Abstract
Multiple sclerosis (MS) is a chronic, inflammatory, neurodegenerative demyelinating disease of the central nervous system (CNS). Inflammation is increased by high-energy Western-style diets, typically high in salt, animal fat, red meat, sugar-sweetened drinks and fried food, and low in fibre, as well as lack of physical exercise. An anti-inflammatory dietary regimen, with or without administration of dietary supplements, supporting the general trend towards an amelioration of inflammatory status, should be considered.
Understanding the role of gut microbiota in health and disease can lay the foundation to treat chronic diseases by modifying the composition of gut microbiota through lifestyle choices, including dietary habits and possibly probiotic supplementation.
Evidence from experimental, epidemiologic and clinical studies supports the potential association between poor vitamin D status and the risk of developing MS, as well as an adverse disease course. Correcting vitamin D status seems plausible in patients with MS.
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, neurodegenerative demyelinating disease of the central nervous system (CNS).Citation1–4 Its onset is more common in young adults and the disease has a female predominance.Citation4 While the aetiology of MS is not completely understood, it seems to be a multifactorial entity that is influenced by both genetic and environmental modifications.Citation1–5 Over the last 20 years evidence has emerged suggesting that distinct immunological pathways drive the progression and relapses of the disease. Thus, immunomodulatory drugs are used in the treatment of MS.Citation6 Some of the risk factors associated with the development or progression of MS have been reported (Table ).
Table 1: Risk factors for the development of multiple sclerosisCitation2,3,5,7,8
At present, MS therapy is not consistently associated with any particular diet, probably due to lack of information on the effects of nutrition on the disease.Citation5,9 However, diets and dietary supplements are frequently used by people with MS in the belief that they might improve disease outcomes in light of the seemingly limited effectiveness and efficacy of conventional treatments.Citation3 Dietary components could, in principle, result in immune modulation and, thus, could be used to obtain beneficial outcomes in such patients.Citation5,10 This review will focus on the interaction between diet and the immune system and inflammation in the context of MS. These effects may happen through direct manipulation of the inflammatory immune response and/or, indirectly, through modulation of the gut microbiota, which is also known to modulate the immune response.Citation1,5,9
Interaction between diet and/or nutrients and the inflammatory response
Different types and amount of dietary factors can interact with enzymes, transcription factors and nuclear receptors of human cells. This may modulate the inflammatory and autoimmune responses in the body.Citation10 The innate immune response is modulated to either the pro-inflammatory Th1/Th17 pathway, releasing pro-inflammatory cytokines (IL-6, IL-10), or to a down-regulation via release of anti-inflammatory cytokines (IL-4, IL-10).Citation9,10
Inflammation is thought to be increased by high-energy Western-style diets, which are typically high in salt, animal fat, red meat, sugar-sweetened drinks and fried food, and low in fibre, as well as a lack of physical exercise. The persistence of this type of diet and lifestyle upregulates the metabolism of human cells toward biosynthetic pathways that favour the production of pro-inflammatory molecules and also promotion of a dysbiotic gut microbiota environment, with alteration of intestinal immunity, which is conducive to low-grade systemic inflammation.Citation2,11–13
On the other hand, exercise and low-energy diets, based on the consumption of vegetables, fruit, legumes, fish, prebiotics and probiotics, act on nuclear receptors and enzymes that upregulate oxidative metabolism, downregulate the synthesis of pro-inflammatory molecules, and restore or maintain a healthy symbiotic gut microbiota pattern.Citation2,9
Oxidative stress, with excessive production of reactive oxygen species, and reduction of antioxidant defence mechanisms, has been implicated in the pathogenesis of MS. Therefore, much of the research into the role of diet and lifestyle in managing MS has focused on inflammation that increases the oxidative burden in the body.Citation14 At present, factors linked to the inflammatory response that are considered to influence the course of the disease include Western-style high-energy diets, low availability and serum levels of vitamin D, postprandial inflammation associated with high animal fat/high sugar and refined carbohydrate diets and obesity (Table ). Obesity has also been associated with a dysbiotic gut microbiota, alteration of intestinal immunity, and low-grade systemic inflammation.Citation2,9,14
Table 2: Pro and anti-inflammatory dietary factors
Gut microbiome and the immune response
The human gut carries, on average, about 540 000 microbial genes, representing the dominant microbes in this ecosystem. Approximately 55% of these genes constitute the core metagenome (i.e. genes shared among at least 50% of individuals), while many other genes appear to be unique and/or present in less than 20% of individuals. This complex ecosystem is an essential part of the human organism and influences both our immune system and our metabolism. Therefore, it has a strong impact on human health.Citation36 The gut microbiota influences health and nutritional status via multiple mechanisms, including eliminating unwanted pathogens, regulating metabolism and influencing the immune response.Citation5 A mounting body of evidence recognises that microbial metabolites have a major influence on host physiology.
