open label study. American Journal of Tropical Medicine and Hygiene. 2010;82(3):488–91.10.4269/ajtmh.2010.09-0529
- Erdoğan Ö , Tanyeri B , Torun E , et al. The comparition of the efficacy of two different probiotics in rotavirus gastroenteritis in children. Journal of Tropical Medicine. 2012; Article ID 787240: 1–5.10.1155/2012/787240
- Billoo AG , Memon MA , Khaskheli SA , et al. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhea. World Journal of Gastroenterology. 2006;12(28):4557–60.10.3748/wjg.v12.i28.4557
- Burande MA . Comparison of efficacy of Saccharomyces boulardii strain in the treatment of acute diarrhea in children: A prospective, single-blind, randomized controlled clinical trial. Journal of Pharmacology and Pharmacotherapeutics. 2013;4(3):205–8.10.4103/0976-500X.114603
- Corrêa NBO , Penna FJ , Lima FLMS , et al. Treatment of Acute Diarrhea with Saccharomyces boulardii in Infants. Journal of Pediatric Gastroenterology and Nutrition. 2011;53:497–501.
- Ozkan TB , Sahin E , Erdemir G , et al. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. The Journal of International Medical Research. 2007;35:201–12.10.1177/147323000703500204
- Dalgic N , Sancar M , Bayraktar B , et al. Probiotic, zinc and lactose-free formula in children with rotavirus diarrhea: Are they effective? Pediatrics International. 2011;53:677–82.10.1111/j.1442-200X.2011.03325.x
- Riaz M , Alam S , Malik A , et al. Efficacy and Safety of Saccharomyces boulardii in acute childhood diarrhea: a double blind randomised controlled trial. Indian Journal of Pediatrics. 2012;79(4):478–482.10.1007/s12098-011-0573-z
- Kurugöl Z , Koturoğlu G . Effects of Saccharomyces boulardii in children with acute diarrhoea. Acta Pædiatrica. 2005;94:44–7.10.1080/08035250410022521
- Htwe K , Yee KS , Tin M , et al. Effect of Saccharomyces boulardii in the Treatment of Acute Watery Diarrhea in Myanmar Children: A Randomized Controlled Study. American Journal of Tropical Medicine and Hygiene. 2008;78(2):214–6.
- Pan J , Hu J , Qi X , et al. A meta-analysis of the efficacy of Saccharomyces boulardii in children with acute diarrhea. African Journal of Pharmacy and Pharmacology. 2012;6(21):1508–1514.
- Lindsey Baker . Concise Medical Dictionary (6th edition). Reference Reviews . 2003; 17(2):32–32. doi:10.1108/09504120310461752.