ABSTRACT
Objective: To present the clinical and laboratory implications of defects or variants of some clotting factors and of thrombomodulin that were discovered during the past few years.
Methods: Data concerning new aspects of FII, FV, FIX and thrombomodulin defects were investigated. This involved the dysprothrombinemias, the East Texas or short FV disorder, a FIX defect and a thrombomodulin abnormality.
Results: the recently reported clotting defects or variants are: (1) the thrombophilic dysprothrombinemias due to Arg596 mutations (Prothrombin Yukuhashi, Belgrade and Padua 2) which are characterized by absence of bleeding and presence of venous thrombosis; (2) the short FV defects due to Ser356Gly (FV East Texas) or Ala863Gly (FV Amsterdam) mutations characterized by a mild bleeding tendency with normal FV and other clotting factors, increased TFPI and no thrombosis; (3) the abnormal FIX (FIX Padua) due to the Arg338Leu mutation which is associated with high levels of FIX activity, lack of bleeding and venous thrombosis; (4) the thrombomodulin Cys537Stop mutation associated with a mild bleeding tendency despite normal clotting factors but increased plasma levels of soluble thrombomodulin and no thrombosis.
Conclusions: these new coagulation defects have great implications in the clinical and laboratory approach to the coagulation disorders. They have demonstrated that a prothrombin defect may be associated with thrombosis, that a mild bleeding tendency may occur despite normal Factor V levels and that high levels of plasmatic thrombomodulin may be associated with mild bleeding.
During the past few years, important new data on the role of some clotting factors or proteins have been discovered. These progresses regard FII, FV, FIX and thrombomodulin (TM). Some dysprothrombinemias, (Prothrombin Yakuhashi, Belgrade and Padua 2) [Citation1–3] and also a mutation in FIX (FIX Padua) have been found to cause venous thrombosis and no bleeding [Citation4]. On the other hand, a peculiar FV abnormality (East Texas or short FV defect) has been found to present a mild bleeding tendency in the presence of normal FV and other clotting factors activity [Citation5,Citation6]. The mild bleeding tendency was due to increased levels of a natural inhibitor, the Tissue Factor pathway inhibitor (TFPI). Finally, a mutation in the TM gene has been associated with a mild bleeding tendency [Citation7,Citation8].
These studies have completely modified our understanding of blood clotting in the sense that they have demonstrated that: (1) prothrombin and FIX defects may be associated with venous thrombosis and not with bleeding; (2) abnormal FV may show a bleeding tendency despite the normal levels of the clotting factors including FV; (3) that a natural inhibitor (TFPI) may play a clinical role and, finally; (4) thrombomodulin abnormalities may play a role in bleeding.
These observations have played a great role in our understanding of the mechanism of blood coagulation and in clarifying some ill-known aspects of it [Citation9,Citation10].
However these observations have not received so far the clinical attention they deserve.
The purpose of the present review is to deal with these new conditions in a systematic way so that the potential clinical implications associated with them become common knowledge for the people interested in blood coagulation.
Thrombophilic abnormalities of FII (antithrombin resistance)
Congenital prothrombin deficiency is one of the rarest coagulation disorders [Citation11,Citation12]. Homozygotes or compound heterozygotes with FII levels of less than 10% of normal, present a severe bleeding tendency [Citation12]. Complete absence of Prothrombin seems incompatible with life. Heterozygotes with FII levels around 40–60% of normal may present occasional bleeding during surgery or tooth extractions [Citation13]. No thrombotic event has ever been reported in ‘true’ Prothrombin deficiency. Recently a few cases of prothrombin abnormalities or dysprothrombinemias have been associated with a thrombotic tendency [Citation1–3,Citation14,Citation15].