The composition of the intestinal microflora is highly individual and is influenced by many factors such as diet, physical activity, stress, medications and age.Citation37−40 Dietary manipulation can regulate gut immunity directly through the formation of either T-cells that suppress immunity, or T-helper 17 cells that stimulate immunity.Citation5
Moreover, dietary intake can directly modulate the microbiome composition and therefore function. A plant-based dietary intake results in a Fermicutes predominance, which ferments non-digestible carbohydrate and contributes to the formation of short-chain fatty acids (SCFA) with anti-inflammatory outcomes.Citation2,5 Conversely, colonic fermentation of animal-based dietary intake (Western diets) leads to bacteria that are more bile-tolerant.Citation5 The latter results in reduced microbial diversityCitation2 and contributes to low-grade inflammation.Citation2 An increase in bile-acid production also results in decreased binding activity for vitamin D receptors, thus decreasing the effectiveness of vitamin D supplementation.Citation2 The most common consequence of a dysbiotic gut microbiota pattern is alteration of the mucosal immune system and the rise of inflammatory, immune, metabolic or degenerative diseases.Citation4,5,41
Both disease-promoting and disease-ameliorating mechanisms can be induced by the gut microbiota, which interacts closely and mutually with the host immune system. The nature of these interactions seems to depend on the composition of the gut microbiome and the immunologic state of the host. On these grounds, understanding the role of gut microbiota in health and disease can lay the foundation to treat chronic diseases by modifying the composition of gut microbiota through the choice of a correct lifestyle, including dietary habits. Whether enterotypes associated with long-term diets can be reversed by changes in the diet remains to be determined.Citation42
It has been argued that a nutritional intervention with anti-inflammatory food and dietary supplements can alleviate possible side effects of immune-modulatory drugs and the symptoms of associated chronic fatigue syndrome and thus favour patient wellness.Citation9 Dietary manipulation can also directly and indirectly affect the immune response, as will be discussed below.
Dietary fat and fatty acids
The relative intakes of different types of dietary polyunsaturated fatty acids (PUFAs) play an important role in determining the inflammatory state of the human body. The human body converts alpha linolenic acid (ALA), the omega-3 fatty acid mostly present in certain plant foods (flaxseeds and flaxseed oil, canola oil, soybeans and soybean oil, pumpkin seeds)Citation43 to the anti-inflammatory precursors EPA (eicosapentaenoic acid) and DHA (docosapentaenoic acid), the omega-3 fatty acids which are usually associated with fish oil. The eicosanoids derived from AA (arachidonic acid) generally promote inflammation, whereas those from EPA and DHA are less inflammatory, inactive or even anti-inflammatory.Citation44 EPA and DHA compete with AA for access to the cyclooxygenase (COX) and lipoxygenase (LOX) enzymes. High intake of long chain omega-3 EPA and DHA in the diet allows for the partial replacement of AA, thus reducing the amount available to form the metabolites that are associated with inflammation and chronic disorders (Figure ).Citation16,17
Figure 1: Metabolism of omega-6 and omega-3 polyunsaturated fatty acids (figure used with permission. COX-2: cyclooxygenase, LOXs: lipoxygenase, TXA2: thromboxane A2 (platelet aggregator, vasoconstrictor), PGI2: prostaglandin I2 (vasodilator, anti-aggregator), PGE2: prostaglandin E2 (immuno-suppressor) Citation44
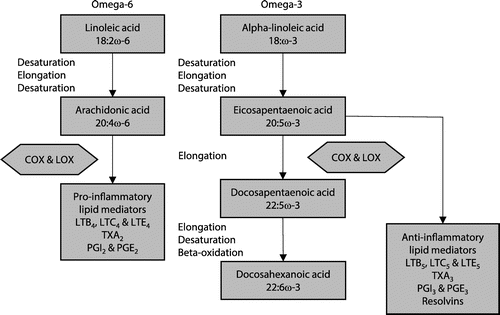
Evidence from some clinical trials on omega-3 supplementation point to the benefits in relapsing-remitting MS. In a systematic review, Farinotti et al. (2012) concluded that omega- 3 fatty acids seem to have no major effect on disease progression of MS, but tend to reduce the frequency of relapses over two years.Citation45 In 2013, based on its anti-inflammatory and neuroprotective action, daily oral supplementation with 4 g of fish oil was found to be highly effective in reducing the levels of pro-inflammatory cytokines and nitric oxide in patients with relapsing-remitting MS (RRMS).Citation14 In another randomised controlled clinical trial, a nutraceutical mixture of omega-3 and omega-6 fatty acids with vitamins was found to significantly reduce the relapse rate and the disability progression in patients with RRMS.Citation43 Secondary analysis of data from the Nurses’ Health Studies I and II found a higher baseline PUFA intake to be associated with a lower risk for MS. This was specific for an inverse association between intake of ALA and risk for MS.Citation7
