Abstract
Background:
Continuous monitoring of glucose (CGM) via subcutaneous patch is an accurate self-monitoring tool of blood glucose, but also introduces a range of additional benefits such as real-time feedback. While its value among pregnant women with gestational diabetes mellitus (GDM) is established in high-income countries, little is known about the feasibility and acceptability among pregnant women without GDM in low-resource settings in low- and middle-income countries.
Objectives:
This study aims to assess the feasibility and acceptability of CGM with pregnant mothers in South Africa and to explore the value of a collected data set for GDM prevention.
Methods:
Ten women between 12 and 18 weeks pregnant were recruited from the antenatal clinic at Chris Hani Baragwanath Academic Hospital into a prospective mixed-methods pilot study. Demographic and anthropometric data, HbA1c and a lipid panel were collected. Women then wore two consecutive Freestyle Libre 2 patches for a total of 28 days. In-depth interviews were undertaken with all 10 women on study exit to explore themes of acceptability and the use of technology during pregnancy. Thematic analysis was performed on the qualitative data while exploratory data-analysis techniques were applied to the CGM data.
Results:
Pregnant women (n = 10) had a mean (SD) age of 29.81 years (4.39), with most being unemployed (8), unmarried (8) and without a tertiary degree (7). Analysis suggests that fear of use was greater than the actual discomfort experienced during use of the CGM patch. The main barrier to use was the patch falling off and women being uncomfortable to reapply it. This was borne out by all 10 women wearing the first patch for at least 12 of the 14 days, but only 4 managing the same with patch two – primarily applied by themselves at home. Women expressed support for the use of technology during pregnancy, especially as it related to feeling that their pregnancy was being monitored and that they were being supported.
Conclusion:
In this pilot study, women overwhelmingly found the wearing of a CGM patch during pregnancy to be acceptable. Feasibility was reasonable with most data being successfully retrieved from the devices over a two-week period. Longer use was found to have additional challenges. The use of CGM patches appear to be a possible candidate for inclusion in GDM prevention or behavioural interventions during pregnancy in South Africa.
Introduction
Point-in-time self-monitoring of blood glucose (SMBC) is a limited technology that fails to offer significant value in understanding the temporal relationship between blood glucose levels and daily activities. Continuous glucose monitors (CGM) fill this gap by automatically recording interstitial glucose concentration levels every few minutes for a period of 14 days.Citation1 These data on direction, magnitude, duration and frequency of glucose-level fluctuation are accessible to both user and healthcare provider in real time. Early CGM systems were inaccurate, costly and hard to come by.Citation2 However, as the technology has improved and costs have reduced, data now suggest that CGM provides similar accuracy to SMBG, but with significant advantages, particularly in the ability to tailor therapy to the needs of each individual.Citation3 The first CGM approved for use by the Food and Drug Administration and carrying the Conformité Européenne mark in the European Union was the Continuous Glucose Monitoring System Gold (CGMS Gold; Medtronic MiniMed, Northridge, CA, USA). Popular products today include the DexaCom G6 (Dexacom, San Diego, CA, USA) and Freestyle Libre 2 (Abbott Laboratories, Alameda, CA, USA), both which use an interstitial patch inserted on the upper arm and a reader that is passed over the patch to retrieve data. Wireless versions of the products also exist that connect to a mobile phone using Bluetooth. Eversense (Senseonics, Germantown, MD, USA) offers a fully implantable sensor that is also approved for use globally.Citation4,Citation5
Mattishent et al.Citation6 tested the feasibility and acceptability of a CGM system among the elderly (65+) suffering from memory loss (abbreviated mental test score ≤ 8) or dementia. The system required the user to place a digital reader over the patch three times per day. Over a two-week period, feasibility was found to be sub-optimal with half of the participants failing to capture more than two out of every three readings (mean 55%). The system was, however, acceptable with qualitative interviews suggesting participants found the device comfortable and that it did not interfere with activities of daily living. Children also stand to benefit from a continuous and automated glucose monitoring system. Assessing the feasibility and acceptability among children, Rai et al.Citation7 found that 65% of the sensors remained in place over the full 14-day study period. Minor discomfort was the only reported side effect with a high rate of acceptance observed. Although showing promise at home in the self-management of diabetes, Wollersheim et al. did not find that CGM systems performed with satisfactory accuracy, feasibility or acceptability in a critical care scenario.Citation8 Among the nursing staff, 79.1% rated the device as not beneficial and 21 of the 31 devices were removed prematurely during this study. Better results have, however, been achieved with monitoring hyperglycaemia during pregnancy.
