ABSTRACT
Background/Objective: Nearly 30% of patients with advanced-stage Hodgkin lymphoma (HL) are not cured. We should better control tumors with initial treatment for patients with advanced stage HL whose interim positron emission tomography/computed tomography (PET/CT) was positive. The objective of our study was to confirm the superiority of autologous hematopoietic stem cell transplantation (ASCT) therapy in these patients.
Methods: Eighty-nine HL patients with stage III-IV, international prognostic score (IPS) ≥3 and Deauville more than 3° at the interim PET/CT were analyzed. Forty five patients received ASCT. The other 44 patients received two cycles DHAP chemotherapy.
Results: The 3-year overall survival (OS) of patients who received ASCT was 91.1%, and for the patients who received chemotherapy, it was 72.7% (P = 0.025). The 3-year progression free survival (PFS) of patients in the ASCT group was 88.9%, but for patients in the chemotherapy group, it was only 70.5%(P = 0.017). No patient died of toxicity from ASCT. Additionally, there was no difference in the rates of secondary malignancies between the ASCT and chemotherapy groups. Extranodal and bone marrow involvement were poor prognostic factors, while ASCT was a good prognostic factor.
Conclusion: The use of ASCT as a first-line consolidation treatment could improve outcome of patients with advanced-stage high risk HL whose interim PET/CT was positive.
Introduction
Classical Hodgkin lymphoma (cHL) is a relatively rare disease [Citation1], and staging of this disease is essential for the choice of optimal therapy [Citation2]. Prognostic models to identify patients at high or low risk for recurrence have been developed, and these models, along with positron emission tomography, are used to provide optimal therapy [Citation3,Citation4]. First-line chemoradiotherapy yields a cure rate of 70% for patients with advanced-stage disease [Citation5]. However, 25% to 30% of these patients are not cured with modern chemoradiotherapy [Citation6]. To improve the outcome of these patients, some studies tried other chemotherapy regimens instead of ABVD. Although BEACOPP revealed better tumor control, severe adverse events have been more frequently reported [Citation7–10]. Until now, ABVD has remained the most commonly used treatment for patients with advanced-stage HL [Citation6,Citation11].
There is no doubt that autologous hematopoietic stem cell transplantation (ASCT) has been the standard treatment for adult patients with relapsed and refractory HL (RR-HL). However, 50% of patients who receive ASCT still relapse or progress within one year after transplant [Citation12,Citation13]. Additionally, about one third of patients with RR-HL are unable to start on salvage therapy or could not finish the salvage program due to poor clinical status or progressive disease [Citation7]. Therefore, the primary treatment should be optimized to cure the largest proportion of patients [Citation10]. Therefore, we attempted ASCT at initial treatment for HL patients with stage III-IV, IPS ≥ 3, and Deauville more than 3° at the interim PET/CT. The aim of our study was to confirm the effectiveness and safety of ASCT therapy in these patients.
Patients and methods
Patients and study design
Eighty-nine patients with newly diagnosed, biopsy-proven HL (World Health Organization, classic type) were retrospectively analyzed. The patients were diagnosed from January 2010 to March 2013 at six hospitals in Southwest China. Staging adhered to the guidelines from the NCCN and included PET/CT and a bone-marrow biopsy. Eligibility criteria were as follows: a diagnosis of stage III-IV HL, an IPS ≥ 3, after 4 cycles of ABVD, the patients were restaged with PET/CT, and Deauville was more than 3°. All of the patients’ PET/CT scans were centrally reviewed in our hospital. Among them, seventy-three patients’ Deauville score was 4° and only 16 patients’ Deauville score was 5°. No patient was regarded to have progressive disease on the PET 4 scan, all patients achieved partial remission (PR). Other criteria for eligibility were a Karnofsky performance score above 70% and adequate cardiac, pulmonary, renal, and liver function. We excluded patients if they were HIV positive or had an infection that was unresponsive to treatment.