Pantzaris et al. (2013) and Farinotti et al. (2012) concluded that the available data are insufficient to assess real benefit or harm from omega-3 supplementation, mostly because of the uncertain quality of the trials.Citation43,45 This was recently confirmed by the 2017 European Society for Clinical Nutrition and Metabolism (ESPEN) evidence- and consensus-based guidelines for neurological states, which state that there is insufficient evidence to recommend omega-3 supplements to MS patients.Citation46 It is, however, interesting to note that DHA is present in high concentrations in the brain and its levels decrease in patients with MS.Citation7,9
Vitamin D
Epidemiological studies have highlighted possible links between vitamin D insufficiency and a wide range of human diseases, including MS.2,4,8,47,48 Based on these studies, vitamin D deficiency seems to impact on both the onset and progression of MS.Citation4 It is believed that these effects are mediated via the immune-modulatory (anti-inflammatory) role of vitamin DCitation4,8,49 and/or via the ability of vitamin D to transcript certain genes associated with MS.Citation4 The clinical importance of this with regard to vitamin D insufficiency and immune-related diseases is discussed in more detail elsewhere.Citation49 The anti-inflammatory properties of vitamin D require the binding of calcitriol to the vitamin D receptor (VDR) of calcitriol [1,25-(OH)2D3]. Once it is formed, the complex VDR-D binds to the Retonoid X receptor (RXR) activated by its ligand retinoic acid (RA), the main metabolite of vitamin A. The heterodimer complex RXR–RA/VDR-D controls the expression of several genes involved in inflammatory and autoimmune processes in chronic diseases by inhibiting the pro-inflammatory transcription factor NF-κB and downregulating the synthesis of inflammatory molecules. For vitamin D supplementation to exert an adequate anti-inflammatory action, it should be administered together with vitamin A.Citation2
Vitamin D also controls tight junctions to maintain mucosal integrity. Vitamin D deficiency can thus result in mucosal permeability and contribute to gut dysbiosis.Citation2
The risk for MS is increased in individuals exposed to vitamin D deficiency during adolescence and early adulthood. Deficiency during pregnancy has also been linked to increased risk for MS in the offspring.Citation4
In individuals with established MS, vitamin D deficiency was also associated with increased disease progression.Citation8 Uncertainty still exists though about the optimal serum level of 25(OH)D in individuals with MS to ensure best outcomes. One such study reported a target of 100 nmol/l (40 ng/ml).Citation4
The role of routine use of vitamin D in MS patients, their first-degree relatives and people at risk of MS is still being debated and varies between different countries and even between practising neurologists working in the same area. Nevertheless, accumulating evidence from experimental, epidemiologic and clinical studies supports the potential link between poor vitamin D status and the risk of developing MS, as well as adverse disease course.Citation9,47
Clinical outcomes of trials with vitamin D supplementation in MS patients are less consistent.Citation50,51 Patients with MS have low levels of vitamin D,Citation47 but this is true for other chronic inflammatory diseases as well.Citation52,53 Studies mostly assessed the mechanism of the protective role of vitamin D in experimental autoimmune encephalomyelitis (EAE). The proposed underlying mechanisms for this relationship are inducing inflammatory cell apoptosisCitation54 i.e. CD4 T-cells,Citation55 suppressing immune cell infiltration into the CNS, i.e. CD 11b monocytes, decreasing inducible nitric oxide synthase,Citation54,55 as well as inhibiting pro-inflammatory cytokine secretion including IL-12 and IFN-γ.Citation56,57
Recommendations for vitamin D supplementation in MS have been put forward by many, including consensus guidelines that answered some important questions on this topic according to the available evidence or experts’ opinions, which was published by Jahromi et al. (2016).Citation51 These recommendations are summarised in Table . Although there is insufficient evidence to implement these guidelines as universal recommendations to reduce the risk for MS development, the recommendations are aimed at individuals with a family history of MS, those with clinically isolated syndrome (CIS) (patients who have experienced a solitary clinical event of demyelination, but not fulfilled the diagnostic criteria for MS or any other related disease)Citation51 and those living in areas where vitamin D deficiency is prevalent.Citation4
Table 3: Proposed management guidelines for Vitamin D and multiple sclerosisCitation2,4,5,8,51–58
The latest 2017 ESPEN guidelines on best medical nutrition therapy in patients with neurological diseases concludes that there is insufficient evidence to recommend vitamin D therapy in MS patients.Citation46 According to ESPEN, there is no clinical evidence on the effects of either vitamin D compared with placebo or high-dose vitamin D compared with low-dose on the relapse rate of patients with MS.Citation46 The studies that evaluate the effect of vitamin D are prone to many sources of bias (selection bias due to diet or sun exposure, reverse causation due to the disease, and effect of the skin type or genetic factors).
