ABSTRACT
Objectives: Detectable minimal residual disease (MRD) after therapy for acute lymphoblastic leukemia (ALL) is the strongest predictor of hematologic relapse. This study evaluated outcomes of patients with B-cell precursor ALL with MRD of ≥10−4
Methods: Study population was from ALL study groups in Europe managed in national study protocols 2000–2014. MRD was measured by polymerase chain reaction or flow cytometry. Patients were age ≥15 years at initial ALL diagnosis. Patients were excluded if exposed to blinatumomab within 18 months of baseline or prior alloHSCT.
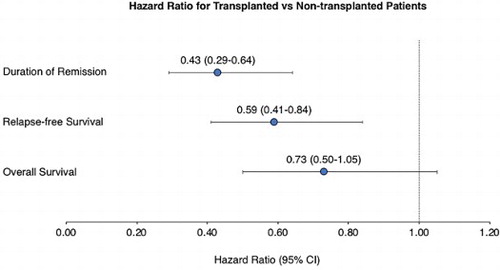
Results: Of 272 patients in CR1, baseline MRD was ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3 in 15 (6%), 71 (26%), 109 (40%), and 77 (28%) patients, respectively. Median duration of complete remission (DoR) was 18.5 months (95% confidence interval [CI], 11.9–27.2), median relapse-free survival (RFS) was 12.4 months (95% CI, 10.0–19.0) and median overall survival (OS) was 32.5 months (95% CI, 23.6–48.0). Lower baseline MRD level (P ≤ .0003) and white blood cell count <30,000/µL at diagnosis (P ≤ .0053) were strong predictors for better RFS and DoR. Allogeneic hematopoietic stem cell transplantation (alloHSCT) was associated with longer RFS (hazard ratio [HR], 0.59; 95% CI, 0.41–0.84) and DoR (HR, 0.43; 95% CI, 0.29–0.64); the association with OS was not significant (HR, 0.72; 95% CI, 0.50–1.05).
Discussion: In conclusion, RFS, DoR, and OS are relatively short in patients with MRD-positive ALL, particularly at higher MRD levels. AlloHSCT may improve survival but has limitations. Alternative approaches are needed to improve outcomes in MRD-positive ALL.
GRAPHICAL ABSTRACT
Introduction
Outcomes for adult patients with acute lymphoblastic leukemia (ALL) have improved. However, some patients remain resistant to standard chemotherapy. Despite hematologic complete remission (CR) rates of 80–90% with intensive induction/consolidation chemotherapy, approximately 30–50% of adult and 10–20% of pediatric patients at CR1 exhibit minimal residual disease (MRD) [Citation1–8]. MRD is defined as the presence of leukemic cells in patients with hematologic CR below 5%, which is the detection level of microscopy. Several methods for standardized and quantitative measurement of MRD at a sensitivity of 10−4 (i.e. 0.01%) are available, such as flow cytometry or real-time quantitative polymerase chain reaction (RQ-PCR) of individual clonal rearrangements of immunoglobulin [Citation9].
MRD indicates resistance to standard chemotherapy and is the most important risk factor for hematologic relapse in adult and pediatric ALL [Citation2,Citation4,Citation10,Citation11–14]. Standards for a description of MRD status have been described [Citation15]. Patients with high MRD levels after induction and consolidation (i.e. MRD persistence) have a higher risk of hematologic relapse (70–100%), whereas patients with complete MRD response are far less likely to have a hematologic relapse (2–8%) [Citation1,Citation3,Citation4–6,Citation10,Citation16]. Conversion from MRD negativity to MRD positivity (i.e. MRD reappearance) is highly predictive of subsequent hematologic relapse [Citation5,Citation7]. Time from MRD positivity to hematologic relapse is also correlated with MRD level [Citation5].
Most study groups recommend allogeneic hematopoietic stem cell transplant (alloHSCT) as the most intensive treatment approach available for the management of MRD [Citation17–19]. Patients who receive alloHSCT have better outcomes compared with those who do not [Citation20]. However, alloHSCT is not always an option due to associated toxicities, lack of suitable donors, or other reasons. Patients may relapse awaiting alloHSCT, particularly those with higher MRD levels. Additionally, patients with detectable MRD before alloHSCT may have a higher risk of relapse after alloHSCT [Citation5,Citation13,Citation21].