We designed a multicenter retrospective cohort study. The primary endpoint was the progression-free survival (PFS) rate. The secondary endpoints were complete response rate at 3 months after the end of treatment, the overall survival (OS) rate and toxicity. According to the data of our center in 2010, the 3-year progression free survival (PFS) of patients in the ASCT group and in the chemotherapy group were about 90% and 62%, respectively. A sample size of a total of at least 86 patients was calculated with a one-side type I error of 0.05 and a statistical power of 80%.
Treatment
According to patient preference, 45 patients received ASCT after interim PET/CT. These patients received stem cell mobilization with MOED chemotherapy, which included mitoxantrone 6 mg/m2 IV on days 1 through 3, vincristine 1.4 mg/m2(maximum, 2 mg) IV on day 1, etoposide 100 mg/m2 IV on days 1 through 3, and dexamethasone 20 mg on days 1 through 5. After chemotherapy, white blood cells (WBC) were remarkably decreased. When WBC began to increase, granulocytecolony-stimulating factor (G-CSF, Filgrastim, Kirin Pharma Co, Ltd, Tokyo, Japan) was subcutaneously administered daily at 10 μg/kg per day for 5–6 days. Starting on the fifth day of G-CSF administration (day 1), donor peripheral blood mononuclear cells were harvested by large-volume leukapheresis using a Fenwall CS3000 (Fenwall, Deerfield, IL, USA). The goal was to collect at least 4 × 108 mononuclear cells and 2 × 106 CD34+ cells per kilogram of patient body weight. Patients received BEAC as a conditioning regimen one week after collection of stem cells, consisting of carmustine 300 mg/m2 IV on day 1, etoposide 200 mg/m2 IV per day on days 2 through 5, cytarabine 300 mg/m2 per day on days 2 through 5, and cyclophosphamide 30 mg/kg per day IV on days 2 through 5. Stem cell reinfusion was performed on day 0, and G-CSF 300 μg was subcutaneously administered once a day from day +1 until engraftment.
The other 44 patients received two cycles DHAP chemotherapy (cisplatinum 100 mg/m2 IV on day 1, cytarabine 2 g/m2 IV q12 h on day 2, dexamethasone 40 mg IV on day days 1 through 4).
Three months after the end of treatment, all patients were evaluated by PET/CT. This study was performed in accordance with the Declaration of Helsinki’ and written informed consent was obtained from the patients. Ethical approval was provided by the Ethics Committee of Xinqiao Hospital (Chongqing, China). All methods were performed in accordance with the relevant guidelines and regulations.
Response assessment and time-to-event end points
Treatment response, including complete remission (CR), partial remission (PR), progressive disease (PD), relapse, and Deauville score were assessed according to previously reported criteria [Citation14]. Response and Deauville score assessment occurred at two time points (after four courses of ABVD and the end of treatment). OS was measured from the time of diagnosis until death from any cause. PFS was defined as the time interval from diagnosis until disease progression or death.
Statistical methods
The differences in baseline characteristics of the disease and patients were evaluated with Mann-Whitney U test, χ2-test and Fisher’s exact test. OS and PFS were estimated according to the Kaplan-Meier method and compared with the log rank test. Multivariate analysis was performed using the Cox proportional regression model. SPSS version 16.0 statistical software was used. The sample size calculation was performed with Stata version 10 software.
Results
Clinicopathologic features
In total, eighty-nine patients were enrolled in our study. After restaging with PET/CT, forty-five patients received ASCT, and the other 44 patients received only two cycles of DHAP chemotherapy. Patient characteristics are summarized in . The baseline characteristics of the two groups did not significantly differ.
Table 1. Characteristics of patients.