Vitamin A
Studies have shown that active vitamin A derivatives suppress the formation of pathogenic T cells in MS patients. Over the last two decades, it has become clear that vitamin A also has important roles in immune functioning, both in immunological tolerance and in adaptive immune responses.Citation59,60 Recently Bitarafan et al. (2015) conducted a randomised placebo-controlled clinical trial to investigate the effect of Vitamin A supplementation (25 000 IU/d retinyl palmitate for 6 month and 10000 IU/d for the next 6 months) on the clinical status, relapse rate and brain lesions in patients with MS. Vitamin A supplementation improved the MS functional composite score (measures the progression of disability as indicated by cognitive, lower and upper limb function), expanded disability status scale (determines lower limb function dominantly), relapse rate and brain lesions.Citation61
Lifestyle factors
Lifestyle factors such as smoking, alcohol use and physical activity may exacerbate or ameliorate MS symptoms by modulating the inflammatory status of the disease.Citation2,9
Although controversial,Citation2 both active and passive cigarette smoking is associated with the development of MS in a dose-dependent manner. Smoking is also associated with a faster deterioration and progression of disability in those with MS.Citation4,8 Smoking has been shown to have a direct and indirect (via gut microbiome) pro-inflammatory effect.Citation2
Studies of the impact of alcohol consumption on MS have produced inconsistent results. However, recent studies do not appear to support alcohol (beer, wine or liquor) consumption being associated with MS risk.Citation2,62,63
Conversely, the role of physical activity in MS is clearer. Activity results in decreased leptin levels.Citation2 Leptin is associated with fewer regulatory T-cells and thus with a more pro-inflammatory state.Citation3,8 Citation7 Through the anti-inflammatory effects of reduced leptin levels, the quality of life is greatly improved.Citation2,64 Less fatigue and a slower progression of debilitating symptoms have also been reported in patients with MS who partake in exercise.Citation2
The physical activity recommendations for individuals for MS are 20–60-minute sessions of moderate-intensity aerobic exercises at least 3–4 days per week, as well as 10–15 minute strength training sessions 2–3 days per week. Daily stretching exercises for 10–15 minutes are also recommended.Citation8
Obesity and weight management
A two- to threefold increased risk for MS has been reported in overweight individuals. These associations have been documented for overweight/obese children and adolescentsCitation4,5 and were more pronounced in females.Citation3−5 Obesity is known to be associated with persistent low-grade inflammation.Citation3−5, 8 It is also inversely associated with a decrease in vitamin D levelsCitation5,46 and increased leptin levels.Citation3,4,9
Gut microbiota have also been reported to be also influenced by body mass index (BMI). With a higher BMI, gut microbiome diversity decreases and the latter has been linked to increased risk for developing MS.Citation5 Low diversity is associated with insulin resistance, hyperlipidaemia and increased inflammatory markers.Citation5
High-energy, Western-type diets with high intake of salt, saturated animal fat, fried food and sugar-sweetened drinks may lead to the onset of postprandial inflammation and systemic low-grade inflammation. Conversely, with energy restriction, insulin release is decreased. Increased insulin levels result in an increased production of AA, which has pro-inflammatory effects (see Figure ).Citation2,9
Diet quality and MS
Better quality of life (QoL) and lower risk of debilitating disabilities are associated with an improved dietary quality (higher intake of fruit, vegetables and fish).Citation5 The association between diet quality and disability status was determined in 6 989 individuals with diagnosed MS. Those with diet quality scores in the highest quintile had lower levels of disability and lower depression scores. The odds of reporting severe fatigue, depression, pain or cognitive impairment were lower for those individuals with composite healthy lifestyle factors.Citation65
Riccio et al. (2016) Citation53 showed that nutritional interventions are well accepted by people with MS and may ameliorate their physical and inflammatory status. A dietary regimen mainly based on principles of Mediterranean diet, with or without administration of dietary supplements, determined an increase of the ratio n-3/n-6 PUFA serum concentration thus supporting the general trend towards an amelioration of inflammatory status. Patients with primary progressive MS (characterised by worsening neurologic function and accumulation of disability from the onset of symptoms) were more responsive to nutritional intervention with fish oil and lipoic acid.Citation9
