ABSTRACT
Objectives: Diffuse large B cell lymphoma (DLBCL) is a heterogeneous lymphoma with a variety of presentations and treatment modalities. With the introduction of immunotherapy as an addition to chemotherapy (CT), there is ongoing debate about the role of radiotherapy (RT) in treatment and a need to clarify differences by specific anatomic locations.
Methods: We identified a cohort of 1929 individuals with limited stage (stage I and II) head and neck DLBCL with extranodal involvement from the National Cancer Data Base. Overall survival (OS) was evaluated by Kaplan-Meier analysis, multivariable Cox proportional hazard regression, and propensity score-matched analysis.
Results: Multi-agent CT plus RT was associated with longer OS (HR, 0.763; 95% CI, 0.614 to 0.948; p = 0.015) when compared with multi-agent CT alone on multivariate analysis. After propensity score matching to account for confounding variables, multi-agent CT plus RT was associated with longer OS than those who received multi-agent CT alone (HR = 0.769; 95% CI, 0.609–0.971; p = 0.027). The survival benefit persisted in patients over the age of 60 years and those who received RT within 180 days of CT. However, there was no significant difference in OS between the two groups in subgroup analysis of patients who received immunotherapy.
Conclusion: The addition of RT to CT resulted in longer OS in patients with limited stage head and neck DLBCL with extranodal involvement.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin Lymphoma (NHL), accounting for 25% of all NHL cases [Citation1]. DLBCL encompasses a range of clinical presentations, genetics, and biological behavior. Patients present at a median age of 64 [Citation2]. The head and neck is a common site of nodal enlargement in patients with DLBCL, and is the second most common site of extranodal lymphoma [Citation3]. A previous study conducted by Sanchez et. al found that patients with primary extranodal head and neck DLBCL (HN-DLBCL) had better overall survival (OS) than those with nodal head and neck DLBCL when treated with standard therapy [Citation4].
DLBCL is an aggressive lymphoma, with survival often measured in months [Citation5]. The International Prognostic Index incorporates staging and other relevant factors into risk groups that correlate with progression-free survival and OS after therapy [Citation6]. The Lugano system, based on the Ann Arbor system, is the current staging system used for NHL patients [Citation7]. Limited or early stage DLBCL (Stage I, Stage II, and Stage II bulky variant) and advanced DLBCL (Stage III and IV) are often treated differently and have very different survival outcomes. Current treatment for limited stage DLBCL often includes chemotherapy (CT), the anti-CD20 antibody rituximab, and possibly radiotherapy (RT), while treatment for advanced stage DLBCL involves CT and rituximab.
With the recent inclusion of Rituximab in the treatment of early stage DLBCL, patient outcomes have improved, while the use of RT has declined [Citation8]. Studies have attempted to understand the role of RT in conjunction with CT versus CT alone, but results remain controversial. Due to the distinct characteristics of different extranodal sites, the benefit of RT in addition to CT is currently uncertain and not well-defined [Citation9,Citation10]. Among the extranodal sites, the head and neck location is of particular interest because RT is often used despite the risk of toxicity to the area [Citation8,Citation11]. Previously, Vargo et al. found OS to be longer with multi-agent CT plus RT than with multi-agent CT alone in a large retrospective cohort of 59,255 patients with early-stage DLBCL from the National Cancer Data Base (NDCB) [Citation8]. However, the study did not perform subgroup analysis on patients with HN-DLBCL and included patients both with and without extranodal involvement. In a retrospective study of 488 patients from 17 cancer centers, Mian et al. found that the addition of RT in remitters of stage I and II HN-DLBCL of several distinct major anatomical locations did not translate into improved 5-year OS rate [Citation11].
The aim of our study was to identify prognostic factors in patients with limited stage HN-DLBCL with extranodal involvement and compare OS of multi-agent CT plus RT and multi-agent CT alone using data from the NCDB.
Materials and methods
The NCDB is a clinical oncology database sourced from hospital registry data from more than 1,500 Commission on Cancer (CoC) accredited facilities, jointly sponsored by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The database consists of patients who received some element of their cancer care (treatment or diagnosis) at a cancer program that is accredited by the CoC. Patients who did not receive care but interacted with a physician at a CoC facility are not reported to the NCDB. Although these cancer programs represent approximately 30% of US hospitals, the NCDB currently captures approximately 70% of all newly diagnosed malignancies in the United States [Citation12]. The data used in this study are derived from a de-identified NCDB data file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or for the conclusions drawn, from these data by the investigators.