Mobilization and engraftment
Peripheral blood stem cells (PBSCs) mobilization and collection in patients was routinely performed, and none of the patients failed to mobilize PBSCs. Patients had a mean PBSCs collection result of 7.96 ± 2.52 × 108 MNCs/kg (range, 4.02–10.94 × 108/kg) and 8.22 ± 6.08 × 106 CD34+ cells/kg (range 3.01–30 × 106/kg). All patients achieved neutrophil and platelet engraftment. The median time to ANC >0.5 × 109/L was 10 days (range, 7–14 days), and to platelets >20 × 109/L, the median was 12 days (range, 8–22 days).
Treatment response and survival
After 3 months of treatment, 39(86.7%) patients achieved CR, and 6 patients achieved PR in the ASCT group. However, 30 (68.2%) patients achieved CR, and 14 patients achieved PR in the chemotherapy group. The rate of CR in patients on ASCT was higher than in those on chemotherapy (P = 0.045). Among the patients who did not achieve CR, some patients with bulky disease received involved site radiation therapy (ISRT), and others switched to another second-line chemotherapy. No patient died of adverse effects from either ASCT or DHAP chemotherapy.
The median follow-up of patients was 54 months (range, 12–84 months). In the ASCT group, 4 patients relapsed or progressed at the time of analysis; however, 13 patients relapsed or progressed in the chemotherapy group. Among relapsed patients, 6 patients terminated their treatment, and 11 patients received another second-line chemotherapy. Two patients in the ASCT group received 12 cycles of anti-CD30 therapy and achieved CR. Then one patient received haploidentical hematopoietic stem cell transplantation (haplo-HSCT) but died of pulmonary invasive fungal infection. Other relapsed patients died of either recurrence or progression.
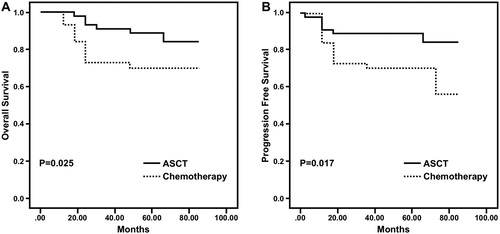
The 3-year OS and PFS of the patients who received ASCT were better than for the patients who received DHAP chemotherapy (). The 3-year OS was 91.1% and 72.7% for patients who received ASCT and chemotherapy, respectively (P = 0.025). Similarly, the 3-year PFS of patients was 88.9% in the ASCT group but only 70.5% in the chemotherapy group (P = 0.017).
Figure 1. A. Kaplan-Meier estimate of OS. The 3-year OS of the patients in ASCT group was 91.1% and that of the patients in chemotherapy group was 72.7% (P = 0.025) B. Kaplan-Meier estimate of PFS. The 3-year PFS of the patients in ASCT group was 88.9% and that of the patients in chemotherapy group was 70.5% (P = 0.017).
Toxicity
The toxicities of ASCT and DHAP chemotherapy are summarized in . There was no hepatic venous occlusive disease, creatinine elevation, hemorrhagic cystitis or tuberculosis (TB) infection in either group. The incidence of transaminase elevation, bilirubin elevation and heart dysfunction were very low in both groups. The incidence of gastrointestinal side effects for patients who received ASCT was significantly higher than for patients who received DHAP chemotherapy. The most common non-hematologic toxicities were nausea and vomiting. All patients had different degrees of nausea and vomiting. Additionally, only a small percentage of patients experienced infection, including bacterial infection, fungal infection, CMV infection, Herpes simplex infection and Herpes Zoster infection. About a quarter of patients in each group experienced bleeding due to thrombocytopenia. However, the main symptom of bleeding was petechial, and only one patient experienced alimentary tract hemorrhage. Myelosuppression was observed in all patients after BEAC conditioning regimen, and the incidence of myelosuppression in the ASCT group was significantly higher than for patients in the chemotherapy group.
Table 2. Toxity of ASCT and chemotherapy.