As various studies report different levels of efficacy, it is difficult to make recommendations based on specific nutrients of food groups. Overall, healthy eating guidelines are therefore currently recommended.Citation5
Probiotic supplements and MS
In a recently published randomised double-blind placebo-controlled clinical trial, Kouchaki et al. (2016) evaluated the effects of probiotic intake on disability, mental health and metabolic condition in subjects with MS.Citation66 The probiotic contained Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum (each 2 x 109 CFU/g). The study demonstrated that the use of probiotic capsules for 12 weeks among subjects with MS had favourable effects on the expanded disability status scale (EDSS), as well as on parameters of mental health, inflammatory factors, markers of insulin resistance, HDL-, total-/HDL-cholesterol and malondialdehyde (MDA), a lipid peroxidation marker, levels.Citation66
Highly selective experimental strategies will be needed to manipulate specific subsets or even single strains of bacteria as a therapeutic approach for MS. Rothhammer and Quintana (2016)Citation67 demonstrated that tryptophanase-positive bacteria, such as Lactobacillus reuteri, generate indole from dietary tryptophan, which is metaboliaed by the host to indole-3-sulfate (I3S), indole-3-propionic acid (I3PA) and indole-3- aldehyde (I3A). Indole, I3S, I3PA and I3A cross the blood–brain barrier and suppress pro-inflammatory activities by activating aryl hydrocarbon receptor (AHR) in astrocytes (dominant glial cells in the brain). Lack of dietary tryptophan or deficiency of AHR in astrocytes caused a failure to recover during chronic stages of EAE. Patients with MS seem to harbour deficits in the generation, uptake or stability of these anti-inflammatory metabolites, resulting in a decrease in their levels and in AHR-dependent immune regulation.Citation68,69
Another more drastic therapeutic approach proposed to restore gut eubiosis, and downregulate inflammation, by faecal microbiota transplantation, a practice that is neither t-tested nor used or recommended in MS.Citation69
Conclusion: current best dietary advice for MS
Insufficient high-quality evidence exists in the scientific literature to inform official clinical recommendations.Citation2,70 However, based on the available data that inflammation plays a role in MS treatment, the anti-inflammatory dietary and lifestyle approach is currently considered the best and safest approach.
Lifestyle modifications (weight management, increased physical exercise and abstinence from smoking) are some of the important interventions that can be implemented to decrease the onset and/or slow the progression of MS.Citation4,8 The current recommendation for MS is an anti-inflammatory diet lower in saturated fats (fatty meat, fried food, confectionery, full-cream dairy products) and high in monounsaturated fats (canola oil, olives and olive oil, nuts, seeds, avocados) and polyunsaturated fats (flaxseed oil, fish and fish oil).Citation8,71,72 Patients who do not eat oily fish at least three times a week can increase their intake of omega-3 fatty acids with supplements. Fish oil is the best source of EPA and DHA.Citation73
Patients who are overweight with a high compliance should follow a low-energy diet (1 600–1 800 kcal) based on vegetables, whole cereals, legumes, fruit and fish, which may decelerate the progression of the disease and improve the wellness of MS patients. Such dietary therapy could also include recommendations on improving the n-3 PUFA, probiotic and vitamins A and D intake of these patients. Probiotics, such as Lactococcus lactis, Bifidobacterium lactis, Clostridium butyricum, Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum, can improve the intestinal microbial balance.Citation9,53
Conflict of interest
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this paper.
References
- Rito Y , Torre-Villalvazo I , Flores J , et al . Epigenetic in multiple sclerosis: molecular mechanisms and dietary intervention. Cent Nerv Syst Agents Med Chem. 2016;16:1–8. https://doi.org/10.2174%2F1871524916666160226131842.
- Riccio P , Rossano R . Diet, gut microbiota and vitamin D and A in multiple sclerosis. Neurotherapies. 2017;15(1):75–91 doi:10.1007/s13311-017-0581-4.