Antithrombin (AT) is a small glycoprotein of a molecular weight of 58,000 Dalton produced by the liver and circulating at a concentration of about 0.12 mg/ml. When coupled with heparin it exerts mainly an anti FII and anti FX activity. Without heparin the activity of antithrombin is markedly reduced. The increased resistance of these abnormal prothrombins to the action of AT creates a condition of prolonged thrombin activity which may cause thrombosis. The condition has been termed ‘antithrombin resistance’ [Citation1].
This is a new clinical entity characterized by a relative decrease of antithrombin activity due to the presence of abnormally resistant prothrombins (thrombins)which have mutations in a special region of the molecule, encoded by exon 14, that is supposed to interact with AT. Due to these mutations the generation of the complex thrombin-antithrombin is defective whereby antithrombin activity is decreased, thrombin persists in the circulation and a thrombophilic state ensues [Citation1–3,Citation14,Citation15].
The first prothrombin abnormality responsible of this effect was reported in 2012 (Prothrombin Yukuhashi) [Citation1]. Subsequently other similar cases were published in Serbia, India and Italy [Citation14–16]. The five families involve three different mutations on the same amino acid, an Arginine: Prothrombin Yukuhashi Arg596Leu [Citation1], Prothrombin Belgrade Arg596Gln [Citation2] and Prothrombin Padua 2, Arg596Trp [Citation3] (Table ). Another Prothrombin abnormality seen in Japan is identical to Prothrombin Belgrade, namely Arg596Gln [Citation14] and Prothrombin Amrita seen in India has also an Arg596Gln mutation [Citation15]. It is still unknown at what level of the codon sequence, a mutation can cause the shifting of a bleeding condition into a thrombophilic one [Citation17].
Table 1. Cases of Dysprothrombinemias due to Arg596 mutations associated with a gain of function toward antithrombin with consequent appearance of a thrombophilic state.
Upstream, using the numbering of aminoacids (a.a.) that includes those of the pre-pro region, we have Prothrombin Greenville (Arg560Gln) [Citation18] and Prothrombin Perija (Gly591Ala) [Citation19]. Downstream, after a.a.596, we have Prothrombin Scranton (heterozygote for Lys599Thr) [Citation20] and Prothrombin Leiden (compound heterozygote for Trp612Stop + ivs2-25C/G) [Citation21].
All these prothrombin defects show a variable bleeding tendency but no thrombosis. It is interesting to note that all patients with dysprothrombinemia and thrombosis are heterozygotes for the mutation. This indirectly confirms the fact that homozygosis for ‘true’ prothrombin deficiency is very severe and sometimes incompatible with life [Citation11,Citation12].
The main laboratory and clinical features of these dysprothrombinemias are gathered in Table . All patients had venous thrombosis; there is no information about arterial thrombosis. It is likely that other cases will be discovered. A clinical suspicion should arise from the following observations: 1) venous thrombosis in a young patient known to have no other known prothrombotic defects (AT, Prot. C etc. deficiencies); 2) slightly decreased or borderline low prothrombin activity level; 3) Prothrombin antigen level higher than the activity counterpart; 4) positive family history for venous thrombosis. Needless to say that genetic analysis is needed to confirm the suspicion.
The occurrence of thrombosis at a young age is of paramount importance. The mean age, excluding the case from India, is 20.1 (range 11-38). The patient from India had a venous thrombosis at the age of 60 [Citation15]. Furthermore there is no information about a familiar predisposition to thrombosis. It is worth noting that so far no abnormality of FX has been described which could generate an impairment in the activity of antithrombin with a consequent antithrombin resistance. On theoretical grounds the possibility that such mutation in the FX gene exist is fully plausible, and even likely.