The objectives of this study were to estimate the duration of hematologic CR (DoR), hematologic relapse-free survival (RFS), and overall survival (OS) for patients with MRD-positive B-cell precursor (BCP) ALL treated with standard of care in Europe. In addition, several statistical methods were used to closely explore how alloHSCT status may influence clinical outcomes.
Methods
Study design
We analyzed a cohort of adults with ALL who had reached CR but had detectable MRD after the standard of care treatment according to national study protocols. The study population was from ALL study groups in Europe (Czech Republic, France, Germany, Italy, Poland, Russia, Spain and the United Kingdom) with protocols that included prospective MRD testing in national reference laboratories. Entry criteria, data collection methods, and analyses were defined in a joint protocol (clinicaltrials.gov identifier: NCT02010931). Ethical approval was obtained according to individual country requirements.
Patient-level data were obtained from clinical databases and entered by central staff of each study group into a study-specific electronic case report form to ensure a standardized and quality-controlled data collection process. Variables included information on demographics, disease characteristics, history of ALL treatment prior to the detection of MRD, diagnostics and level of MRD, details of transplantation for patients who underwent alloHSCT after their MRD detection, and clinical event data including occurrence of hematologic relapse, date of death, or date last known to be alive.
Patient eligibility
Patients were eligible if they had Ph/BCR-ABL–negative BCP ALL in hematologic CR (<5% blasts in bone marrow after ≥3 intensive chemotherapy blocks) and no extramedullary involvement. Other key eligibility criteria were: MRD of ≥10−4 by RQ-PCR of clonally rearranged immunoglobulin or ≥10−3 by flow cytometry at a reference lab; age ≥15 years at initial ALL diagnosis; initial ALL diagnosis in the years 2000–2014; availability of data for history of ALL treatment (including response to first therapy and number of prior relapses); and relapse status and disease follow-up after MRD detection. To focus on standard therapies, patients were excluded if exposed to blinatumomab within 18 months of MRD detection. Patients with MRD after prior alloHSCT were excluded.
Statistical analyses
Patients were included in all analyses regardless of whether they received a transplant after MRD detection. The primary outcome, RFS, was defined as the time from baseline MRD detection until hematologic relapse or death due to any cause; patients alive without relapse were censored at their last disease assessment. The secondary outcome, OS, was defined as the time from baseline MRD detection until death; patients without a recorded death were censored at last follow-up. The analysis of DoR was defined as the time from baseline MRD detection until relapse; patients who died in CR were censored at the date of death. RFS, OS, and DoR were summarized with Kaplan-Meier curves and proportions; point estimates were accompanied by 2-sided 95% confidence intervals (CI).
Baseline MRD was the first measurement of MRD-positivity after CR. Baseline MRD status was defined as MRD persistence (MRD positivity without prior documented negativity) or MRD reappearance (return to MRD positivity after achievement of complete MRD response) [Citation15]. In univariate Cox regression models, the potential association between RFS, DoR, and OS with each of the following factors was investigated: age at diagnosis (15–34, 35–54, 55–64, or ≥65 years); sex (female vs. male), MRD status (persistence vs. reappearance); MRD at baseline (≥10−1, 10−2 to <10−1, 10−3 to <10−2, or 10−4 to <10−3), and white blood cell (WBC) count at diagnosis (≥30,000/µL vs. <30,000/µL). Using forward selection, factors significant at P < .10 were included in a multivariable Cox regression model describing RFS, DoR or OS.
Several methods were employed to explore the effect of receiving alloHSCT after detection of MRD. (1) Conditional landmark analyses of alloHSCT defined the landmark at 3 months, which was approximately the median time to alloHSCT from baseline (3.4 months; interquartile range, 1.7–6.3); additionally, a landmark at 6 months was assessed which included most patients that underwent a transplant. For each analysis, patients with an event (DoR, RFS, OS) before the defined landmark were excluded, transplant status was assessed at the time of the landmark (3 months and 6 months), wherein a patient was considered to be transplanted only if the transplant was conducted on or before the landmark time. Any patients who underwent a transplant after the landmark were included in the nontransplant group. (2) Mantel–Byar analysis handles alloHSCT as a time-dependent covariate. A patient is attributed to the nontransplant group until the time of transplant, once transplanted, is considered part of the transplant group. No patients were excluded from this analysis as in the conditional landmark analysis. This method accounts for time bias observed in fixed-time analyses because patients undergoing alloHSCT must survive long enough to receive alloHSCT [Citation22]. The Simon–Makuch method (with and without a 3-month landmark) was used to graphically display results from the Mantel–Byar test [Citation23]. (3) A multivariate Cox proportional hazards model, adjusting for the baseline factors that were identified in the multivariate Cox model as described above, included alloHSCT as a time-dependent covariate. Statistical significance was measured at P < .05. No adjustment was considered for multiplicity.
