ABSTRACT
Background
Immunosuppressive therapy (IST) composed of antithymocyte globulin (ATG) and cyclosporine A (CSA) is one of the standard therapies in pediatric patients with acquired aplastic anemia (AA), but predictors of IST are lack of consensus.
Procedures
Ninety-four patients from two pediatric medical centers in China were included between January 2005 and March 2018. Clinical factors associated with the efficacy were analyzed according to multivariate logistic regression model previously established.
Results
We discovered that overall responsiveness was 77.66%. Five out of 35 factors were statistically significant in univariate analysis. Based on the cutoff point chosen by receiver operating characteristic (ROC) curve, 5 continuous variables were made categorical, among which 3 variables with significance were employed to establish the logistic regression equation. Based on these 3 variables, we found that starting IST within 126 days of the first appearance of symptoms (X1, p = .003), absolute neutrophil count (ANC) higher than 0.435×109/L (X2, p = .012), and rate of decreased actual lymphocyte count (ALC) higher than 59.2% within the 1st week after IST (X3, p = .001) were three independent risk factors for response to IST. The rate of decreased ALC higher than 59.2% after IST was the most significant variable (OR = 9.355, Log (P) = −2.161 + 2.149X1 + 1.662X2 + 2.236X3). The accuracy, sensitivity, and specificity of the model were 86.2%, 94.5% and 57.1%, respectively.
Conclusion
Duration of AA, ANC and decreased ALC rate after IST might predict the response to IST, among which the rate of decreased ALC after IST is the most important predictive factor.
Introduction
Aplastic anemia (AA) is characterized by pancytopenia in peripheral blood and bone marrow failure. The annual morbidity of AA was reported to be 2–6 per million population in North America and Europe. In Asia, including China, the value is tripled or even quadrupled [Citation1,Citation2]. According to the International Diagnosis Criteria for Aplastic Anemia, the disease can be divided into three types: nonsevere AA (NSAA), severe AA (SAA) and very severe AA (VSAA) [Citation3]. Hematopoietic stem cell transplantation (HSCT) is considered as a standard therapy for SAA/VSAA patients with suitably matched sibling donors (MSD) [Citation3]. But for patients without histocompatible sibling donors or transfusion-dependent NSAA patients, immunosuppressive therapy (IST) composed of antithymocyte globulin (ATG) and cyclosporine (CSA) is a preferred treatment [Citation3–7] because of its equal efficacy to HSCT [Citation8–11] and low toxicity.
Several factors have been proposed to predict the responsiveness to IST in adult patients with AA, include younger age [Citation12,Citation13], a higher absolute neutrophil count (ANC) [Citation14], a higher absolute reticulocyte count (ARC), a higher actual lymphocyte count (ALC) [Citation15], a higher baseline platelet count [Citation16], a shorter interval between diagnosis and IST [Citation13,Citation17], and higher CSA levels during the 1st-2nd week of IST [Citation18]. However, less data are available from pediatric patients with AA in terms of their responsiveness and risk factors to IST.
Here, we study retrospectively the major factors that determine the response to IST for childhood AA by establishing a logistic regression model as well as evaluating the sensitivity, specificity and accuracy of the risk model. We aim to provide data that can improve the efficacy of IST while selecting alternative therapy such as unrelated matched HSCT.
Materials and methods
Patients
In this retrospective study, 94 pediatric patients with AA received IST composed of ATG and CSA between January 2005 and March 2018, including 46 male and 48 female cases, were recruited. The median age was 7 year (range, 3–14 yr). Sixty cases of SAA, 19 cases of VSAA, and 15 cases of transfusion-dependent NSAA were included. The data were derived from two pediatric medical centers in China - 65 cases from Tongji Hospital of Tongji University and 29 cases from Children's Hospital of Fudan University. All patients were diagnosed by following the International Diagnosis Criteria for Aplastic Anemia [Citation3,Citation4,Citation19], including clinical manifestations, peripheral blood cell counts, and bone marrow hyperplasia, while excluding other hematopoietic diseases manifesting as pancytopenia.