Gestational diabetes mellitus (GDM) is defined as any glucose impairment that first emerges during pregnancy.Citation9 It affects 3–10%) of pregnant women and is a risk factor for multiple maternal and foetal complications.Citation10 Acutely, GDM elevates the risk of birth trauma and foetal macrosomia. Longer term implications of GDM include the increased risk of diabetes mellitus type 2 and obesity in the mother, as well as diabetes mellitus type 2 and metabolic syndrome in children born to mothers with GDM.Citation11,Citation12 In South Africa, GDM prevalence has been found to be 9.1%, a significant figure considering the impact of type 2 diabetes for a health system that is already overburdened.Citation13,Citation14 A recent systematic review of CGM during pregnancies complicated by GDM found mixed evidence for its effectiveness.Citation15 Two randomised trials reported no significance in macrosomia, birthweight and gestational age at delivery between a group of women using CGM or another SMBG. However, an observational study found CGM increased the detection of both hypoglycaemic and hyperglycaemic episodes. The review concludes by noting that research remains necessary to understand whether CGM may have application in the screening and prediction of GDM. None of the 29 studies identified was from a low- or middle-income country (LMIC).
The lack of studies exploring the use of CGM during pregnancy in LMIC populations is significant as there is abundant evidence to suggest that digital health interventions require adaptation to achieve cultural and practical acceptability and feasibility. Potential benefits to the management of GDM using modern CGM systems that wirelessly transmit sensor data to the participant’s phone, before uploading to a care provider’s dashboard, may be limited by several potential barriers. In most cases of technology-based interventions, primary challenges hinge around Internet access and power. Technical capacity may also limit the use of technology. Patients need to know how to use the technology properly, such as how to connect a glucose metre to their phone and execute an upload. There may also be provider-related barriers that limit technology use for diabetes management. Providers may not, for different reasons, endorse or recommend the use of electronic management tools by their patients. They could doubt the potential benefits, have concerns that use will create uncompensated work for them or think that they will be responsible for more data than they are able to keep up with.Citation16–19 While CGMs are a central component in the glucose management of type 1 diabetes and insulin-dependent type 2 diabetes, little is known about how common health technology barriers may impact on pregnant women using a CGM system in South Africa. The aims of this study were to (1) describe CGM in terms of feasibility and acceptability to pregnant women and (2) explore the use of these data in GDM prevention.
Methods
Study design and sampling
This prospective mixed-methods pilot study used an explanatory design consisting of two sequential phases. In the first phase, physiological, survey and continuous glucose monitoring data were collected. These data were used to inform the design of the second phase, during which in-depth interviews were conducted. A stratified convenience sampling strategy was followed to recruit woman between 12 and 18 weeks pregnant. To be considered for inclusion the pregnant woman was required to have a first antenatal booking BMI of greater than 18.5 kg/m2. Recruitment was undertaken at the antenatal clinic in the Chris Hani Baragwanath Academic Hospital (CHBAH), Soweto. Women were classified either as overweight or obese (BMI > 25 kg/m2) or within a normal range (18.5 kg/m2 to 24.9 kg/m2). Recruitment began in 2019 and was severely impacted by Sars-Cov2 in 2020 when study enrolment was closed. A total of 10 women consented to participate in this study.
Setting
CHBAH is the largest hospital in Africa and is situated in Soweto, an urban area in Johannesburg, South Africa. Participant recruitment was undertaken at the antenatal clinic while all other study procedures including glucose patch installation, assessments and in-depth interviews took place at the SAMRC/Wits Developmental Pathways for Health Research Unit (DPHRU), based within the grounds of CHBAH. The facilities provided by the unit include office space, a laboratory, and an examination and interview room. The on-site laboratory was used to analyse collected samples.