Results
Patient population
Data were captured from 287 patients. Analyses of baseline characteristics included 272 patients with quantifiable baseline MRD and excluded 15 patients whose MRD was qualitatively reported to be ≥10−4 but had non-quantifiable MRD. Of the 272 patients, time-to-event analyses for DoR, RFS, or OS included 270 patients and excluded 2 patients with quantifiable baseline MRD missing the date for MRD.
Patient characteristics are shown in and Supplemental Table 1. The median age at diagnosis was 33 years (IQR, 24–45). Slightly more than half of the patients were male (58%) and aged <35 years (56%). MRD was assessed by RQ-PCR in most patients (79%) and the baseline MRD was ≥10−3 in 72%. Most patients (80%) had MRD persistence; 19% had MRD reappearance and 1% had an unknown MRD status. Median time from initial ALL diagnosis to MRD positivity was 4.2 months. Median follow-up was 23.0 months for patients included in time-to-event analyses.
Table 1. Patient demographics, disease characteristics, and MRD status.
Duration of remission
Median DoR was 18.5 months (95% CI, 11.9–27.2) after baseline MRD assessment ((A)). At 36 months, 38% of patients remained in hematologic CR (95% CI, 32% to 45%). Median DoR was longer in patients with lower baseline MRD: 2.0, 10.9, 18.5, and 42.4 months after baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, respectively (P < .0001; (A) and ). Median DoR was also longer in patients with WBC <30,000/µL vs. ≥30,000/µL at diagnosis (P = .003), younger vs. older patients (P = .21), and patients with MRD persistence vs. MRD reappearance (P = .03; and Supplemental Figure 1). In a multivariable model, younger age (P = .08), lower WBC count at diagnosis (P = .005), lower baseline MRD (P = .002), and MRD persistence vs. reappearance (P = .03) were associated with longer DoR (). Year of diagnosis, which was not a significant factor for DoR in the univariate analysis (P = .86), was not included in the multivariable model. Median DoR was slightly shorter when patients were censored at HSCT (Supplemental Figure 2).
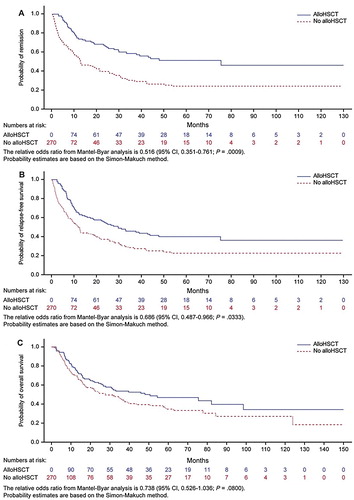
Figure 1. Outcomes among patients with MRD. (A) DoR. (B) RFS. (C) OS. Note: Censored patients are indicated by vertical bars.
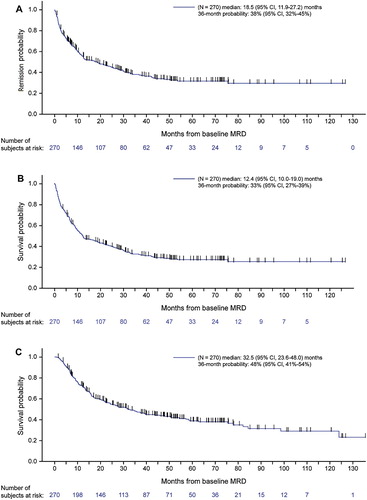
Figure 2. Kaplan–Meier plots of outcomes by baseline MRD level. (A) DoR by all MRD levels. (B) RFS by all MRD levels. (C) OS by all MRD levels. (D) DoR by MRD ≥10−3 or <10−3. (E) RFS by MRD ≥10−3 or <10−3. (F) OS by MRD ≥10−3 or <10−3. Note: Censored patients are indicated by vertical bars.