Peripheral blood cell counts met at least two of the three following criteria: (1) hemoglobin (HB) <100 g/L; (2) platelet <50 × 109/L; (3) ANC <1.5 × 109/L. The severity of AA was classified in accordance with Camitta’s criteria [Citation4]. SAA was defined by meeting at least two of the three following: (1) percentage of reticulocyte (Ret) <1% or ARC <20 × 109/L; (2) ANC <0.5 × 109/L; (3) platelet <20 × 109/L. VSAA was considered if cases met the diagnosis criteria of SAA while ANC <0.2 × 109/L. Patients with inherited bone marrow failure (IBMF), such as the Fanconi anemia (FA) and congenital dyskeratosis (DC) were excluded.
Peripheral blood cell counts, immune indexes, and ferritin were measured before IST. After IST, CSA concentration was measured once a week for the first month and once a month until the last section of treatment, whereas ALC was monitored from day 1 to day 28 after IST.
We collected data on the following parameters: demographics (age and gender), pretherapy factors (duration of AA, diagnostic category, peripheral blood cell counts, transfusion, and laboratory indicators), IST-related characteristics (type of ATG, ATG-induced adverse reactions, CSA level and decreased ALC after IST), and the clinical outcomes.
Treatment
Totally 94 patients received a combination of ATG and CSA for IST. Two different types of ATG were commercially available in China: Jurkat cell-reactive anti-T lymphocyte globulin (ATG-F, Germany, Fresenius Company) and rabbit antithymocyte globulin (R-ATG, the United States, Genzyme Corporation). When both of the drugs were available, we randomly selected the type of ATG. Thirty-one and 63 patients received ATG-F and R-ATG, respectively ().
Table 1. Characteristics of patients.
Five milligram ATG-F or 2.5 mg R-ATG was intravenously infused as an allergenic test before the initiation of ATG treatment. If the allergenic test was negative, then ATG-F (5 mg/kg/d) or R-ATG (3.75 mg/kg/d) was given by slow intravenous infusion for 1–5 consecutive days. CSA was administered orally at a dose of 5 mg/kg/d and continued for at least 6 months. According to the recommended range of pediatric AA from the guidelines [Citation3,Citation19], CSA level was maintained at 150–200 ng/mL.
Methylprednisolone (30 mg/m2/day) was given on the first week after IST to prevent allergic reactions, after that the dose was reduced half every 3 days until 1 mg/kg/day. The total duration of glucocorticoid was 3–4 weeks. Red blood cell (RBC) transfusion was performed when patients could not tolerate the symptom of anemia or HB <60 g/dL. Platelets transfusion was administered to maintain peripheral platelet count >20 × 109/L before and during ATG treatment. During the period of follow-up, platelet transfusion was administered in all patients with a platelet count <10 × 109/L or obvious hemorrhagic tendency. Antibiotics and granulocyte colony-stimulating factor (G-CSF) was given to patients with infections or severe neutropenia.
Determination of responsiveness
We determined the efficacy of IST according to Camitta’s criteria [Citation3, Citation4]. Complete response (CR) was defined as HB normal for age, ANC >1.5 × 109/L, and platelet count >150 × 109/L. Partial response (PR) for SAA was defined as transfusion independence associated with no longer meeting criteria for severe disease. For NSAA patients, PR should meet one of the following five criteria: (1) Transfusion independence; (2) doubling or normalization of at least one cell line; (3) increase of baseline HB >30 g/L; (4) increase of baseline ANC >0.5 × 109/L; (5) increase of baseline platelets of >20 × 109/L. Still severe or worse was considered no response (NR). Both CR and PR were considered as a positive response.
Follow-up
All the patients were evaluated including peripheral blood cell count, transfusion dependence/independence, transfusion frequency once a month in the first 6 months after IST and once 3–6 months during the rest of follow-up period. CD55 and CD59 were monitored and bone marrow aspiration was performed every 6 months to observe the development of late clonal complications post-IST. The follow-up period expired in August 2018.