Short FV or East Texas bleeding disorder
This bleeding condition was reported, as an autosomal dominant disorder, in a large kindred from East Texas in 2001. The patients had a mild bleeding tendency but all clotting factors were normal and no diagnosis was reached [Citation22,Citation23]. The nature of the defect was clarified several years later [Citation16,Citation24]. It was due to the presence of a short FV that lacked (a.a.756-14580 of the B domain). This short FV has a great avidity for Tissue Factor Pathway Inhibitor (TFPI). As a consequence levels of TFPI in these patients are about 10 times greater than in normal subjects. The high level of TFPI retards or inhibits the function of the tissue factor-FVII complex and, indirectly, of FX activation and this explains the mild bleeding tendency. The condition is also known as ‘short FV defect’ [Citation11,Citation16]. The mutation in exon 13 responsible for the defect is Ser756Gly.
Since the B domain is not required for FV activity, this explains the normal levels of FV present in these patients [Citation16,Citation24]. However the B domain is essential in maintaining FV in an inactivated state. Once it is removed or reduced, the resulting FV shows a great avidity for TFPI [Citation16,Citation24]. The gain of function of this abnormal FV refers to its binding to TFPI. This leads to prolongation of TFPI life and to its increased plasma levels with consequent bleeding tendency. In other words the high TFPI levels interfere with the aFVII-Tissue Factor formation and activity with a consequent appearance of a hypocoagulable state.
Recently, another similar FV defect was reported (FV Amsterdam) [Citation25]. This is also a short FV but is different from East Texas FV in that only 63 aa. of the B domain are missing. The number of missing aa in FV East Texas are 702. In this case the deletion of most of the B domain results in a greatly increased binding of TFPI. In the case of FV Amsterdam the increased of TFPI is less pronounced [Citation16,Citation25] but the clinical and laboratory picture is similar to that of East Texas FV. The mutation is Ala863Gly always in exon 13.
Because there is no geographical or familial relation between these two families and because of the different mutation, it is highly probable that they represent separate founder effects. It is likely, therefore, that new cases will be discovered. From a diagnostic point of view the most important suspicion should arise when a mild bleeding tendency in a family is not explained by any known defect. The suspicion has to be confirmed by the finding of high levels of TFPI and the correct diagnosis clinched by genetic studies. The significance of an elevated level of TFPI had never been suspected to play a role in the diagnosis of a bleeding tendency in humans [Citation24]. TFPI is a polipeptide that has two isoforms TFPIα and TFPIβ which show different inhibitory activities. TFPI congenital deficiency has never been described in humans but the inhibitor is at present time the object of great scientific interest [Citation26].
Abnormal FIX defect (FIX Padua)
An abnormal FIX defect characterized by an elevated FIX clotting activity in the presence of normal or only slightly increased antigen was described in a patient with venous thrombosis. The defect was X-linked and was named FIX Padua [Citation4].
The hemizygous mutation found in this patient was Arg338Leu in exon 8. The propositus was a 25 year old male who presented a right femoral-popliteal vein thrombosis. He was treated with LMWH and warfarin with a complete recanalization of the involved veins. A brother was similarly affected but was asymptomatic whereas the mother was a carrier and was also asymptomatic [Citation4].
The main laboratory feature was a 10 fold increase in FIX clotting activity. This discovery prompted research to try and produce chimeric FIX Padua concentrates for potential use in the treatment of hemophilia B patient [Citation27–29].
Epidemiological studies in unrelated groups of patients with venous thrombosis in the Netherlands and Brasil failed to discover other cases with this FIX abnormality. This indicates that the defect is rare [Citation30,Citation31] and probably contributes only a little to the understanding of the causes underlying the group of still unexplained familiar venous thromboses. However, since the total number of the patients with venous thrombosis so far investigated is limited (333 patients) [Citation30,Citation31], the actual impact of the abnormality has to be considered still unknown.