Procedures
After recruitment and initial screening, participants were provided with a convenient appointment date to return for their scheduled visit at the DPRHU site. If eligible and consenting, participants completed (1) written informed consent, (2) a baseline demographic including date of birth, marital status, occupation status, education and home description, (3) height, which was measured without shoes to the nearest 0.1 cm (0.04 in) using a Seca Stadiometer (Seca, Birmingham, UK) and weight measured on an electronic scale to the nearest 0.1 kg (0.22 pounds), (4) blood pressure, measured in accordance with the American Heart Association recommendations using Omron HBP-1300-E devices (Omron Global, Tokyo, Japan), (5) 10 ml of venous blood was drawn to establish non-fasting HbA1c, adiponectin and cholesterol levels, (6) the Freestyle Libre (Abbott Laboratories, Alameda, CA, USA) continuous glucose monitoring patch was applied to the participant’s upper arm. The Libre 2 can store 14 days’ worth of continuous data on persistent in-device memory. A second patch was given to participants, and they were asked to apply it after removing the first patch after 14 days. Feasibility was assessed by reviewing the amount of data collected from these two modes of application. This allowed for a thorough test of whether wearing a CGM patch could be reasonably achieved by pregnant women in Soweto.
Participants were given the telephone number of a research assistant who was able to help with this procedure if needed. Both patches were then either collected by the research assistant or returned by the participant at a scheduled clinic visit. Compliance was measured as the number of days, out of the 28 possible days, on which the participant wore the patch. Number of days wearing the patch was established by downloading data from the patch and assessing how many days had a complete set of readings. Participants were reimbursed for travel expenses to attend the scheduled data collection session. Within six months of returning the CGM patches, telephonic in-depth-interviews (IDIs) were undertaken with participants to understand their experience of using the continuous glucose patch and to establish their views on how technology can support antenatal care. Interviews were conducted by a female research assistant trained in qualitative research methods. All interviews were conducted in the participant’s mother tongue (either Sesotho or isiZulu) and then transcribed and translated into English. Participants were provided with a pseudonym for use during the interview to protect their identity. Interviews ranged from 20 to 48 minutes. The study received approval by the University of the Witwatersrand Ethics Committee (M190318).
Survey scales and interview guide
A health survey was administered at enrolment and included several validated scales. Among them was the Healthcare Technology Self-Efficacy (HTSE) scale, consisting of three self-efficacy factors, to assess individuals’ attitudes toward healthcare technology.Citation20 At a follow-up visit conducted within one month of completing the testing of the CGM patch, individuals completed the same health survey as administered at enrolment (post-test data not presented in this paper). At six months a qualitative interview was completed with participants. A semi-structured guide was developed for this visit in order to explore two primary topics of interest. First, the acceptability of wearing a CGM during pregnancy among women without gestational diabetes and second, their views on how technology could be used to support antenatal care. Interview questions covered topics pertaining to comfort of device, feasibility of wearing the CGM, understanding of what was being recorded, what information they wished technology could provide about their pregnancy, and any barriers they encountered.
Analysis plan
Telephonic interviews were audio-recorded using a mixture of mobile phone app (Automatic Call Recorder, Appliqato; https://www.appliqato.com/) and a digital audio recorder placed next to the telephone with its loudspeaker activated. These recordings were transcribed, translated to English and loaded into ATLAS.ti 8 (https://atlasti.com/), a qualitative software package for data management, coding and analysis. The first author (AVH) reviewed a subsample of transcripts and generated a list of inductive and deductive codes that mapped to the themes present in the text. Deductive codes included themes based on the interview guide questions and the inductive codes were developed to identify new themes that emerged through the process of review. Throughout the coding process, the study aims were revisited to steer the analysis towards the intended outcome. CGM data was analysed using SPSS version 24 (IBM Corp, Armonk, NY, USA). An exploratory data analysis approach was decided upon to identify and describe patterns in the continuous glucose data and the relationship to participants demographics.