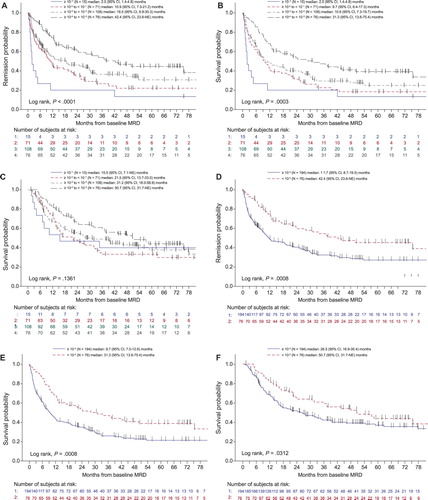
Table 2. Median DoR, RFS, and OS according to different patient factors.
Table 3 . Multivariable analysisa of baseline factors associated with RFS, DoR, and OS.
Relapse-free survival
Median RFS was 12.4 months (95% CI, 10.0–19.0) after baseline MRD assessment ((B)). At 36 months, estimated RFS was 33% (95% CI, 27–39%). Median RFS was longer in patients with a lower baseline MRD: 2.0, 9.7, 10.6, and 31.3 months after baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, respectively (P = .0003; (B) and ). Lower WBC count at original ALL diagnosis was also associated with longer median RFS (P = .005; and Supplemental Figure 1). In a multivariable model, lower baseline MRD (P = .003), lower WBC at diagnosis (P = .007), MRD persistence vs. reappearance (P = .11) and younger vs. older age (P = .05) were associated with longer RFS (). As with DoR, year of diagnosis was not significant in the univariate analysis (P = .91) and was not included in the multivariable model. Median RFS was slightly shorter when patients were censored at HSCT (Supplemental Figure 2).
Overall survival
Median OS was 32.5 months (95% CI, 23.6–48.0) after baseline MRD assessment ((C)), with 48% of patients alive at 36 months (95% CI, 41–54%). Among 272 patients with quantifiable baseline MRD, 155 (57%) patients died, including 23 (8%) without documented relapse (Supplemental Table 2). Median OS was longer in patients with a lower baseline MRD (15.5, 21.5, 31.2, and 50.7 months after baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, respectively) but the trend was not statistically significant (P = .14; (C) and ). When patients with baseline MRD ≥10−3 or <10−3 were compared, those with higher baseline MRD had significantly worse OS (50.7 vs. 26.5 months; P = .03; ). Younger age (P = .002) and lower WBC at diagnosis (P = .02) were associated with longer median OS (Supplemental Figure 1). In a multivariable model, lower WBC at diagnosis (P = .002), younger age (P = .0005), and later year of diagnosis (P = .06) were associated with longer median OS (). Median OS was slightly shorter when patients were censored at HSCT (Supplemental Figure 2).
Other analyses
WBC at diagnosis, which was prognostic for RFS, DoR, and OS, was cross-tabulated with other predictors for patient outcomes. Age, sex, baseline MRD, and transplant status were not significantly different between patients with higher (≥30,000/µL) or lower (<30,000/µL) WBC at diagnosis (Supplemental Table 3). However, patients with a year of diagnosis after 2010 had higher WBC count than those diagnosed in 2004–2010 (P = .02).
Transplant outcomes
A total of 110 patients (40%) received alloHSCT in ongoing hematologic CR and 162 (60%) did not (Supplemental Table 4). Patients undergoing transplant were slightly younger at diagnosis compared to nontransplanted patients: 64% vs. 50% were age <35 years (P = .06) and median age was 31.5 vs. 33.0 years. Median time from baseline MRD assessment to alloHSCT was 3.4 months (range, 0.1–39.8). From year 2005 onward, proportionally more patients underwent transplant compared to patients diagnosed in the years 2000 to 2004 (45% [67/149] vs. 31% [31/97]; P = .06).