Thrombomodulin defects
Thrombomodulin (TM) is a transmembrane glycoprotein composed of 557 aminoacids. It has a great affinity for binding to thrombin and to transform it from a coagulant to an anticoagulant compound [Citation9,Citation32]. The complex thrombin-thrombomodulin (T-TM) has two main actions: 1) it activates Protein C which by its turn, once activated, downregulates FVa and FVIIIa namely it behaves as an anticoagulant. and 2) it activates also the thrombin activable fibrinolysis inhibitor (TAFI) [Citation9,Citation32]. Activated TAFI inhibits the adherence of plasminogen to the surface of a fibrin clot thereby decreasing the effect of plasmin-induced fibrinolysis. As a consequence, TM plays an important role in regulating the structure and resistance of the fibrin clot [Citation32]. Congenital TM deficiency has never been described. In 2014 and 2015 two families with a mild bleeding tendency but normal coagulation tests were reported [Citation7,Citation8]. Extensive and elegant investigations demonstrated that the defect was due to greatly elevated levels of soluble TM. Genetic analysis of the TM gene showed the presence of the same mutation, namely Cys537Stop in both families [Citation7,Citation8]. Such mutation is responsible for the gain of function effect of TM. It has to be remembered that another TM mutation (Asp468Tyr) has been associated with venous thrombosis. This observation has not been confirmed yet. Should it be, TM defect should be included, together with FII, FV and FIX, among the coagulation defects that may cause both bleeding and thrombosis.
The greatly increased levels of TM are maintained to be responsible for the bleeding tendency. Bleeding is usually mild but it may become severe after trauma or surgical procedures which generate Tissue Factor [Citation7,Citation8] ().
Figure 1. Schematic representation of mutated TM (Cys573Stop) on the clotting mechanism. The end result is the appearance of a mild bleeding tendency that may worsen after trauma or surgery which increase tissue factor and thrombin formation.
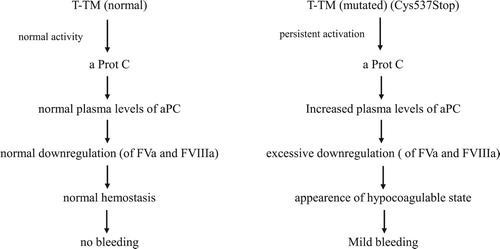
The exact mechanism of bleeding is still not fully explained but it seems that in these patients the effect of the T-TM complex on FV and FVIII (potentially hemorrhagic action) prevails on the effect on TAFI (potentially procoagulant action).
The assay method used to evaluate circulating TM is an Elisa method.
The continuous shedding of TM into the circulation, once bound to thrombin, causes a persistent activation of Protein C with secondary downregulation of FVa, FVIIIa) and an inhibition of thrombin generation within the hemostatic clot that, as a consequence, is weak.
These patients show also a decreased prothrombin consumption which has not been explained [Citation7,Citation8].
The description of these two families with the same mutation has considerably spurred the interest in the T-TM complex activities [Citation32,Citation33].
Discussion
The discovery of these new defects has complicated the laboratory investigation and diagnosis of blood coagulation disorders.
The dysprothrombinemias show slightly reduced FII levels, no bleeding and venous thrombosis: an unknown combination. The ‘short’ FV defects show normal clotting tests, including normal FV activity together with a mild bleeding tendency and, for the first time, increased levels of a natural inhibitor the TFPI that had been in the past only of little importance, if any.
Finally, a mutation in TM, always considered only a protein capable of binding thrombin, is found to be responsible for a mild bleeding tendency due to increased plasma levels of soluble TM. TM, a trans membrane protein, plays a role also in plasmatic clotting tests and becomes responsible for a mild bleeding tendency.
Because of these considerations the diagnostic approach to clotting tests has to be modified. Assays of natural anticoagulants (TFPI) or of soluble TM, have to be included. These assays are so far immunological, Elisa test assays.
It is likely that ‘activity clotting’ assays could be developed in the future.
This would eliminated the possibility that discrepancies might exist between ‘activity’ and protein assays. This has already been demonstrated to occur for several clotting factors, for example, for FII and FVII [Citation34,Citation35].
The worsening of bleeding after trauma or surgery in TM disorders is due to liberation of T.F. and thrombin. This causes a continuous activation of Protein C with consequent persistent and extensive downregulation of FVa and FVIIIa namely a neutralization of their precoagulant activity.