Results
Overall sample summary
Of the 10 women enrolled, the mean (SD) age was 29.81 years (4.39). NineCitation9 of the women had a BMI above 25 kg/m2, 2 were married, 2 employed and 3 had completed a tertiary degree. An elevated HbA1c (> 7.8 mmol/l) was noted in none of the women, and blood pressure was similarly normal (less than 120 mmHg systolic and less than 80 mmHg diastolic) for all women in the sample. More detail is provided in . All but one of the participants owned a smartphone. Only one participant had heard about smartwatches, and she was also the only one to have heard about fitness trackers. None had health apps installed on their mobile phones.
Table 1: Demographic and health characteristics of participating women
CGM patch acceptability
Perceptions and reality of device use were found to be discordant. Before applying the patch, participants reported feeling apprehensive, worried about the pain and fearing needles. These fears were in part raised by both the explanation given to participants about how the CGM patch worked and them seeing it with their own eyes ().
‘I thought it was just a patch when I saw it. Its only then when I realized it will have something inside me.’ (ADL006)
‘When it was first explained and demonstrated to me I was worried because the needle looked so big and I was scared it was going to be painful even worse leave a mark after it was removed. It turned out to not be painful at all, its even better than a finger prick.’ (ADL009)
‘I was scared about the application but it turned out to not be painful at all and I did not even feel it.’ (ADL008)
‘At first I was sceptical but after it was on me it wasn’t painful, it wasn’t annoying, you couldn’t even feel that it was there and it made me to be aware of what I eat and how much exercise I do, its actually the one that made me to be more conscious about what I do and when do I relax, it was a lovely experience and I learnt a lot from it.’ (ADL007)
Problems experienced during the 28 days included, most commonly, that it fell off. Other challenges included participants not being aware that the patch was water resistant and removing it during bathing, and scepticism from partners and family about the true purpose of the CGM patch. However, for the vast majority of women the device gave no problems, and they quickly became accustomed to wearing it to the point that they were no longer consciously aware of the patch being on their arm.
‘Yes, it peeled off on its own within a couple of days and I did not put on the second one because my husband was uncomfortable, I told him it’s for HIV so he did not believe me when I finally told him the truth.’ (ADL006)
‘Yes my 3 year old daughter kept on wanting to see it and ask what it was so she always reminded me that there was something on my arm.’ (ADL008)
‘No, I completely forgot about it, unless I accidentally touch it while undressing or getting dressed.’ (ADL004)
Feasibility of CGM patch among women living in Soweto
Continuous glucose readings were obtained from all 10 women with varying degrees of success. Two participants chose only to wear one of the two provided sensor patches. In the case of ADL002, she fell ill and chose not to continue participation, while for ADL011 her partner was not happy for her to be a part of the research study and she chose to complete activities at the research site but not at home. Of the days participants were asked to wear the CGM patch, compliance ranged from 42% through to 100%. Of the days when the patch was worn, data were collected every 15 minutes, stored and retrievable with near 100% fidelity (99–100%). The minimum recorded blood glucose from the sample of women was 2.2 mmol/l and the maximum reading observed was 7.9 mmol/l with a mean of 4.0 (SD 0.9). introduces key descriptive statistics for each of the women in the study. While the sensor did not remain attached to some women (e.g. ADL007), when it was secured the sensor produced a nearly perfect number of readings. a is an aggregate plot of all CGM readings recorded by the patch during the course of the study for all women, standardised to a start date of January 1, 2021. b presents the Locally Weighted Scatterplot Smoothing (LOWESS) for a single participant and c a smoothed line plot of readings (mmol/l) disaggregated by normal weight vs. overweight or obese.
Figure 2: Daily CGM readings: (a) all readings from all women in the study standardised to a start date of 2021-01-01; (b) ADL002 with Locally Weighted Scatterplot Smoothing (LOWESS); (c) women classified as either normal weight (n = 1) or overweight or obese (n = 9).