Secondary malignancies
Secondary malignancies were observed in two patients in the ASCT arm; one occurred as non-Hodgkin’s lymphoma (NHL) after ASCT 48 months, and another occurred acute myeloid leukemia (AML) after ASCT 66 months. In the chemotherapy arm, one patient experienced NHL after the end of treatment, at 73 months, and another patient experienced lung adenocarcinoma after the end of treatment, at 80 months. There was no difference in the incidence of secondary malignant tumors at the time of analysis. However, the follow-up of the 33 surviving patients was less than 5 years.
Causes of death
In the ASCT arm, 2 patients died of recurrence or progression, 1 patient died of invasive
fungal infection after haplo-HSCT and 2 patients died of secondary malignancies. In the chemotherapy arm, all 13 patients died of recurrence or progression. No patients died of toxicity from ASCT and chemotherapy.
Impact of various prognostic factors on survival
Compared with chemotherapy, ASCT significantly improved OS (P = 0.025) and PFS (P = 0.017), as determined by a univariate analysis. For OS, the univariate analysis identified elevated sLDH (P = 0.002), extranodal involvement (P < 0.001), bone marrow involvement (P = 0.001) and ESR ≥ 50 mm/h (P = 0.008) as negative factors. Gender, age, B symptoms, chronic disease and bulky disease did not significantly influence patient OS. Similarly, for PFS, elevated sLDH (P = 0.005), extranodal involvement (P < 0.001), bone marrow involvement (P < 0.001) and ESR ≥ 50 mm/h (P = 0.017) were found to be significant.
Multivariate analysis identified extranodal and bone marrow involvement as poor prognostic factors and ASCT as a good prognostic factor. However, the influence of elevated sLDH and ESR ≥ 50 mm/h was not confirmed by multivariate analysis ().
Table 3. Results of multivariate analysis of OS and PFS.
Discussion
Staging of HL is essential for the optimal choice of therapy. Patients with advanced-stage HL commonly receive a more prolonged course of combination chemotherapy, and most patients can be cured [Citation2]. However, nearly 30% of these patients are not cured and show either primary refractoriness or relapse [Citation6]. To improve the outcome of these patients, some studies have tried other chemotherapy regimens. Some clinical trials have compared the Stanford V regimen to ABVD and reported similar response rates, similar adverse events, and similar failure-free and overall survival [Citation15,Citation16]. Additionally, the GHSG developed a standard-dose and an escalated-dose BEACOPP for patients with advanced-stage HL [Citation17]. Some studies revealed better tumor control, PFS and OS for escalated BEACOPP [Citation7–9]. However, severe adverse events are more frequent in patients receiving BEACOPP than in ABVD-treated patients [Citation10]. One randomized study showed that compared with ABVD, treatment with BEACOPP resulted in better initial tumor control, but the long-term clinical outcome did not significantly differ because ASCT was sufficiently effective [Citation7]. This plan prevents all patients from receiving intensive initial treatment such as escalated BEACOPP [Citation7]. Presently, ABVD remains the most commonly used treatment for patients with advanced-stage HL [Citation6,Citation11].
The interim functional assessment of treatment response by PET/CT is the strongest predictor of treatment failure in advanced Hodgkin’s lymphoma, proving to be superior to the IPS [Citation18]. PET-negative patients with low total metabolic tumor volume had an excellent outcome, whereas patients with high total metabolic tumor volume had a moderate outcome [Citation19]. While treatment is being given, an interim PET scan that is positive may result in intensification of treatment, whereas treatment may be decreased if PET is negative [Citation20]. Thus, we should better control tumors during initial treatments for patients with advanced stage HL whose interim PET was positive while not increasing toxicity.
In our study, we applied ASCT at initial treatment for HL patients with stage III-IV, IPS ≥ 3, and Deauville more than 3° after 4 cycles of ABVD. We retrospectively compared the outcome of ASCT and second-line salvage high-dose chemotherapy. The objective of our study was to confirm the superiority of ASCT therapy in these patients and to determine whether this superiority would translate into higher rates of PFS and OS.