Transplant status was associated with different causes of death. At last follow-up, 58% of transplanted patients and 33% of nontransplanted patients remained alive. Death without documented relapse was reported for 14% of transplanted patients and 5% of nontransplanted patients, while 28% of transplanted patients and 62% of nontransplanted patients died after relapse (P < .0001).
Median DoR in all transplanted patients was 68.4 months (95% CI, 34.1 to NE) and 36-month DoR was 59% (95% CI, 48–69%). In transplanted patients with baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, median DoR was not reached, 33.1 months, not reached, and 75 months, respectively. Median DoR in all nontransplanted patients was 7.4 months (95% CI, 4.8–10.9) and 36-month DoR was 22% (95% CI, 15–28%).
Median RFS from the date of alloHSCT in all transplanted patients was 34.4 months (95% CI, 21.8 to NE) and 36-month RFS was 49% (95% CI, 39–60%). In transplanted patients with baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, median RFS from date of alloHSCT was not reached, 27.7, 47.9, and 75.4 months, respectively. In nontransplanted patients, median RFS was 6.7 months overall (95% CI, 4.6–9.2) and 36-month RFS was 20% (95% CI, 13–26%).
Median OS from the date of alloHSCT in transplanted patients was 76.1 months overall (95% CI, 41.1 to NE) and 36-month OS was 61% (95% CI, 51–71%). In transplanted patients with baseline MRD of ≥10−1, 10−2 to <10−1, 10−3 to <10−2, and 10−4 to <10−3, median OS from date of alloHSCT was not reached, 33.0, 54.4, and 75.4 months, respectively. In nontransplanted patients, median OS was 20.4 months overall (95% CI, 15.3–31.7) and 36-month OS was 38% (95% CI, 30–46%).
Mantel–Byar analysis indicated improved outcomes among transplanted patients for DoR (odds ratio [OR], 0.49; 95% CI, 0.33–0.73; P = .0004), RFS (OR, 0.65; 95% CI, 0.47–0.92; P = .02), and OS (OR, 0.72; 95% CI, 0.51–1.01; P = .06; ). Mantel–Byar test results were visualized with Simon–Makuch plots, illustrating longer DoR, RFS, and OS among transplanted patients (), similar results were observed with a 3-month landmark (Supplemental Figure 3). Conditional landmark analyses which considered patients undergoing alloHSCT up to month 3 suggested longer DoR, RFS, and OS for patients that underwent alloHSCT. However, no difference was observed in transplanted patients up to month 6 (Supplemental Figure 4). In a time-dependent Cox model adjusting for baseline factors, similar improvement after alloHSCT was observed for DoR (HR, 0.43; 95% CI, 0.29–0.64; P < .0001; ) and RFS (HR, 0.59; 95% CI, 0.41–0.84; P = .003). Transplanted patients also appeared to have longer OS than nontransplanted patients, although these findings were not statistically significant (HR, 0.72; 95% CI, 0.50–1.05; P = .08).
Table 4. Analysis of outcomes for transplanted vs. nontransplanted patients.
Discussion
This was an analysis of a large dataset of patients with Ph-negative BCP-ALL and measurable MRD who received standard of care therapy at European academic study group protocols or at major centers. A broad cross-section of patients were included which increased robustness and generalizability of these study results. Baseline MRD level appeared to influence DoR, RFS, and OS, demonstrating the prognostic impact of MRD and highlighting the importance of incorporating MRD assessment into routine care. Patients with very high baseline MRD (≥10−1) had very short DoR, whereas those with MRD between 10−4 and 10−3 had much longer DoR. This finding underlines that MRD detection is a surrogate for disease burden and is consistent with previous research showing that progressively higher MRD are associated with higher relapse rates [Citation5,Citation10]. In patients with MRD above 10−2, the median time until hematologic relapse was weeks (1.6–3.9 months) in this study, indicating that the time window to change treatment strategy (eg, alloHSCT) based on MRD is limited. With higher levels of MRD, the outcome even with alloHSCT is not nearly as beneficial compared to lower levels of MRD or undetectable MRD [Citation5,Citation24].