Statistical analyses
The overall response included CR and PR. Overall survival (OS) was the length of time from IST to death or the end of follow-up. Event-free survival (EFS) was defined as the time from IST to the first event occur or the end of follow-up. Events included nonresponse, relapse, clonal evolution, death and performing HCST. The values of quantitative variables, if in normal distribution, were presented as ‘mean ± standard error’ and two independent sample t-test was used to determine the statistical significance. The values of variables in skew distribution were presented as ‘median and range’ and Mann–Whitney U-test was used to determine the statistical significance. Differences in the distribution of categorical variables, including gender, type of ATG, diagnostic category, and ATG-induced adverse reactions, were tested with the chi-square test.
To identify whether the cutoff points stratify the patients according to the outcome of IST, we adopted the receiver operating characteristic (ROC) method. Based on the cutoff view, all the continuous variables were made categorical and compared by logistic regression analysis. The efficacy of IST was considered as dependent variable using non-response cases as a reference group. Significant factors from the univariate analysis were conducted by logistic regression analysis using a backward conditional procedure for multivariate analysis to establish the logistic regression model. Then, training samples were used to test the logistic prediction equation.
All statistical analyses were carried out by SPSS statistical software (SPSS, version 17.0, Chicago, IL). P-values <0.05 indicated statistical significance.
Results
Characteristics of patients
Totally 73 out of 94 (77.66%) patients responded to IST, among which 49 (52.13%) patients achieved CR and 24 (25.53%) achieved PR. The median duration from IST to cellular recovery was 180 (85–295) days. The response rates at 90 days, 180 days, and 365 days after IST were 8.51%, 46.81%, and 77.66%, respectively. The remaining 21 (22.34%) cases failed to respond to IST (NR). Before IST, the median of hematological test results were HB: 71.5 (31–107) g/L, RBC: 2.39 (1.4–3.29) × 1012/L, ARC: 9.36 (0–48.64) × 109/L, white blood cell (WBC) count: 3.06 (0.4–12.66) × 109/L, ANC: 0.6 (0–2.7) × 109/L, ALC: 2.1 (0.15–10.8) × 109/L and platelet count: 31.5 (2–67) × 109/L (). There was no death during ATG treatment. Among 21 non-responders, 4 of them received unrelated donor HSCT (MUD-HSCT) and achieved hematopoietic reconstitution with normal peripheral blood counts, 10 patients died because of infections and 6 of hemorrhage, the rest one patient survived with periodic transfusion. The OS rate was 82.98%. With a median follow-up duration of 104 months, 2 patients relapsed in the second year after IST. No case developed clonal evolution. The EFS rate was 75.53%. ().
Comparison between the response group and NR group
Seventy-three patients in the response group and 21 in NR group were compared by the following clinical factors: age, gender, type of ATG, duration of AA, transfusion before and after IST, peripheral blood index and other laboratory examinations before IST, mean CSA level at first to second week after IST, peripheral ALC changes in first week after IST, and ATG-induced adverse reactions.
As shown in , interval from symptoms occur to IST (p = .000), peripheral ANC (p = .012), CD4/CD8 (p = .019) before IST, mean CSA level at 1–2 week (p = .004), and rate of decreased ALC in 1st week (p = .001) after IST were potential factors associated with the overall response rate. Furthermore, shorter interval from symptoms occur to IST, higher ANC, lower CD4/CD8, higher CSA level and higher rate of decreased ALC were positive. Other factors between the two groups were no significant difference.
Table 2. Comparison of clinical factors between response and NR groups.
ROC analysis
Through ROC curve analysis, the optimum cutoff of five predictors were ANC > 0.435 × 109/L (sensitivity = 73.97%, specificity = 61.90%) (), interval from symptoms occur to IST < 126 d (sensitivity = 80.85%, specificity = 53.29%), CD4/CD8 < 0.408 (sensitivity = 100%, specificity = 31.51%), mean CSA level at 1–2 week >207.6 ng/mL (sensitivity = 43.84%, specificity = 95.24%), and rate of decreased ALC in 1st week > 59.12% (sensitivity = 90.41%, specificity = 57.14%). The area under ROC curve was showed in .