All patients were examined by PET/CT 3 months after the end of treatment in our study. Our results showed that the rate of CR in patients who received ASCT was significantly higher than in patients who received chemotherapy. With respect to toxicity, our results showed that compared with patients who received DHAP, only the rates of gastrointestinal side effects and myelosuppression were higher in patients who received the BEAC conditioning regimen. However, there was no difference in other adverse events, including infection, bleeding, or heart dysfunction. Most importantly, no patients died from ASCT toxicity at an early stage. This outcome may be due to the development of ASCT, the prevention of adverse effects, environmental protection provided by a laminar flow ward and the support of hematopoietic stem cells (HSCs). Although initial studies reported an average treatment-related mortality (TRM) of approximately 10%, recent randomized studies reported a lower TRM 3–4%, likely due to better supportive care, the use of PBSCs instead of bone marrow (BM), and earlier referral of patients to autografting [Citation21,Citation22]. Additionally, there was no difference in rate of secondary malignancies between ASCT and chemotherapy. At a minimum follow-up of 45 months, there were two patients who experienced secondary malignancies in each group. In our study, the 3-year OS and PFS of patients in the ASCT arm were both approximately 90%, which was better than the patients in the DHAP arm. Although different second-line chemotherapy may lead to different toxicity, the duration of follow-up was not sufficient to make these conclusions; nevertheless, our results still indicated that ASCT, as consolidation therapy at initial treatment, could increase CR rate and did not increase severe toxicity. This advantage could translate into a higher rate of survival.
The German Score, which incorporates 3 variables, including anemia, stage III-IV, and time to relapse less than 12 months, was validated by the randomized HDR2 study, which showed a 3-year PFS of 81%, 70%, 50%, and 14% in patients with adverse factors ranging from 1 to 4, respectively [Citation23,Citation24]. Majhail et al. analyzed 141 patients and identified the three following variables as being predictive of outcome: chemoresistance, B symptoms at relapse, and persistence of disease at transplant [Citation25]. However, in our study, extranodal and bone marrow involvement were poor prognostic factors, and ASCT was a good prognostic factor. This inconsistency in prognostic factors may be explained by differences in patient populations, the duration of follow-up, and different chemotherapy regimens.
The goal of treatment for patients with HL is to cure the disease, but limit long-term complications [Citation2]. The use of factors that identify patients who are at high risk for relapse is critical for defining the optimal intensity and duration of treatment [Citation2]. This process ensures adequate treatment, avoiding overtreatment for some patients and undertreatment for others [Citation2]. Prognostic factors for patients with advanced-stage disease focus less on disease bulk and more on evidence of systemic involvement [Citation2]. Thus, to avoid overtreatment, the enrollment criterion in our study was very strict, including stage III-IV, IPS ≥ 3 and Deauville more than 3° after 4 cycles of ABVD. Additionally, NCCN guidelines advised patients with stage III-IV HL to receive 6 cycles of escalated BEACOPP chemotherapy if the interim PET/CT was positive. The toxic effects of ASCT are similar to the toxicity of escalated BEACOPP therapy, which was related to a 3% TRM and a 2–3% secondary malignant rate [Citation10]. Further, ASCT may be safer than BEACOPP due to HSCs support.
There is no doubt that novel agents are a huge challenge for ASCT. An increasing number of studies have shown that the combination of brentuximab vedotin, programed cell death protein 1(PD-1) inhibitor or lenalidomide significantly improved PFS or OS in patients with RR-HL post-ASCT [Citation26–29]. Furthermore, more recent approaches have focused on adding novel new agents to standard chemotherapy [Citation30]. However, presently, we do not have strong evidence that we can depend solely on novel agents to cure HL. Therefore, we should explore better treatment strategies to decrease the rate of relapse and increase PFS or OS for patients with advanced stage HL.