As a result of this protracted downregulation of FVa and FVIIIa a hypocoagulable state appears together with bleeding.
The study on the ‘short’ FV and on Tm mutations have underscored the importance of natural inhibitors in blood coagulation.
So far, only antithrombin, antiplasmin and Plasminogen activator inhibitor had a clear, clinical significance. Now TFPI, TAFI and soluble TM have entered the play.
Furthermore the central role of a Protein C has received confirmation [Citation9]. Finally a transmembrane protein as TM has been demonstrated to play a double role, both at the endothelial level and in the circulating plasma.
These new achievements have also important implication in the diagnosis of bleeding disorders. There may be patients who show a mild bleeding tendency despite the fact that all known clotting factors are normal. This was typical of non-natural circulating anticoagulants.
Now it could occur even with natural anticoagulants due to a mutation in a clotting factor (‘short’ FV) or a mutation in the clotting protein (TM).
The diagnosis of bleeding disorders should include now even the evaluation of natural anticoagulants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Miyawaki Y, Suzuki A, Fujita J, et al. Thrombosis from a prothrombin mutation conveying antithrombin resistance. N Engl J Med. 2012;366:2390–2396. doi: 10.1056/NEJMoa1201994
- Djordjevic V, Kovac M, Miljic P, et al. A novel prothrombin mutation in two families with prominent thrombophilia – the first cases of antithrombin resistance in a Caucasian population. J Thromb Haemost. 2013;11:1936–1939.
- Bulato C, Radu CM, Campello E, et al. New prothrombin mutation (Arg596Trp, prothrombin Padua 2) associated with venous thromboembolism. Arterioscler Thromb Vasc Biol. 2016;36:1022–1029.
- Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361:1671–1675. doi: 10.1056/NEJMoa0904377
- Vincent LM, Tran S, Livaja R, et al. Coagulation factor V(A2440G) causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123:3777–3787.
- Cunha ML, Bakhtiari K, Peter J, et al. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125(11):1822–1825.
- Langdown J, Luddington RJ, Huntington JA, et al. A hereditary bleeding disorder resulting from a premature stop codon in thrombomodulin (p.Cys537Stop). Blood. 2014;124:1951–1956.
- Dargaud Y, Scoazec JY, Wielders SJ, et al. Characterization of an autosomal dominant bleeding disorder caused by a thrombomodulin mutation. Blood. 2015;125:1497–1501. doi: 10.1182/blood-2014-10-604553
- Esmon CT. The protein C pathway. Chest. 2003;124(Suppl 3):26S–32S. doi: 10.1378/chest.124.3_suppl.26S
- Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38 (Suppl 1):4–11. doi: 10.1111/ijlh.12508
- Girolami A, Scarano L, Saggiorato G, et al. Congenital deficiencies and abnormalities of prothrombin. Blood Coagul Fibrinolysis. 1998;9:557–569. doi: 10.1097/00001721-199810000-00001
- Lancellotti S, Basso M, De Cristofaro R. Congenital prothrombin deficiency: an update. Semin Thromb Hemost. 2013;39:596–606. doi: 10.1055/s-0033-1348948
- Girolami A, Santarossa C, Cosi E, et al. Bleeding manifestations in heterozygotes with prothrombin deficiency or abnormalities vs. unaffected family members as observed during a long follow-up study. Blood Coagul Fibrinolysis. 2017;28:623–626.
- Kishimoto M, Suzuki N, Murata M, et al. The first case of antithrombin-resistant prothrombin Belgrade mutation in Japanese. Ann Hematol. 2016;95:541–542. doi: 10.1007/s00277-015-2533-6
- Sivasundar S, Oommen AT, Prakash O, et al. Molecular defect of ‘prothrombin Amrita’: substitution of arginine by glutamine (Arg553 to Gln) near the Na(+) binding loop of prothrombin. Blood Cells Mol Dis 2013; 50: 182–183.