Patients with MRD reappearance in our study population had worse outcomes compared to those with MRD persistence. Detecting MRD reappearance strongly depends on the frequency of follow-up MRD testing in patients with complete MRD response. Follow-up evaluation to detect MRD reappearance is not routine in many countries and explains the relatively low number of patients with MRD reappearance in the studied population. The management of MRD reappearance may differ from MRD persistence. Whereas MRD persistence is often detected early after consolidation and patients usually receive continued intensive treatment, this is often not the case in MRD reappearance, when patients are off treatment or in the maintenance phase, which may contribute to the high risk of immediate hematologic relapse [Citation5,Citation11].
Other factors that were associated with outcomes, included WBC and age at initial ALL diagnosis. High WBC at diagnosis is an established factor predicting poorer prognosis [Citation25]. Although the biologic effect behind this clinical factor is not described thus far, it seems that the adverse impact of elevated WBC count at diagnosis is maintained at the time of MRD measurement, as it confers more aggressive disease. Similarly, in relapsed/refractory ALL, the WBC count at first diagnosis maintains an impact on survival [Citation26]. The youngest patients had the longest OS, whereas the oldest patients had the shortest OS, consistent with published literature [Citation10]. Possible explanations are decreased transplant realization rate and lower-intensity chemotherapy in older patients and increased alloHSCT-related mortality in the middle age group.
With no recognized standard of care for MRD-positive ALL during the study period, DoR and RFS remained relatively stable over the evaluated time periods from 2000 to 2014. However, more recently diagnosed patients (2011 or later) appeared to have slightly longer OS compared to patients diagnosed in earlier years. An increasing proportion of patients underwent alloHSCT in general [Citation27] and in this study. This may be due to the fact that the negative impact of MRD was acknowledged to an increasing extent, leading to a clear indication for alloHSCT, which is now part of different guidelines and first-line protocols [Citation18]. Improvements in alloHSCT may have reduced risk of non-relapse mortality for younger patients [Citation28]. It is possible that with more follow-up data, the effect of alloHSCT on survival among MRD-positive patients could be even greater than observed.
Several different statistical analysis techniques were used to examine the potential role of alloHSCT and showed a trend toward better outcomes with alloHSCT. However, these analyses did not adjust for potential bias such as differential patient characteristics for transplant (e.g. younger age). In time-varying Cox models, Mantel–Byar analysis, and Simon–Makuch plots, alloHSCT was a time-dependent covariate and reduced bias introduced by different on-study time to alloHSCT. Conditional landmark analyses suggested benefit for patients surviving without event until month three. A later landmark at month six didn’t show an advantage for transplanted patients. Patients surviving without transplantation until this later time-point may represent a positive selected patient population, which would include patients who do not require a transplant. However, this is not the usual situation in clinical practice where transplantation is performed earlier. Limitations of this method are the definition of an adequate landmark as patients with an event before the landmark are excluded from the analysis, reducing total evaluable patients. Furthermore, patients receiving a transplant after the landmark are considered nontransplanted, further attenuating the results.
Overall, patients with persistent or recurrent MRD who underwent alloHSCT achieved a better outcome in terms of DoR and RFS and was assessed by multiple methods. Patients receiving alloHSCT also had longer OS, but was not statistically significant. A possibility is that some non-transplanted patients survived after relapse accounting for the smaller difference in OS. But these events were few and relapse events were captured in the DoR and RFS analyses. This study did not collect detailed information on transplant donor type or non-relapse related mortality. However, the patient population receiving a transplant was relatively young and mortality in CR was limited (though higher than that in nontransplanted patients). Also, risk of hematologic relapse was higher in nontransplanted patients. Overall benefits of DoR, RFS, and OS favored patients undergoing a transplant.
MRD has become a routine part of disease monitoring; in prognostic models, MRD is an important factor in guiding the development of new protocols/therapies. Current guidelines from National Comprehensive Cancer Network [Citation19] and European Society for Medical Oncology [Citation18] mandate MRD testing for post-induction follow-up and risk stratification. AlloHSCT is often recommended to improve outcomes of patients with MRD [Citation1,Citation2,Citation5,Citation29]. MRD detection between flow cytometry or RQ-PCR are highly concordant [Citation30], so the most readily available standardized assay should be used. Persistent MRD has been reported as the only risk factor predictive of a significant effect of alloHSCT in first CR [Citation20]. MRD status immediately before subsequent alloHSCT was not documented in our study. Others have reported, however, that less MRD prior to transplant was associated with improved OS and RFS [Citation13,Citation21,Citation24]. In addition, many MRD-positive patients develop overt hematologic relapse despite continued chemotherapy while alloHSCT is prepared. This is also evident from the high relapse rate in MRD-positive patients in our study. Currently, many new trials are targeting MRD negativity as the primary endpoint.