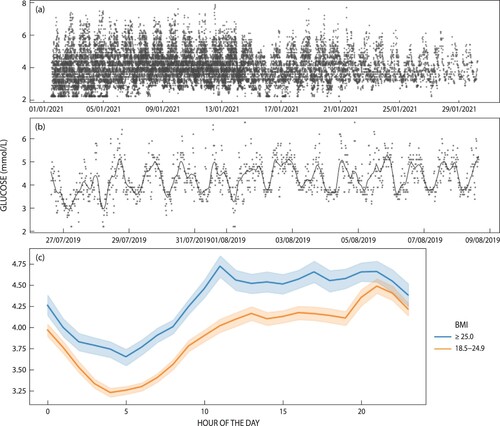
Table 2: Feasibility and some results regarding GDM sensor data
Monitoring device preferences during pregnancy
Women found access to the information provided by the patch both curious and fascinating. Many participants reported drawing comfort from believing that a clinician was monitoring their data in real time and would react if their data suggested there was an emergency with the pregnancy. Although this was not the case in this study, the idea appears to be very compelling to pregnant mothers, who carry the burden of both their own health and that of their unborn child. Women spoke about wishing for a similar device that could monitor blood pressure, their baby’s heart rate, HIV and their mental health. These data would give the assurance that all was well and support them to get the care and attention they needed.
‘I would like to know how my baby is because I had to have a premature baby because of the dangers that I had since they discovered that I got high blood pressure. Also the babies heartbeat and vitals. Knowing that my baby will be safe and I will also be checked and be referred if I need more care and attention.’ (ADL004)
‘What I wish is technology … . Knowing that my baby will be safe and I will also be automatically checked and be referred if I need more care and attention.’ (ADL007)
‘Having high blood pressure meant that I constantly worry about my health and that of my baby so I felt like this would be a great feature to calm me down. Pregnancy can be very challenging but it helps to have support from family and everyone who loves you so I would like to have a device that might pick up my moods and feelings.’ (ADL003)
Support was also expressed for the use of wearables during pregnancy, particularly in the monitoring of both maternal and child health. The only reason stated as a barrier to using a wearable device during pregnancy was by a woman who stated that her husband had a concern about her wearing a device that might give off radio waves, which could impact foetal development.
‘I would be excited if there was something that can tell how the baby is growing and developing inside of me.’ (ADL003)
‘A device would help knowing that my baby will be safe, and I will also be checked and be referred if I need more care and attention. Knowing that I have something that shows me that the baby is okay and I am also okay. If I’m not fine, I am able to know and get ways to prevent it from happening again.’ (ADL004)
Tracking and monitoring of growth and development, blood pressure, nutrition and exercise, mental health and foetal health all surfaced as targets for a wearable device designed for pregnant women.
‘Yes, it would be nice to know how the baby is growing and developing. The baby's heart beat and the baby's weight [would be nice to know] because I had a very big girl. Actually, all the vital signs so that there are no surprises for both me and the baby.’ (ADL003)
‘Whether [I’m] eating correctly, if there’s anything life threatening to me I would like to know about that, what do I need to lookout for health wise and mentally. Is the baby growing well is she eating well is there anything affecting the baby and anything that might be alarming.’ (ADL011)
‘I would also like to know that I do the right amount of exercises. It must tell me my level of fitness and how I can improve it so that I’m ready for labour even some simple monthly exercises would be great and also to know if I’m overdoing it because pregnancy can be a fragile process.’ (ADL004)
‘Am I health[y] and is the baby healthy. Do I have any deficiencies that I need to look out for while I am pregnant. I would like to know what I can do to make sure that I’m getting enough nutrition because I’m no longer just looking out for myself only and sometimes we stick to diets that are not very helpful when it comes to pregnancy. I think knowing all the above would really improve the mental health state of any pregnant mom who has had multiple pregnancies and is constantly worried about everything.’ (ADL008)
When considering the design of these devices, women suggested a band or watch, which could be of any colour. Charging and battery life were priorities in the design, with women suggesting that the device be worn throughout the pregnancy. The data would ideally both be available to the mother and be automatically transmitted to a clinician who would monitor the data for warning signs.