- Vincent LM, Tran S, Livaja R, et al. Coagulation factor V (A2440G) causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123:3777–3787.
- Girolami A, Ferrari S, Cosi E, et al. Congenital prothrombin defects: they are not only associated with bleeding but also with thrombosis: a new classification is needed. Hematology. 2018;23:105–110. doi: 10.1080/10245332.2017.1359900
- Henriksen RA, Dunham CK, Miller LD, et al. Prothrombin Greenville, Arg517 Gln, identified in an individual heterozygous for dysprothrombinemia. Blood. 1998;91:2026–2031.
- Sekine O, Sugo T, Ebisawa K, et al. Substitution of Gly-548 to Ala in the substrate binding pocket of prothrombin Perijá leads to the loss of thrombin proteolytic activity. Thromb Haemost. 2002;87:282–287.
- Sun WY, Smirnow D, Jenkins ML, et al. Prothrombin Scranton: substitution of an amino acid residue involved in the binding of Na+ (LYS-556 to THR) leads to dysprothrombinemia. Thromb Haemost. 2001;85:651–654.
- Poort SR, Njo KT, Vos HL, et al. Two novel mutations in the prothrombin gene cause severe bleeding in a compound heterozygous patient. Blood Coagul Fibrinolysis. 1998;9:761–764. doi: 10.1097/00001721-199811000-00007
- Kuang SQ, Hasham S, Phillips MD, et al. Characterization of a novel autosomal dominant bleeding disorder in a large kindred from east Texas. Blood. 2001;97:1549–1554. doi: 10.1182/blood.V97.6.1549
- Broze GJ Jr, Girard TJ. Factor V, tissue factor pathway inhibitor, and east Texas bleeding disorder. J Clin Invest. 2013;123:3710–3712. doi: 10.1172/JCI71220
- Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38(Suppl 1):4–11. doi: 10.1111/ijlh.12508
- Cunha ML, Bakhtiari K, Peter J, et al. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125:1822–1825.
- Wood JP, Ellery PE, Maroney SA, et al. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764
- Lozier JN. Gene therapy. Factor IX Padua: them that have, give. Blood. 2012:29(120):4452–4453. doi: 10.1182/blood-2012-09-452821
- Cantore A, Nair N, Della Valle P, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012:29(120):4517–4520.
- Monahan PE, Sun J, Gui T, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther. 2015;26:69–81.
- Koenderman JS, Bertina RM, Reitsma PH, et al. Factor IX-R338L (Factor IX Padua) screening in a Dutch population of sibpairs with early onset venous thromboembolism. Thromb Res. 2011;128:603.
- Mazetto Bde M, Orsi FL, Siqueira LH, et al. Prevalence of Factor IX-R338L (Factor IX Padua) in a cohort of patients with venous thromboembolism and mild elevation of factor IX levels. Thromb Res. 2010;126:165.
- Anastasiou G, Gialeraki A, Merkouri E, et al. Thrombomodulin as a regulator of the anticoagulant pathway: implication in the development of thrombosis. Blood Coagul Fibrinolysis. 2012;23:1–10. doi: 10.1097/MBC.0b013e32834cb271
- Ohlin AK, Marlar RA. The first mutation identified in the thrombomodulin gene in a 45-year-old man presenting with thromboembolic disease. Blood. 1995;85:330–336.
- Girolami A, Fabris F, Dal Bo Zanon R, et al. Factor VII Padua: a congenital coagulation disorder due to an abnormal factor VII with a peculiar activation pattern. J Lab Clin Med. 1978;91:387–395.
- Girolami A, Molaro G, Lazzarin M, et al. A ‘new’ congenital haemorrhagic condition due to the presence of an abnormal factor X (factor X friuli): study of a large kindred. Br J Haematol. 1970 Aug;19:179–192. doi: 10.1111/j.1365-2141.1970.tb01615.x