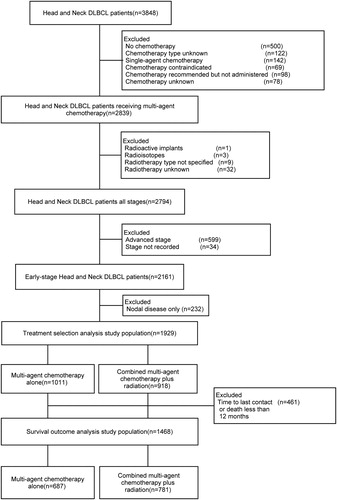
Our study population consisted of patients who were diagnosed with early stage (defined as Stage I and II) HN-DLBCL with extranodal involvement between 2004 and 2014 from the NCDB. We excluded patients who did not receive multi-agent CT, or those who received RT modalities other than external beam RT. Patients with unknown treatment status, or unrecorded stage were also excluded. A patient selection flowchart describing the cohort is outlined in . We compared the clinical and demographic characteristics between included and excluded patients (Supplemental Table 5).
Patients’ co-morbidity was evaluated by the Charlson-Deyo score. Charlson-Deyo score (0, 1, or 2) was assigned according to NCDB guidelines based on how many co-morbid conditions were reported and their relative severity. Location was further subdivided into five subregions: Waldeyer’s ring, parotid and salivary glands, palate and oral cavity, pharynx and more than one involved site [Citation11].
All statistical analyses were performed using SPSS V22.0 (SPSS Inc., Chicago, IL). The primary endpoint was OS, defined as date of diagnosis to the date of death or last contact (censored). To account for immortal time bias, the conditional landmark method was used. We restricted survival analysis to patients with survival time of 12.0 months or greater (the allowed time from diagnosis to RT) [Citation13]. Categorical variables were compared using chi-square tests, and continuous variables were compared using independent sample t tests. Univariate hazard estimates of OS were generated with unadjusted Cox proportional hazards models. Covariates demonstrating significance (P < 0.05) on univariate analysis were included in the multivariate model. To balance confounding factors between groups, propensity-score matching was performed using independent factors that had association with survival on Univariate Cox regression (Matchlt package in R). Matching was performed using independent factors that had an association with survival on univariate Cox regression. The caliper width was 0.05 times the standard deviation of the logit of the propensity score, which is estimated to eliminate greater than 99% of the bias due to confounding variables [Citation14]. Next, the same survival analyses were performed in the matched cohorts. Subgroup analyses based on an age cut-off of 60 years and in patients who started RT within 180 days of the CT start date were performed. We also compared OS between multi-agent CT alone versus multi-agent CT plus RT in patients who received immunotherapy.
Results
The final study cohort consisted of 1,929 stage I or II HN-DLBCL patients who received multi-agent CT and/or RT as the primary treatment modality. Baseline patient, tumor, and treatment characteristics for the included patients are summarized in . Comparison of demographic factors among included and excluded patients is shown in supplementary Table 5. Significant statistical differences were found in insurance status and B symptoms between the two groups. In terms of Ann Arbor staging, 56.7% of the patients were stage I and 43.3% stage II. The median age at diagnosis was 64.5 years (range:2–90 years); 65.4% were older than 60 years; the male to female ratio was 1.3:1. Among all patients, 52.4% (n = 1,011) patients received multi-agent CT alone, and 47.6% (n = 918) patients received multi-agent CT plus RT.
Table 1. Comparison of baseline characteristics between patients who received multi-agent CT alone and those who received multi-agent CT plus RT.
Univariable and multivariable Cox regression analyses for OS are shown in . On multivariate analysis, increasing age (HR, 3.878; 95% CI,2.843 to 5.288;p < 0.001) and Charlson-Deyo score ≥ 1 (HR,1.914; 95% CI,1.499 to 2.444;p < 0.001) were associated with shorter OS, while multi-agent CT plus RT was associated with longer OS when compared to multi-agent CT alone (HR, 0.763; 95% CI, 0.614 to 0.948; p = 0.015) ( and ). After adjusting for immortal time and indication bias, multi-agent CT plus RT was still associated with longer OS (HR, 0.797; 95% CI, 0.614 to 0.990; p = 0.040) when compared with multi-agent CT alone ().