Figure 1. Receiver operating characteristic curve analysis for absolute neutrophil count correlated with response to immunosuppressive therapy. AUC, area under the curve.
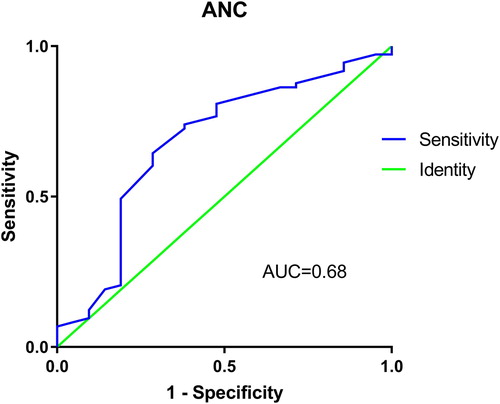
Table 3. Receiver operating characteristic curve analysis, optimum cutoff value and predict performance for factors affecting overall response to IST.
Univariate logistic regression analysis
A total of 94 training samples were analyzed using univariate logistic regression analysis. The outcome of IST was considered as dependent variable (response = 1, non-response = 0). The following variables were entered in logistic regression analysis: interval from symptoms occur to IST < 106d, ANC > 0.435 × 109/L, CD4/CD8 < 0.408, mean CSA level at 1–2 week after IST > 207.6 ng/mL, and rate of decreased ALC in 1st week after IST > 59.2%. Mean CSA level at 1–2 week after IST > 207.6 ng/mL was excluded (p = .058). Other factors had p-value <.05 therefore remained in the analysis ().
Table 4. Factors affecting overall response to IST: univariate analysis.
Multivariate logistic regression analysis
The 4 variables that were significant in the univariate analyses, interval from symptoms occur to IST < 126 d (YES = 1, NO = 0), ANC > 0.435×109/L (YES = 1, NO = 0), CD4/CD8 < 0.408 (YES = 1, NO = 0), and rate of decreased ALC in 1st week after IST >59.12% (YES = 1, NO = 0), were chosen as independent variables included in the multivariate logistic regression analysis.
As a result, three variables were analyzed by logistic regression equation, among which the maximum contribution was the rate of decreased ALC in 1st week after IST. The probability of response to IST was significantly higher in patients who received IST timely [interval from symptoms occur to IST < 126 d (X1), OR = 8.579, p = .003], ANC > 0.435 × 109/L (X2, OR = 5.271, p = .012), and achieve high rate of decreased ALC >59.2% (X3, OR = 9.355, p = .001) (). The logistic regression equation was then established as logit (p) = −2.161 + 2.149X1 + 1.662X2 + 2.236X3.
Table 5. Factors affecting overall response to IST: multivariate logistic regression analysis (Backward, Wald, α = 0.05).
Evaluation of predictive model
Training samples were tested in the predictive model, which showed that the accuracy, sensitivity, and specificity were 86.2%, 94.5%, and 57.1%, respectively.
Discussion
Both HSCT and IST are standard therapies for treating adult and pediatric patients with AA, whereas MSD-HSCT is considered to be the first-line treatment for pediatric patients, because its long-term survival rate was reported to be over 90% [Citation20]. When MSD-HSCT is unavailable, IST is recommended as the second choice [Citation3,Citation5–7,Citation20,Citation21]. For patients who failed IST, matched unrelated donor HSCT (MUD-HSCT) is the only choice left. MUD-HSCT is historically considered with high incidence of graft failure, high incidence of graft-versus-host disease (GVHD), and low response rate [Citation1,Citation22], but outcomes have improved because of advances in the supportive care, the selection of high-resolution human leukocyte antigen (HLA) matched donors and modification of the conditioning regimen [Citation23,Citation24]. Some scholars recommend MUD-HSCT should be considered alongside IST when a matched donor is available because of the outcome of upfront MUD-HSCT shows similar even superior to IST in their studies [Citation25,Citation26]. In China, MSD-HSCT is not accessible to the majority of patients with AA as the result of ‘one child policy’. Moreover, MUD-HSCT is hard to proceed because of the difficulty in finding a suitable unrelated donor and the time of arranging a donation. Thus, IST might be the only treatment can be executed in the short term after diagnosed with SAA. Therefore it is essential to develop an effective predictor-specific estimation tool for patients treated with IST. Base on the clinical data from two pediatric medical centers, we used the multivariate logistic regression model to identify the determining factors of the responsiveness of IST.