‘As soon as they find out that they are pregnant and to use it at the beginning of every trimester. I would tell them that these devices are very important and usefull especially to pregnant mothers who might have some health concerns they help not to worry a lot because you know you have something that will let you know if something is wrong. It can be something like a watch or a band that the mom can wear.’ (ADL008)
‘It could work via the phone but phones sometimes run out of batteries so at least with a watch you always wearing it on your wrist. I know it’s hard to have something that won’t need to be charged so it would need a long battery life so that it does not need to be charged every day.’ (ADL004)
‘It can be a wearable device. It would definitely have to have a separate battery so that I can continue to use the watch while the battery charges. I would like it to track the baby's heart beat and help mommy know her blood pressure. The device can use data or SMS or even telephonically as long as the mother can have access. All data or information can come directly to me and my doctor or whoever is monitoring it. I would like my partner and doctor or nurse to know this data as I’m scared I might panic especially if I am lone.’ (ADL0011)
Discussion
The acceptability and feasibility of CGM among women in the second trimester of their pregnancy was assessed as a first step towards including CGM as part of a behavioural intervention to reduce GDM. Both acceptability and feasibility were high in this small sample of women. A primary barrier to uptake was initial fear and concern about the pain caused by applying the patch. Reframing how the device works and refraining from using the word ‘needles’ might reduce anxiety and improve uptake. Although there is currently mixed evidence as to the clinical outcomes value of CGM in pregnancy,Citation21 our data suggest another benefit. We found that acceptability may be further enhanced by focusing on the psychological relief that CGM patches and other monitoring technologies may afford pregnant women. With the help of a research assistant, all 10 women wore the first CGM patch for more than 85% of the expected 14 days and a minimum of 99% of the data was retrieved from the CGM patch. This suggests both high acceptability and feasibility if the devices are applied in a controlled environment by a trained professional. The home application was less well tolerated with only 4 out of the 10 wearing the patch successfully in the second two-week period. For those that did, data were again easily retrieved from the patch.
Wearable devices to support the pregnancy were found to be of interest to all the pregnant women in the sample with only one expressing a reservation related to her husband’s concerns about the impact of wearable electronics on mother and child health. Health interventions that could be supported by wearables include mental health, nutrition and exercise, and foetal health and development. These areas were all cause for substantial concern during pregnancy. Devices that could alleviate these fears and provide reassurance or evidence that ‘all was well’ would be highly acceptable in this population. This type of pregnancy-related anxiety (PRA) is well described in the literature with socioeconomic status and social support being key drivers of PRA.Citation22,Citation23
A key limitation of this study is the small sample size. Although sufficient for a qualitative study, especially because even with this small sample we reached saturation in response to acceptability of the device, more data would have been helpful in assessing feasibility in this context. Data collection was unavoidably interrupted by the Sars-Cov2 pandemic, which halted most non-essential research in 2020. This meant that only 10 women could be recruited and IDIs completed. This small sample and the convenience sampling approach is partially responsible for 9 out of the 10 women happening to be overweight or obese. This limitation is moderated by a strength of this study, which is that these data clearly indicate that the next steps can be taken towards integrating CGM in behavioural monitoring and intervention. No obvious barriers exist that need to be further addressed.
Although not highly acceptable in inpatient critical care settings,Citation8 the feasibility and acceptability of CGM during pregnancy in women both with and without GDM is well described.Citation15,Citation24,Citation25 The use of these devices in Soweto, South Africa is less well known, although their potential is evident from the high rates of GDM in this community.Citation14 In other settings, feasibility and acceptability have been good, with the majority of woman finding it easy to use (92%), beneficial for self-glycaemic control (90%), and being willing to wear a CGM patch again, with 77% of women reporting its benefit outweighed its inconvenience. Concerns include minor discomfort at the sensor site and, in older systems, the need to carry the patch reader around with them. CGM has been found to increase information missing in self-monitored blood glucose diaries by 62%.Citation15,Citation26 The qualitative data collected in this study support these findings with the CGM patch being acceptable when applied with the help of a trained professional. Not yet reported in the literature is the extent to which the CGM data may introduce a level of psychological comfort among women in knowing that their bodies are being monitored for signs of distress. Women also report wishing the technology could be extended to continuously detect blood pressure, heart rate and HIV infection.