Figure 2. Comparison of overall survival between patients who received multi-agent CT alone and those who received multi-agent CT plus RT (a) before propensity-score matching (b) after propensity-score matching and (c) after adjusting for immortal time and indication bias.
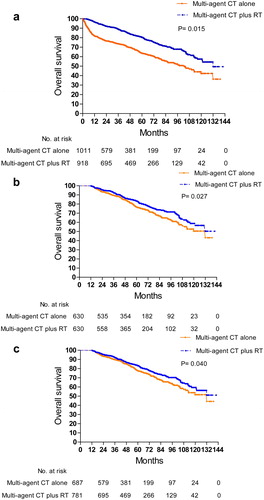
Table 2. Univariate and multivariate cox proportional hazards analyses of overall survival among all patients.
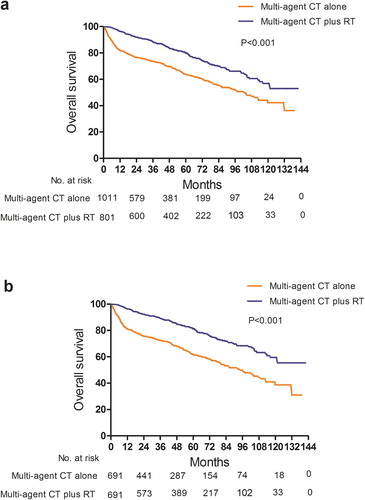
A comparison of clinical and demographic characteristics between younger and older patients are shown in Supplementary Table 1. Univariable and multivariable Cox regression analyses for OS of the age subgroups are shown in Supplementary Tables 2 and 3. In patients aged < 60 years, there was a trend towards longer OS with the addition of RT for patients who received multi-agent CT (HR, 0.640; 95% CI, 0.405–1.013;p = 0.057) (). In patients aged ≥ 60, multi-agent CT plus RT was significantly associated with longer OS (HR, 0.486; 95% CI, 0.400–0.591;p < 0.001) (). As shown in Supplementary , the vast majority of patients started RT within 180 days of the CT start date, indicating concurrent or sequential RT. Subgroup analysis of patients who received RT within 180 days of CT (HR, 0.522; 95% CI, 0.433–0.629;p < 0.001) demonstrated longer OS in patients who received multi-agent CT plus RT when compared to those who received multi-agent CT alone (). In the 371 patients who received immunotherapy, there was no significant difference in OS between patients who received multi-agent CT alone versus multi-agent CT plus RT (HR, 0.974; 95% CI, 0.584–1.625; p = 0.919) (Supplementary Figure 1a and Supplementary Table 4).
Figure 3. Comparison of overall survival between multi-agent CT alone and multi-agent plus RT for patients aged <60 years (a) before and (b) after propensity-score matching and patients aged ≥ 60 (c) before and (d) after propensity-score matching.
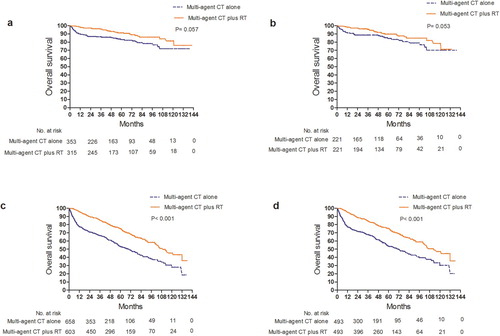
Figure 4. Comparison of overall survival between multi-agent CT alone and multi-agent CT plus RT for patients who received RT within 180 days of CT (a) before and (b) after propensity-score matching.
The two treatment groups were matched on propensity score of age, race and Charlson-Deyo score. On comparison of 630 patients who received multi-agent CT alone and 630 matched patients who received multi-agent CT plus RT, the mortality was decreased for patients treated with multi-agent CT plus RT (HR = 0.769; 95% CI, 0.609–0.971; p = 0.027) (). The same results persisted in analyses of age subgroups, patients who received RT within 180 days of CT, and those who received immunotherapy on propensity-score matched analyses.