The overall response rate of IST in this retrospective study was 77.66%, which was similar to previous studies [Citation8–12], indicating that IST is an effective therapy for treating patients with AA. Interval form symptoms occur to IST (p = .000), ANC (p = .012), value of CD4/CD8 (p = .019), mean CSA level at 1–2 week after IST (p = .004), and rate of decreased ALC (p = .001) were associated with the high response rate. Predictive factors that were confirmed in other studies include younger age, higher ARC, higher ALC, higher platelet counts, and shorter interval between diagnosis and initiation of IST. However, these factors showed no significance in our analysis. ATG-F and R-ATG were showed to be similar in pharmacokinetic parameters, rate of lymphocyte clearance, efficacy and adverse effects in the first-line treatment of pediatric patients with AA in our previous studies [Citation27,Citation28]. Similarly, in this double-center study, the data indicated that the type of ATG (ATG-F or R-ATG) had no relationship with the high response rate of IST. Other clinical factors, such as transfusion, CD index, ferritin, and ATG-induced adverse reaction, were not significantly different between response group and NR group.
Based on the cutoff value calculated by ROC curve analysis, we made the duration of AA, ANC, CD4/CD8, mean CSA level, and rate of decreased ALC into dichotomous variables. In univariate logistic regression analysis, the results showed that interval from symptoms occur to IST <126d, ANC > 0.435 × 109/L, CD4/CD8 < 0.408 and rate of decreased ALC after IST >59.12% were predictive factors of responsiveness to IST. In the study of Moo-Kon Song et al. [Citation18], mean CSA levels in 1–2 week after IST >200 ng/mL was associated with higher overall response rate. However, our univariate logistic regression analysis showed that mean CSA level during first to second week of IST more than 207.6 ng/mL was not a positive factor. The contradictory results might reflect the difference between pediatric and adult patients with AA. Thus, it is essential to carry out further studies with large cohorts.
In the multivariate logistic regression analysis, three out of four independent predictive factors that achieved significance therefore entered logistic regression equation. Higher rate of decreased ALC (>59.12%), higher ANC (>0.435 × 109/L), and shorter interval from symptoms occur to IST (<126 d) were positive factors associated with overall response rate. The maximum contributor was the rate of decreased ALC in the first week during IST, followed by interval from symptoms occur to IST and ANC.
Controversially, baseline hematological parameters could serve as simple predictors of response of IST. Sheinberg and colleagues found that ARC and ALC were associated with high response to IST [Citation15], while ANC and platelet were found to be predictive of response rate in other studies [Citation16,Citation29]. Our study demonstrated that responders to IST had higher ANC than non-responders, yet ARC and ANC did not show statistical significance. Age, ethnicity, ATG formulations, and dosage of Thymoglobulin might underlie the difference between our study and the others. A recent randomized clinical study showed that addition of growth factor to IST led to faster and higher ANC recovery and reduced infection rate, but had no effect on overall response [Citation8]. This study suggested that high baseline ANC (especially over 0.435 × 109/L) was a strong predictor of response. Thus, further studies should include the outcome of adding growth factor before IST.
Duration from diagnosis to IST also affected the clinical outcome. A clinical multicenter study reported by Bacigalupo et al. showed that the interval between diagnosis and treatment was the major predictor of response, indicating patients treated within 1 month after diagnosis might achieve the best response [Citation13]. Median duration from diagnosis in our cohort was 25 and 35 days in the response group and NR group, respectively. It appeared that responders received treatment more timely than non-responders but the result was not statistically significant. However, our data demonstrated that shorter interval from symptoms occur to IST was predictive of response rate, and the optimum cutoff was 126 days. Pediatric patients with AA often have atypical clinical manifestations due to their stronger bone marrow hematopoiesis potential than adults. Before typical pancytopenia occurs, they might only have reduction in one or two lineages of peripheral blood cells, which leads to difficulties in AA diagnosis. The results in this study revealed that the impact of the duration from diagnosis to IST might be marginal, while the duration between symptoms occur with IST was significant. Therefore, it highlighted the importance of early diagnosis and treatment in AA patients.