Altogether, these data indicate the need for improved treatments for patients with MRD-positive ALL. Since patients with persistent MRD demonstrate resistance to chemotherapy, new drugs with different mechanisms of action are of interest. A recent trial with the bispecific antibody blinatumomab in MRD-positive BCP-ALL included patients with MRD in CR1 but in one-third of the patients in CR2/CR3. The median age was 45 years and the MRD level was above 10−3. In this unfavorable patient population, blinatumomab yielded conversion to MRD-negative among 78% of patients and median survival of 36.5 months. Additionally, longer survival was observed for patients with complete MRD response compared to patients who did not respond [Citation31]. Blinatumomab was recently approved by the US Food and Drug Administration and European Medicines Agency for the treatment of >10−3 MRD in ALL. Trials of blinatumomab and inotuzumab in relapsed/refractory ALL demonstrated that CR often was accompanied by MRD response [Citation32,Citation33].
The primary limitation of this study is that it was retrospective. Each country or study group followed their protocol, which specified when to conduct MRD assessments. Treatments and procedures for relapse were not captured or analyzed in this study and data collection around alloHSCT was limited. Conditioning regimen, donor type or match can be associated with survival [Citation34], but these details were not available. Lack of these data likely biased study results to non-significance rather than causing the observed effects. The use of multiple statistical techniques was necessary because no single method is best for analyzing time-varying covariates such as alloHSCT. There may be some uncertainty on the size of the effect of alloHSCT, but not on the directional improvement of outcomes. This study included patients over a 14-year span. Changes in alloHSCT may not be fully realized in this study, as the effect of alloHSCT on outcomes was averaged over that time span. The major effect was probably the higher realization rate of alloHSCT over the years. Finally, the study sample size was modest, which limited the power to detect differences with P-values <.05.
In conclusion, MRD is a strong prognostic factor for clinical outcomes. This data provides a reference for outcomes of adults with MRD-positive BCP-ALL who receive standard therapies including alloHSCT. Achievement of MRD negativity is a goal of the modern treatment strategy, even patients allocated to alloHSCT consolidation. Use of alloHSCT may improve outcomes in these patients, but there is room for further improvement, possibly use of targeted therapies to enable more MRD-negative alloHSCT. Although the patient population in this study was relatively young, these conclusions may be even more relevant for older patients with persistent MRD who have the worst prognoses, likely due to not having the option to undergo alloHSCT or further intensification of chemotherapy.
Registered at www.clinicaltrials.gov as #NCT02010931.
Supplemental Material
Download MS Word (556.3 KB)Disclosure statement
N.G. has received research funds and honoraria and has served on advisory boards, for Amgen, Pfizer, Celgene and Novartis. H.D. has received honoraria and/or research funding from Amgen, Agios, Seattle Genetics, Celgene, Sunesis, Roche, Pfizer, Ambit-Daiichi Sankyo, Shire-Baxalta, Ariad-Incyte, Karyopharm, Abbvie, Novartis, Kite, Otsuka, Celator-Jazz, Astellas, Menarini, Cellectis, Janssen, ImmunoGen, and Servier. S.G. has served on advisory boards and received honoraria from Amgen. M.B. has served as an advisor for Amgen and Incyte and on speakers’ bureaus for Amgen, Pfizer and Roche, and received research funding from Amgen, Affimed, and Regeneron. R.F. has been on advisory boards and speakers bureaus for Amgen, Pfizer, Janssen, Novartis, AbbVie, Celgene, Sandoz, Celltrion, Roche, Incyte. C.K., M.S., J.M.S, and G.Z. are employees and stockholders of Amgen. G.M. has served as an advisor to Amgen, Ariad/Incyte, Pfizer, Roche, Celgene, Janssen, and Jazz Pharmaceuticals, on speakers’ bureaus for Novartis, Pfizer, and Celgene, and received travel compensation from Daiichi Sankyo, Roche, and Shire. A.R. received support from Amgen, Pfizer, Novartis, and Celgene for travel, sponsored lectures, and advisory board meetings. J.-M.R. has received research funds and honoraria, and served on advisory boards, for Amgen, Pfizer, Shire, and Ariad. R.B. received honoraria for participation in advisory boards for Amgen, Pfizer, Shire, and Incyte. E.P. received support for advisory board meetings from Amgen, Roche and Celgene. M.D., D.H. declare no conflicts of interest.