- Guerrero-García JdJ , Carrera-Quintanar L , López-Roa RI , et al . Multiple sclerosis and obesity: possible roles of adipokines. Mediators Inflamm. 2016:ID4036232. doi:10.1155/2016/4036232.
- Amato MP , Derfuss T , Hemmer B , et al . Environmental modifiable risk factors for multiple sclerosis: Report from the 2016 ECTRIMS focused workshop. Multiple Sclerosis Journal 2017 Jan 6:1352458516686847. doi:10.1177/1352458516686847.
- Altowaijri G , Fryman A & Yadav V. Dietary interventions and multiple sclerosis. Curr Neurol Neurosci Rep. 2017; 17(3):Article 28. DOI 10.1007/s11910-017-0732-3
- Dendrou CA , Fugger L , Friese MA . Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015 Sep 15;15(9):545–58. doi:10.1038/nri3871. Epub 2015 Aug 7.
- Bjørnevik K , Chitnis T , Ascherio A , et al . Polyunsaturated fatty acids and the risk of multiple sclerosis. Multiple Sclerosis Journal. 2017;23(14):1830–8. doi:10.1177/1352458517691150.
- Moss BP , Rensel MR , Hersh CM . Wellness and the role of comorbidities in multiple sclerosis. Neurotherapeutics. 2017;14:999–1017. doi:10.1007/s13311-017-0563-6.
- Riccio P , Rossano R . Nutrition facts in multiple sclerosis. ASN Neuro. [Internet]. 2015;7(1):1–20 https://doi.org/10.1177%2F1759091414568185.
- Desvergne B , Michalik L , Wahli E. Transcriptional regulation of metabolism Physiol Rev. 2006 Apr; 86(2):465–514. https://doi.org/10.1152%2Fphysrev.00025.2005
- Erridge C , Attina T , Spickett CM , et al . A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86(5):1286–92.10.1093/ajcn/86.5.1286
- Ghanim H , Abuaysheh S , Sia CL , et al . Increase in plasma Endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care [Internet]. American Diabetes Association; 2009 Sep 15;32(12):2281–7. https://doi.org/10.2337/dc09 10.2337/dc09-0979
- Margioris AN . Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:129–37. doi:10.1097/mco.0b013e3283232a11.
- Ramirez-Ramirez V , Macias-Islas MA , Ortiz GG , et al . Efficacy of fish oil on serum of TNFα, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon Beta-1b. Oxidative Medicine and Cellular Longevity. Hindawi Limited; 2013;2013:1–8. https://doi.org/10.1155/2013/ 10.1155/2013/709493
- Okada Y , Tsuzuki Y , Ueda T , et al . Trans fatty acids in diets act as a precipitating factor for gut inflammation? J Gastroenterol Hepatol. 2013;28(Suppl. 4):29–32. doi:10.1111/jgh.12270.
- Li B , Reynolds JM , Stout RD , et al . Regulation of Th17 Differentiation by Epidermal Fatty Acid-Binding Protein. J Immunol. The American Association of Immunologists. 2009; 3;182(12):7625–33. https://doi.org/10.4049/jimmunol.
- Li Y-H , Yang L-H , Sha K-H , et al . Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21(22):7008–13.10.3748/wjg.v21.i22.7008
- Serhan CN , Dalli J , Colas RA , et al . Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome Biochim Biophys Acta. 1851;2015:397–413. doi:10.1016/j.bbalip.2014.08.006.
- Montonen J , Boeing H , Fritsche A , et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2012; 18; 52(1):337–345. https://doi.org/10.1007/s00394-012-0340-6.
- Stenson WF . The universe of arachidonic acid metabolites in inflammatory bowel disease: Can we tell the good from the bad? Current Opinion in Gastroenterology 2014;30:347–51. doi:10.1097/mog.0000000000000075.
- Williams R , Buchheit CL , Berman NEJ , et al . Pathogenic implications of iron accumulation in multiple sclerosis. J Neurochem. 2011;120 (1):7–25. https://doi.org/10.1111/j.1471-4159.2011.07536.x
- Wu C , Yosef N , Thalhamer T , et al . Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7. doi:10.1038/nature11984.
- Kleinewietfeld M , Manzel A , Titze J , et al . Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. doi:10.1038/nature11868.
- Erridge C , Attina T , Spickett CM , et al . A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286–92.10.1093/ajcn/86.5.1286
- Thaipisuttikul P , Galvin JE . Use of medical foods and nutritional approaches in the treatment of Alzheimer’s disease. Clinical Practice [Internet]. OMICS Publishing Group 2012 Mar;9(2):199–209. doi:10.2217/cpr.12.3.