Discussion
Our retrospective analysis of 1,929 Stage I and II HN-DLBCL patients with extranodal involvement suggests that multi-agent CT plus RT is associated with improved OS when compared to multi-agent CT alone. Furthermore, the survival benefit persisted after propensity score matching to account for differences in age, race and Charlson-Deyo score between the two groups. This survival advantage was especially prominent in patients older than 60 and persisted in subgroup analysis of patients who started RT within 180 days of the CT start time.
With regard to our primary study objective of adjuvant RT and CT in early stage extranodal HN-DLBCL patients, our study follows considerable discussion on the role of RT in DLBCL patients [Citation10,Citation15,Citation16]. Before the introduction of Rituximab, several randomized trials yielded mixed results on multi-agent CT plus RT versus CT alone [Citation8,Citation10,Citation15–30]
To this end, one approach is to differentiate between different sites of extranodal involvement, though standardized protocols remain limited. In our study, focusing on the head and neck region is important because HN-DLBCL is associated with a higher use of consolidation RT [Citation29]. Head and neck is also a vulnerable site where significant toxicity might otherwise disfavor RT as a treatment option. To our knowledge, few studies have looked at early stage HN-DLBCL with extranodal involvement specifically. In 2014, Mian et al. found that OS of early stage HN-DLBCL patients was not improved in patients receiving RT in addition to CT when compared to patients receiving CT alone [Citation11]. An important distinction between this retrospective study of 488 patients and our analysis is that Mian et al. compared the two groups only in remitters after CT, whereas the majority of patients in our cohort likely received RT for as consolidation therapy. Murawski et al. analyzed the results of 11 prospective clinical studies conducted by the German High-Grade Non-Hodgkin Lymphoma Study Group and found no improved survival for 145 DLBCL patients with extralymphatic craniofacial involvement receiving RT in addition to CT compared to 57 patients not receiving RT [Citation31]. However, the conclusion is limited due to small cohort size.
In contrast to these earlier studies on HN-DLBCL, some recent studies have provided evidence in support of the use of RT in conjunction with CT in early stage HN-DLBCL. Kwak et al. conducted a retrospective cohort study of 56 patients to examine relapse-free survival, OS, and local control rate, finding excellent local control and survival rates with R-CHOP followed by RT [Citation32]. A limitation of this study is that all patients received CT followed by RT, so there was no treatment comparison. Lee et al. conducted a retrospective cohort study of 35 patients and found that RT was associated with improved OS and event free survival in early stage DLBCL of the Waldeyer ring, the most common extranodal site of HN-DLBCL [Citation33,Citation34]. Treatment toxicity of RT was studied and found to be acceptable [Citation33]. Of note, both studies had relatively small sample sizes, so definitive conclusions could not be drawn.
In contrast with previous literature, our sample size of 1,929 allowed us to perform subgroup analysis. We performed subgroup analysis in patients who started RT within 180 days of the CT start date because R-CHOP often takes approximately 12–18 weeks. This allowed us to absolutely capture patients who received RT for consolidation purposes rather than salvage since we did not have data on response to systemic chemotherapy. The OS benefit for the addition of RT to multi-agent CT was more prominent in patients older than 60 than those younger than 60. This may be related to the potential greater response of younger patients to salvage therapy [Citation35]. In contrast to Mian et al., even with our larger sample size, we failed to see any significant differences in OS when comparing the anatomic locations of Waldeyer’s ring, parotid and salivary glands, palate and oral cavity, and the pharynx [Citation11].
We acknowledge several limitations of our study. First, we acknowledge the limitations of the data set. The NCDB is based on a population of patients with cancer who seek care at a facility accredited by the CoC. Therefore, approximately 30% of the US cancer population was not included in the NCDB. Although CoC accreditation is widely perceived to be a quality metric, as of 2016, several prominent and high-volume hospitals were not accredited by the CoC [Citation36]. More specifically, we note several ways in which the dataset may vary from the national population, potentially affecting interpretation of the data. First, demographically, compared with the data reported in the United States Cancer Statistics (USCS), only 50% of cancers in people of Hispanic ethnicity are captured compared with 65% in patients who are white, black, or Asian. Second, the NCDB captures a greater percentage of younger patients (73% of those <65years) compared with older patients (63% of patients ≥ 65 years) [Citation37]. Finally, we excluded patients with unknown treatment status and unrecorded stage, even though they made up nearly 15% of our final cohort. We found significant differences in insurance status and B symptoms between the inclusion and exclusion groups, both factors with the potential to influence results. Nevertheless, most registry studies have limitations with regard to representativeness. The NCDB represents a diverse group of CoC accredited hospitals from a high percentage of states representing various stages of economic development. Therefore, we are confident that the NCDB PUF offers a unique and important perspective on cancer care in the United States.