While registrational safety trials are complete for the first implantable CGM from Eversense,Citation27 the work described here should be extended to better describe people’s views as to the acceptability of implanting the sensor below the skin. Conspiracy theories abound as to how the COVID-19 vaccine is part of a global conspiracy to implant a ‘chip’ into the body. These types of concerns need to be surfaced and further understood before moving to this potentially more convenient form of sensor. Due to the volume of data generated by continuous monitoring, future work should focus on applying machine learning to these data sets to evaluate blood glucose fluctuations and alert care providers to the possible early signs of GDM.
Conclusions
While evidence is mixed as to the predictive value and clinical significance of continuous glucose monitoring over traditional self-monitoring of blood glucose, many of the studies finding no value are now almost 10 years old.Citation28,Citation29 This study suggests that CGM patches are acceptable to South African women and feasible in a context of intermittent electricity and high mobile phone data costs. Feasibility produced mixed results with shorter use (< 14 days), but the device being applied by a trained research assistant worked reasonably well for all participants. Patients attaching the patch themselves produced more instances of the device falling off, making long-term feasibility less certain. As an intervention, the mental health of mothers could be improved by the knowledge that their pregnant body is being remotely monitored. Modern machine learning approaches could support this monitoring, analysis and feedback.
Author contributions
AVH, SN and SK conceived the idea and designed the study. All the authors contributed to planning, logistics, data analysis and drafting of the manuscript.
Acknowledgements
The authors would like to thank the participants who continued to support this research during the SARS-CoV2 pandemic of 2020.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. https://linkinghub.elsevier.com/retrieve/pii/S0168822720307592.
- Kompala T, Neinstein A. A new era: increasing continuous glucose monitoring use in type 2 diabetes. Am J Manag Care. 2019;25(4 Spec No.):SP123–6. http://www.ncbi.nlm.nih.gov/pubmed/30933461.
- American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S71–80. http://care.diabetesjournals.org/lookup/doi/10.2337dc19-S007.
- Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231–9. http://care.diabetesjournals.org/cgi/doi/10.2337diacare.28.5.1231.
- Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in selfmonitoring of diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2018;12(2):181–7. https://linkinghub.elsevier.com/retrieve/pii/S187140211730303X.
- Mattishent K, Lane K, Salter C, et al. Continuous glucose monitoring in older people with diabetes and memory problems: a mixedmethods feasibility study in the UK. BMJ Open. 2019;9(11):e032037. https://bmjopen.bmj.com/lookup/doi/10.1136bmjopen-2019-032037.
- Rai S, Hulse A, Kumar P. Feasibility and acceptability of ambulatory glucose profile in children with Type 1 diabetes mellitus: A pilot study. Indian J Endocrinol Metab. 2016;20(6):790. https://doi.org/10.4103/2230-8210.192894.
- Wollersheim T, Engelhardt LJ, Pachulla J. Accuracy, reliability, feasibility and nurse acceptance of a subcutaneous continuous glucose management system in critically ill patients: a prospective clinical trial. Ann Intensive Care. 2016;6(1):70. http://annalsofintensivecare.springeropen.com/articles/10.1186s13613-016-0167-z.
- World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: a report of WHO/IDF consultation. Geneva: World Health Organization; 2006.
- Wan CS, Abell S, Aroni R. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: A comparison between immigrant ethnic Chinese women and Australian born Caucasian women in Australia. J Diabetes. 2019;11(10):809–17. https://onlinelibrary.wiley.com/doi/abs/10.11111753-0407.12909.
- Damm P, Houshmand-Oeregaard A, Kelstrup L. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–9. http://link.springer.com/10.1007s00125-016-3985-5.
- Dugas C, Perron J, Kearney M. Postnatal prevention of childhood obesity in offspring prenatally exposed to gestational diabetes mellitus: where are we now? Obes Facts. 2017;10(4):396–406. https://www.karger.com/Article/FullText/477407.