Although the pathogenesis of AA is still unclear, it is widely accepted that the main cause of AA is the immunological attack of bone marrow hematopoietic cells by dysfunctional T-lymphocytes [Citation20,Citation22,Citation30,Citation31]. ATG can ablate abnormal T lymphocytes via specific antibody-mediated cytotoxicity and reverse the ‘immune-mediated’ pathogenic mechanisms in aplastic anemia [Citation27]. Immunosuppression is mediated by the consumption and modulation of lymphocytes. This provides a reasonable hypothesis that ALC during or after IST might be a clinical factor related to the efficacy of ATG. In this study, ALC obviously decreased on day 2 and achieved the lowest count in the first week after IST, which was similar to our previous studies [Citation27,Citation28]. The result of the statistical analysis showed that the decreased rate of peripheral lymphocyte count after IST was a major predictive factor of overall response rate. Patients achieved ALC decreased rate more than 59.12% have 9.355 times higher possibilities of response to IST than patients who did not achieve it. Since cellar recovery post-ATG costs at least 3–4 months, it takes several months to evaluate the efficacy of IST. In our study, the range of duration from IST to cellar recovery was 85–295 days and 29 out of 73 responders showed positive results after 6 months of treatment, which indicated the precise time of response is uncertain. Thus, it is difficult to determine the exact time point to evaluate the responsiveness of IST. Although the decreased rate of ALC after IST is a post-treatment factor, it might shorten the observation time of IST responsiveness from several months to one week. If ALC does not achieve significantly reduce during 1st week after IST, a rescue treatment should rapidly be considered. We recommend that an urgent unrelated donor search should be made for HSCT if patients failed to achieve ALC decreased rate more than 59.12% after IST.
Moreover, we found that logistic regression was a multivariate analysis method appropriate for studying the relationship between observation dichotomies and influencing factors, which could be applied to the evaluation of IST in patients with AA. The accuracy and sensitivity of the logistic regression equation established in our study was 86.2% and 94.5%, respectively. The predictive model could provide evidence for forecasting the rate of response to IST and improving the efficacy of treatment in AA.
Our study is however limited in several aspects, including a relatively small number of cohort and the retrospective nature of the study. We propose to test the model developed in our study in more multi-center, large-scale, randomized studies with proper controls.
In conclusion, our study showed that the duration of AA, ANC, and decreased ALC rate after IST were predictors of response to IST in pediatric patients with AA, and the rate of decreased ALC was the major predictive factor.
Acknowledgements
The authors would like to thank Dr. Qian Zhang for the assistance and suggestion of this article. We also thank all of the patients, families, nursing and medical staff for their collaboration.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Changjuan Gu http://orcid.org/0000-0002-3173-8169
References
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777
- Jeong DC, Chung NG, Kang HJ, et al. Epidemiology and clinical long-term outcome of childhood aplastic anemia in Korea for 15 years: retrospective study of the Korean Society of Pediatric Hematology Oncology (KSPHO). J Pediatr Hematol Oncol. 2011;33:172–178. doi: 10.1097/MPH.0b013e31820826a8
- Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x
- Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–363.
- Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428–1436. doi: 10.1182/blood-2016-08-693481
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. doi: 10.1111/bjh.13853
- Barone A, Lucarelli A, Onofrillo D, et al. Diagnosis and management of acquired aplastic anemia in childhood. Guidelines from the Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP). Blood Cells Mol Dis. 2015;55:40–47. doi: 10.1016/j.bcmd.2015.03.007
- Tichelli A, Schrezenmeier H, Socie G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA working party of the European group for blood and marrow transplantation. Blood. 2011;117:4434–4441. doi: 10.1182/blood-2010-08-304071
- Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99:1784–1791. doi: 10.3324/haematol.2014.109355
- Nair V, Sondhi V, Sharma A, Das S, Sharma S. Survival after immunosuppressive therapy in children with aplastic anemia. Indian Pediatr. 2012;49:371–376. doi: 10.1007/s13312-012-0086-5
- Bacigalupo A. Aplastic anemia: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2007;2007(1):23–28. doi: 10.1182/asheducation-2007.1.23
- Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075
- Bacigalupo A, Oneto R, Schrezenmeier H, et al. First line treatment of aplastic anemia with thymoglobuline in Europe and Asia: outcome of 955 patients treated 2001-2012. Am J Hematol. 2018;93:643–648. doi: 10.1002/ajh.25081
- Chang MH, Kim KH, Kim HS, et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol. 2010;84:154–159. doi: 10.1111/j.1600-0609.2009.01378.x
- Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144:206–216. doi: 10.1111/j.1365-2141.2008.07450.x
- Boddu P, Garcia-Manero G, Ravandi F, et al. Clinical outcomes in adult patients with aplastic anemia: A single institution experience. Am J Hematol. 2017;92:1295–1302. doi: 10.1002/ajh.24897
- Peffault DLR, Tabrizi R, Marcais A, et al. Nationwide survey on the use of horse antithymocyte globulins (ATGAM) in patients with acquired aplastic anemia: A report on behalf of the French Reference Center for Aplastic Anemia. Am J Hematol. 2018;93:635–642. doi: 10.1002/ajh.25050
- Song MK, Chung JS, Joo YD, et al. Is the early cyclosporine A level predictive of the outcome of immunosuppressive therapy in severe aplastic anemia? Eur J Haematol. 2009;83:72–78. doi: 10.1111/j.1600-0609.2009.01237.x
- Marsh JC, Ball SE, Darbyshire P, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123:782–801. doi: 10.1046/j.1365-2141.2003.04721.x
- Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transpl. 2010;16:S119–S125. doi: 10.1016/j.bbmt.2009.09.013
- Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. BLOOD. 2000;96:2049–2054.
- Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162-168. doi: 10.1097/MOH.0b013e3282fa7470
- Marsh JC, Gupta V, Lim Z, et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft-versus-host disease after allogeneic stem cell transplantation for acquired aplastic anemia. BLOOD. 2011;118:2351–2357. doi: 10.1182/blood-2010-12-327536
- Marsh JC, Pearce RM, Koh MB, et al. Retrospective study of alemtuzumab vs ATG-based conditioning without irradiation for unrelated and matched sibling donor transplants in acquired severe aplastic anemia: a study from the British Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:42–48. doi: 10.1038/bmt.2013.115
- Choi YB, Yi ES, Lee JW, et al. Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched familial donor. Bone Marrow Transplant. 2017;52:47–52. doi: 10.1038/bmt.2016.223
- Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric diseases Working Party and severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171:585–594. doi: 10.1111/bjh.13614
- Xie X, Zhao H, Qin D, Qiao X. Pharmacokinetics and pharmacodynamics of two antithymocyte globulins in treatment of pediatric aplastic anemia. Int J Clin Exp Med. 2015;8:4349–4355.
- Xie X, Shi W, Zhou X, Shao Y, Qiao X. Comparison of rabbit antithymocyte globulin and Jurkat cell-reactive anti-T lymphocyte globulin as a first-line treatment for children with aplastic anemia. Exp Hematol. 2014;42:431–438. doi: 10.1016/j.exphem.2014.02.003
- Jalaeikhoo H, Khajeh-Mehrizi A. Immunosuppressive therapy in patients with aplastic anemia: a single-center retrospective study. Plos One. 2015;10:e126925. doi: 10.1371/journal.pone.0126925
- Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4 + CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146
- Song EY, Kang HJ, Shin HY, et al. Association of human leukocyte antigen class II alleles with response to immunosuppressive therapy in Korean aplastic anemia patients. Hum Immunol. 2010;71:88–92. doi: 10.1016/j.humimm.2009.10.002