Second, in a similar vein regarding limitations of the database, key information on treatment regimens and completion is unavailable through the database that would have allowed for further analysis and nuance. For example, our analysis of patients who received immunotherapy did not show multi-agent CT plus RT to be superior to multi-agent CT alone, which can be due to the relatively small number of patients with immunotherapy data in the database versus reduced effect of RT in patients who also received immunotherapy. Further study is needed to distinguish the two possibilities. Finally, the NCDB does not capture relapse after treatment. Thus, remission after CT cannot be assessed. However, with the vast majority of our patients receiving RT within 180 days of the CT start date (95.2%), RT was likely used for consolidation in this setting.
Third, even though propensity score matching was done to account for known confounders, allocation into groups wasn’t random, and other confounders may exist.
In conclusion, the results of our study suggest improved OS associated with multi-agent CT plus RT in early stage HN-DLBCL with extranodal involvement when compared to multi-agent CT alone. This survival advantage was more prominent in patients older than 60 and persisted in subgroup analysis of patients who started RT within 180 days of the CT start time.
Supplemental Material
Download (633.9 KB)Acknowledgements
This work was supported by the Natural Science Foundation of Hunan province to LY(2018JJ2257).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Swerdlow SH, Campo E, Harris NL, et al. World health organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008.
- Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011;117(11):2530–2540. doi: 10.1002/cncr.25765
- Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005;9(6):340–350. doi: 10.1016/j.anndiagpath.2005.09.020
- Sanchez LA, Redondo AM, Munez OB, et al. Extranodal and nodal diffuse large B cell lymphoma of the head and neck: two different entities? Ann Hematol. 2015;94(4):609–616. doi: 10.1007/s00277-014-2256-0
- Miyazaki K. Treatment of diffuse large B-cell lymphoma. J Clin Exp Hematopathol. 2016;56(2):79–88. doi: 10.3960/jslrt.56.79
- International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. New Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402
- Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin’s lymphomas. Cancer Treat Rep. 1977;61(6):1023–1027.
- Vargo JA, Gill BS, Balasubramani GK, et al. Treatment selection and survival outcomes in early-stage diffuse large B-cell lymphoma: do we still need consolidative radiotherapy? J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(32):3710–3717. doi: 10.1200/JCO.2015.61.7654
- Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):11–31. doi: 10.1016/j.ijrobp.2015.01.009
- Ng AK, Dabaja BS, Hoppe RT, et al. Re-examining the role of radiation therapy for diffuse large B-cell lymphoma in the modern Era. J Clini Oncol: Off J Am Soc Clini Oncol. 2016;34(13):1443–1447. doi: 10.1200/JCO.2015.64.9418
- Mian M, Capello D, Ventre MB, et al. Early-stage diffuse large B cell lymphoma of the head and neck: clinico-biological characterization and 18 year follow-up of 488 patients (IELSG 23 study). Ann Hematol. 2014;93(2):221–231. doi: 10.1007/s00277-013-1856-4
- American College of Surgeons. National Cancer Database. [cited 2017 May 20] Available from: https://www.facs.org/quality-programs/cancer/ncdb.
- Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clini Oncol: Off J Am Soc Clin Oncol. 2013;31(23):2963–2969. doi: 10.1200/JCO.2013.49.5283
- Rusthoven CG, Jones BL, Flaig TW, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(24):2835–2842. doi: 10.1200/JCO.2016.67.4788
- Sehn LH. Chemotherapy alone for localized diffuse large B-cell lymphoma. Cancer J (Sudbury, Mass). 2012;18(5):421–426. doi: 10.1097/PPO.0b013e31826c5907
- Longo DL. Combined-modality therapy for early-stage diffuse large B-cell lymphoma: knowing when to quit. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(32):3684–3685. doi: 10.1200/JCO.2015.63.0285
- Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. New Engl J Med. 1998;339(1):21–26. doi: 10.1056/NEJM199807023390104
- Reyes F, Lepage E, Ganem G, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. New Engl J Med. 2005;352(12):1197–1205. doi: 10.1056/NEJMoa042040
- Miller TP, Leblanc M, Spier C, et al. CHOP alone compared to CHOP plus radiotherapy for early aggressive non-Hodgkin’s lymphoma: update of the Southwest oncology group (SWOG) randomized trial. Blood. 2001;98(11):724–725.