- Margioris AN . Fatty acids and postprandial inflammation. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12:129–37. doi:10.1097/mco.0b013e3283232a11.
- Salinthone S , Yadav V , Schillace RV , et al . Lipoic acid attenuates inflammation via cAMP and protein kinase A signaling. In: Bonini MG , editor. PLoS ONE [Internet]. Public Library of Science (PLoS). 2010 Sep 28;5(9):e13058. https://doi.org/10.1371/journal.pone.0013058 10.1371/journal.pone.0013058
- Smolders J , Damoiseaux J , Menheere P , et al . Vitamin D as an immune modulator in multiple sclerosis, a review. Journal of Neuroimmunology. 2008;194(1–2):7–17. doi:10.1016/j.jneuroim.2007.11.014.
- Pierrot-Deseilligny C . Clinical implications of a possible role of vitamin D in multiple sclerosis. J Neurol. 2009;256:1468–79. doi:10.1007/s00415-009-5139-x.
- Cantorna MT . Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–6. doi:10.1016/j.abb.2011.11.001.
- Shivappa N , Hebert JR , Behrooz M , et al . Dietary inflammatory index and risk of multiple sclerosis in a case-control study from Iran. Neuroepidemiology. 2016;47(1) 26–31. doi:10.1159/000445874.
- Cheng G , Zhang X , Gao D , et al . Resveratrol inhibits MMP- 9 expression by up-regulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci Lett. 2009;451:105–8. doi:10.1016/j.neulet.2008.12.045.
- Wang S , Moustaid-Moussa N , Chen L , et al . Novel insights of dietary polyphenols and obesity. The Journal of Nutritional Biochemistry 2014;25(1):1–18. doi:10.1016/j.jnutbio.2013.09.001.
- Das S , Das DK . Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–73. doi:10.2174/187152807781696464.
- Shokryazdan P , Jahromi MF , Navidshad B , et al . Effects of prebiotics on immune system and cytokine expression. Review. Med Microbiol Immunol, 2016; 4;206 (1):1–9. https://doi.org/10.1007/s00430-016-0481
- Ríos-Covián D , Ruas-Madiedo P , Margolles A . Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Frontiers in Microbiology. Front Microbiol. 2016;7: Article 185. doi:10.3389/fmicb.2016.00185.
- Cani PD , Delzenne NM . The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–58. doi:10.2174/138161209788168164.
- Wu GD , Chen J , Hoffmann C et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science [Internet]. American Association for the Advancement of Science (AAAS). 2011;334(6052):105–8. https://doi.org/10.1126/science.1208344
- Tremaroli V , Baừckhed F . Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi:10.1038/nature11552.
- Panda S , Guarner F , Manichanh C . Structure and functions of the gut microbiome. Endocrine, Metabolic & Immune Disorders-Drug Targets [Internet]. 2014; 12;14(4):290–9. https://doi.org/10.2174/1871530314666140714120744
- Chassaing B , Gewirtz AT . Gut Microbiota, Low-grade Inflammation, and Metabolic Syndrome. Toxicol Pathol. 2013;42(1):49–53. https://doi.org/10.1177/0192623313508481
- Honda K , Littman DR . The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–95. doi:10.1146/annurev-immunol-020711-074937.
- Pantzaris MC , Loukaides GN , Ntzani EE . A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open BMJ. 2013;3(4):e002170. https://doi.org/10.1136/bmjopen-2012-002170
- Donoghue V , Spruyt M , Blaauw R . Use of intravenous fat emulsions in adult critically ill patients: does omega 3 make a difference? S Afr J Clin Nutr. 2017;30(3):38–48 http://www.sajcn.co.za/index.php/SAJCN/article/view/1281.
- Farinotti M , Simi S , Confalonieri P , et al . Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev. 2012 Dec 12;12:CD004192. https://doi.org/10.1002/14651858.cd004192
- Burgos R , et al . ESPEN guideline clinical nutrition in neurology. Clin Nutr. 2017; 1–43. doi:10.1016/j.clnu.2017.09.003.
- Ascherio A , Munger KL , White R , et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurology American Medical Association (AMA). 2014;71(3):306–14. https://doi.org/10.1001/jamaneurol.2013.5993
- Yin K , Agrawal DK . Vitamin D and inflammatory diseases. Journal of Inflammation Research 2014;7:69–87 https://doi.org/10.2147%2Fjir.s63898.