In conclusion, our results indicated that ASCT as a first-line consolidation treatment for patients with advanced stage HL whose interim PET/CT was positive not only significantly increased the rate of CR but also did not increase toxicity. More importantly, this advantage could translate to better PFS and OS. However, the true efficacy of a given treatment is only evident after prolonged follow-up; thus, long-term prospective analyses rather than retrospective data are needed.
Author contributions
All authors have contributed to the data preparation, drafting, and revising of the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208
- Ansell SM. Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90:1574–1583. doi: 10.1016/j.mayocp.2015.07.005
- Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positronemission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin’s disease. Haematologica. 2001;86:266–273.
- Agostinelli C, Gallamini A, Stracqualursi L, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin's lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol. 2016;3:e467–e479. doi: 10.1016/S2352-3026(16)30108-9
- Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067
- Canellos GP, Rosenberg SA, Friedberg JW, et al. Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32:163–168. doi: 10.1200/JCO.2013.53.1194
- Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340
- Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5
- Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:943–952. doi: 10.1016/S1470-2045(13)70341-3
- Connors JM. Hodgkin’s lymphoma – the great teacher [editorial]. N Engl J Med. 2011;365:264–265. doi: 10.1056/NEJMe1104576
- Schwenkglenks M, Pettengell R, Szucs TD, et al. Hodgkin lymphoma treatment with ABVD in the US and the EU: neutropenia occurrence and impaired chemotherapy delivery. J Hematol Oncol. 2010;3:27–32. doi: 10.1186/1756-8722-3-27
- Gopal AK, Metcalfe TL, Gooley TA, et al. High-dose therapy and autologous stem cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle experience. Cancer. 2008;113:1344–1350. doi: 10.1002/cncr.23715
- Viviani S, Di Nicola M, Bonfante V, et al. Long term results of high-dose chemotherapy with autologous bone marrow or peripheral stem cell transplant as first salvage treatment for relapsed or refractory Hodgkin lymphoma: a single institution experience. Leuk Lymphoma. 2010;51:1251–1259. doi: 10.3109/10428194.2010.486090
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas: NCI Sponsored international working group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244
- Chisesi T, Bellei M, Luminari S, et al. Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin's lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol. 2011;29:4227–4233. doi: 10.1200/JCO.2010.30.9799
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma: United Kingdom National Cancer research Institute lymphoma group study ISRCTN64141244. J Clin Oncol. 2009;27:5390–5396. doi: 10.1200/JCO.2009.23.3239
- Diehl V, Sieber M, Rüffer U, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin’s disease. Ann Oncol. 1997;8:143–148. doi: 10.1023/A:1008294312741
- Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-Deoxy-D-Glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525
- Meignan M. Baseline metabolic tumour volume in Hodgkin lymphoma: the prognostic value of accessory cells. Eur J Nucl Med Mol Imaging. 2014;41:1732–1734. doi: 10.1007/s00259-014-2815-6
- Radford J, Illidge T, Barrington S. PET-directed therapy for Hodgkin’s lymphoma. N Engl J Med. 2015;373:392. doi: 10.1056/NEJMe1511947
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9
- Josting A, Müller H, Borchmann P, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin's lymphoma. J Clin Oncol. 2010;28:5074–5080. doi: 10.1200/JCO.2010.30.5771
- Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221–230.
- Josting A, Rueffer U, Franklin J, et al. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin lymphoma study group. Blood. 2000;96:1280–1286.
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. doi: 10.1016/j.bbmt.2006.06.006
- Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive Lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087
- Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24–31. doi: 10.1186/1756-8722-7-24
- Mandac I, Kolonic SO. Lenalidomide induced good clinical response in a patient with multiple relapsed and refractory Hodgkin's lymphoma. J Hematol Oncol. 2010;3:20–22. doi: 10.1186/1756-8722-3-20
- Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14:1348–1356. doi: 10.1016/S1470-2045(13)70501-1