- Haque W, Dabaja B, Tann A, et al. Changes in treatment patterns and impact of radiotherapy for early stage diffuse large B cell lymphoma after Rituximab: a population-based analysis. Radiother Oncol: J Eur Soc Ther Radiol Oncol. 2016;120(1):150–155. doi: 10.1016/j.radonc.2016.05.027
- Ballonoff A, Rusthoven KE, Schwer A, et al. Outcomes and effect of radiotherapy in patients with stage I or II diffuse large B-cell lymphoma: a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2008;72(5):1465–1471. doi: 10.1016/j.ijrobp.2008.02.068
- Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(27):4170–4176. doi: 10.1200/JCO.2009.27.3441
- Marcheselli L, Marcheselli R, Bari A, et al. Radiation therapy improves treatment outcome in patients with diffuse large B-cell lymphoma. Leukemia Lymphoma. 2011;52(10):1867–1872. doi: 10.3109/10428194.2011.585526
- Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(32):4115–4122. doi: 10.1200/JCO.2012.48.0467
- Shi Z, Das S, Okwan-Duodu D, et al. Patterns of failure in advanced stage diffuse large B-cell lymphoma patients after complete response to R-CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):569–577. doi: 10.1016/j.ijrobp.2013.02.007
- Held G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(11):1112–1118. doi: 10.1200/JCO.2013.51.4505
- Dabaja BS, Vanderplas AM, Crosby-Thompson AL, et al. Radiation for diffuse large B-cell lymphoma in the rituximab era: analysis of the national comprehensive cancer network lymphoma outcomes project. Cancer. 2015;121(7):1032–1039. doi: 10.1002/cncr.29113
- Kwon J, Kim IH, Kim BH, et al. Additional survival benefit of involved-lesion radiation therapy after R-CHOP chemotherapy in limited stage diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2015;92(1):91–98. doi: 10.1016/j.ijrobp.2014.12.042
- Parikh RR, Yahalom J. Older patients with early-stage diffuse large B-cell lymphoma: the role of consolidation radiotherapy after chemoimmunotherapy. Leukemia Lymphoma. 2017;58(3):614–622. doi: 10.1080/10428194.2016.1205739
- Hu CD C, Zou W, Zhang G, et al. The role of consolidative radiotherapy after a complete response to chemotherapy in the treatment of diffuse large B-cell lymphoma in the Rituximab era: results from a systematic review with a meta-analysis. Acta Haematol. 2015;134:111–118. doi: 10.1159/000370096
- Murawski N, Held G, Ziepert M, et al. The role of radiotherapy and intrathecal CNS prophylaxis in extralymphatic craniofacial aggressive B-cell lymphomas. Blood. 2014;124(5):720–728. doi: 10.1182/blood-2013-10-535021
- Lee SF, Ng TY, Wong FCS, et al. The role of radiotherapy in early-stage primary diffuse large B-cell lymphoma of the Waldeyer ring: a retrospective cohort study. Am J Clin Oncol. 2018;41(8):802–806. doi: 10.1097/COC.0000000000000375
- Niemiec M, Stryjewska-Makuch G, Janik M, et al. Head and neck lymphomas – a retrospective ten-year observation. Contemp Oncol (Poznan, Poland). 2017;21(1):66–69.
- Kwak YK, Choi BO, Kim SH, et al. Treatment outcome of diffuse large B-cell lymphoma involving the head and neck: two-institutional study for the significance of radiotherapy after R-CHOP chemotherapy. Medicine. 2017;96(25):e7268. doi: 10.1097/MD.0000000000007268
- Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. (0923-7534 (Print)). Ann Oncology. 1 July 2002;13(7):1099–1107. doi: 10.1093/annonc/mdf175
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905
- Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. doi: 10.1245/s10434-013-2901-1