- Macaulay S, Dunger DB, Norris SA. Gestational diabetes mellitus in Africa: a systematic review. Schillaci G, editor. PLoS One. 2014;9(6):e97871. https://dx.plos.org/10.1371journal.pone.0097871.
- Macaulay S, Ngobeni M, Dunger DB. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract. 2018;139:278–87. https://linkinghub.elsevier.com/retrieve/pii/S0168822717317370.
- Yu Q, Aris IM, Tan KH, et al. Application and utility of continuous glucose monitoring in pregnancy: a systematic review. Front Endocrinol (Lausanne). 2019;10. https://www.frontiersin.org/article/10.3389fendo.2019.00697/full.
- Beane A, De Silva AP, Athapattu PL. Addressing the information deficit in global health: lessons from a digital acute care platform in Sri Lanka. BMJ Glob Heal. 2019;4(1):e001134. https://gh.bmj.com/lookup/doi/10.1136bmjgh-2018-001134.
- van Olmen J, Erwin E, García-Ulloa AC. Implementation barriers for mHealth for non-communicable diseases management in low and middle income countries: a scoping review and field-based views from implementers. Wellcome Open Res. 2020;5:7. https://wellcomeopenresearch.org/articles/5-7/v2.
- Mechael P, Batavia H, Kaonga N. Barriers and gaps affecting mHealth in low and middle income countries: policy white paper. New York, New York, USA; 2010. https://ghdonline.org/uploads/Barriers__Gaps_to_mHealth_in_LMICs_-_White_Paper_-_May_2010.pdf.
- Jewkes R, Dartnall E. More research is needed on digital technologies in violence against women. Lancet Public Heal. 2019;4(6):e270–1. https://linkinghub.elsevier.com/retrieve/pii/S2468266719300763.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
- Yu W, Wu N, Li L. A review of research progress on glycemic variability and gestational diabetes. Diabetes Metab Syndr Obes Targets Ther. 2020;13:2729–41. https://www.dovepress.com/a-review-of-research-progress-on-glycemic-variabilityand-gestational–peer-reviewed-article-DMSO
- van Heyningen T, Honikman S, Myer L. Prevalence and predictors of anxiety disorders amongst low-income pregnant women in urban South Africa: a cross-sectional study. Arch Womens Ment Health. 2017;20(6):765–75. http://link.springer.com/10.1007s00737-017-0768-z.
- Wall V, Premji SS, Letourneau N. Factors associated with pregnancy-related anxiety in Tanzanian women: a cross sectional study. BMJ Open. 2018;8(6):e020056. https://bmjopen.bmj.com/lookup/doi/10.1136bmjopen-2017-020056.
- Allen NA, Fain JA, Braun B. Continuous glucose monitoring in non–insulin-using individuals with type 2 diabetes: acceptability, feasibility, and teaching opportunities. Diabetes Technol Ther. 2009;11(3):151–8. http://www.liebertpub.com/doi/10.1089dia.2008.0053.
- Nally LM, Bondy N, Doiev J. A feasibility study to detect neonatal hypoglycemia in infants of diabetic mothers using real-time continuous glucose monitoring. Diabetes Technol Ther. 2019;21(4):170–6. https://www.liebertpub.com/doi/10.1089dia.2018.0337.
- McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust New Zeal J Obstet Gynaecol. 2007;47(3):186–90. http://doi.wiley.com/10.1111j.1479-828X.2007.00716.x.
- Deiss D, Irace C, Carlson G. Real-world safety of an implantable continuous glucose sensor over multiple cycles of use: a post-market registry study. Diabetes Technol Ther. 2020;22(1):48–52. https://www.liebertpub.com/doi/10.1089dia.2019.0159.
- Secher AL, Ringholm L, Andersen HU. The effect of realtime continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013;36(7):1877–83. http://care.diabetesjournals.org/cgi/doi/10.2337dc12-2360.
- Cordua S, Secher AL, Ringholm L. Real-time continuous glucose monitoring during labour and delivery in women with Type 1 diabetes – observations from a randomized controlled trial. Diabet Med. 2013;30(11):1374–81. http://doi.wiley.com/10.1111dme.12246.