- Hewison M . Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–79 https://doi.org/10.1016%2Fj.rdc.2012.03.012.10.1016/j.ecl.2010.02.010
- Thouvenot E , Orsini M , Daures J-P , et al . Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur J Neurol. 2014;22(3):564–9. https://doi.org/10.1111/ene.12617
- Jahromi SR , Sahraian MA , Togha M , et al . Iranian consensus on use of vitamin D in patients with multiple sclerosis. Curr Opin Immunol . 2016;16(1). https://doi.org/10.1186/s12883-016-0586
- Agrawal D , Yin K . Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi:10.2147/jir.s63898.
- Riccio P , Rossano R , Larocca M , et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: A pilot study. Exp Biol Med. 2016: 241(6):620–35. https://doi.org/10.1177/1535370215618462
- Jiao Z , Fu Y , Fu J , et al . 1, 25-dihydroxy vitamin D3 promotes the apoptosis of inflammatory cells in acute experimental autoimmune encephalomyelitis: experiment with rats. Zhonghua Yi Xue Za Zhi. 2008;88(33):2350–4.
- Pedersen LB , Nashold FE , Spach KM , et al . 1, 25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85(11):2480–90. doi:10.1002/jnr.21382.
- Mattner F , Smiroldo S , Galbiati F , et al . Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D3. Eur J Immunol. 2000;30(2):498–508. doi:10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q.
- Muthian G , Raikwar HP , Rajasingh J , et al . 1, 25 dihydroxyvitamin‐D3 modulates JAK–STAT pathway in IL‐12/IFNγ axis leading to Th1 response inexperimental allergic encephalomyelitis. J Neurosci Res. 2006; 83(7):1299–1309. https://doi.org/10.1002%2Fjnr.20826
- Holmøy T , Øivind Torkildsen Ø . Can vitamin D reduce inflammation in relapsing-remitting multiple sclerosis? Expert Rev Neurother. 2016;3: 233–5. doi:10.1586/14737175.2016.1146134.
- Mullin GE . Vitamin A and immunity. Nutr Clin Pract. 2011;26: 495–6. doi:10.1177/0884533611411583.
- Hall JA , Grainger JR , Spencer SP , et al . The role of retinoic acid in tolerance and immunity. Immunity. 2011;35(1):13–22. doi:10.1016/j.immuni.2011.07.002.
- Bitarafan S , Saboor-Yaraghi A , Sahraian MA , et al . Impact of Vitamin A supplementation on disease progression in patients with multiple sclerosis. Arch Iran Med. 2015 Jul;18(7):435–440.
- Massa J , O’Reilly EJ , Munger KL , et al . Caffeine and alcohol intakes have no association with risk of multiple sclerosis. Multiple Sclerosis. 2015;19:53–8. doi:10.1177/1352458512448108.
- Hedstro¨m, AK , Hillert, J , Olsson, T , et al Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurology. 2014; 71:300–5. https://doi.org/10.1001/jamaneurol.2013.5858
- Florindo M . Inflammatory cytokines and physical activity in multiple sclerosis. ISRN Neurology. 2014;1: 8151–572. doi:10.1155/2014/151572.
- Fitzgerald KC , Tyry T , Salter A , et al . Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology. 2018;90:e1–11. doi:10.1212/WNL.0000000000004768.
- Kouchaki E , Tamtaji OR , Salami M , et al . Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition. 2016;1–5. doi:10.1016/j.clnu.2016.08.015.
- Rothhammer V , Quintana FJ . Environmental control of autoimmune inflammation in the central nervous system RSS. Current Opinion in Immunology. 2016;43:46–53. doi:10.1016/j.coi.2016.09.002.
- Lamas B , Richard ML , Leducq V , et al . CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nature Medicine. 2016;22(6):598–605. doi:10.1038/nm.4102.
- Smits LP , Bouter KE , de Vos WM , et al . Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013 Nov;145(5):946–53. doi:10.1053/j.gastro.2013.08.058.
- Sumowski JF , McDonnell GV , Bourdette D . Diet in multiple sclerosis. Science takes a seat at the table. Neurology. 2018;90:1–2. doi:10.1212/WNL.0000000000004775.
- Baily J . Multiple sclerosis society of Canada. Healthy Eating: A guide for people with MS. 2008; doi:10.1057/9781137457066.0008.
- National Multiple Sclerosis Society . Diet & Nutrition. Eating healthy to take charge of your health. [cited 2015 Jul 15]. Available from: http://www.nationalmssociety.org/Living-Well-With-MS/Health-Wellness/Diet-Nutrition#section-0
- Opperman M . What health professionals should know about omega-3 fatty acid supplements. S Afr J Clin Nutr. 2013;26(2):6–11. doi:10.1080/16070658.2013.11734444.