ORCID
Michael Doubek http://orcid.org/0000-0002-1269-6282
Elena Parovichnikova http://orcid.org/0000-0001-6177-3566
Josep-Maria Ribera http://orcid.org/0000-0003-1042-6024
Additional information
Funding
References
- Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–4162. doi: 10.1182/blood-2008-11-185132
- Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749. doi: 10.1182/blood-2014-01-547695
- Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–1123. doi: 10.1182/blood-2005-07-2708
- Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146
- Gokbuget N, Kneba M, Raff T, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868–1876. doi: 10.1182/blood-2011-09-377713
- Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish adult leukemia group ALL 4-2002 MRD study. Br J Haematol. 2008;142(2):227–237. doi: 10.1111/j.1365-2141.2008.07185.x
- Raff T, Gokbuget N, Luschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910–915. doi: 10.1182/blood-2006-07-037093
- van Dongen JJ, Seriu T, Panzer-Grumayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731–1738. doi: 10.1016/S0140-6736(98)04058-6
- van Dongen JJ, van der Velden VH, Bruggemann M, et al. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996–4009. doi: 10.1182/blood-2015-03-580027
- Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi: 10.1001/jamaoncol.2017.0580
- Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from children’s Oncology group study AALL0232. Blood. 2015;126(8):964–971. doi: 10.1182/blood-2015-03-633685
- Bruggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120(23):4470–4481. doi: 10.1182/blood-2012-06-379040
- Sutton R, Shaw PJ, Venn NC, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. 2015;168(3):395–404. doi: 10.1111/bjh.13142
- Van der Velden VH, Corral L, Valsecchi MG, et al. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23(6):1073–1079. doi: 10.1038/leu.2009.17
- Bruggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: proceedings of the second international symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010;24(3):521–535. doi: 10.1038/leu.2009.268
- Sutton R, Venn NC, Tolisano J, et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol. 2009;146(3):292–299. doi: 10.1111/j.1365-2141.2009.07744.x
- Hoelzer D. Monitoring and managing minimal residual disease in acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book. 2013;2013:290–293. doi: 10.1200/EdBook_AM.2013.33.290
- Hoelzer D, Bassan R, Dombret H, et al. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69–v82.
- Brown PA, Shah B, Fathi A, et al. NCCN guidelines insights: acute lymphoblastic leukemia, Version 1.2017. J Natl Compr Canc Netw. 2017;15(9):1091–1102. doi: 10.6004/jnccn.2017.0147
- Dhedin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486–2496. doi: 10.1182/blood-2014-09-599894
- Kotrova M, van der Velden VHJ, van Dongen JJM, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52(7):962–968. doi: 10.1038/bmt.2017.16
- Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81–86. doi: 10.1080/01621459.1974.10480131
- Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106
- Bassan R, Spinelli O, Oldani E, et al. Different molecular levels of post-induction minimal residual disease may predict hematopoietic stem cell transplantation outcome in adult Philadelphia-negative acute lymphoblastic leukemia. Blood Cancer J. 2014;4:e225. doi: 10.1038/bcj.2014.48
- Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53
- Gokbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101(12):1524–1533. doi: 10.3324/haematol.2016.144311
- Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51(6):786–792. doi: 10.1038/bmt.2016.20
- Giebel S, Labopin M, Socie G, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the acute leukemia Working Party of the European Society for blood and marrow transplantation. Haematologica. 2017;102(1):139–149. doi: 10.3324/haematol.2016.145631
- Leung W, Pui CH, Coustan-Smith E, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120(2):468–472. doi: 10.1182/blood-2012-02-409813
- Huang YJ, Coustan-Smith E, Kao HW, et al. Concordance of two approaches in monitoring of minimal residual disease in B-precursor acute lymphoblastic leukemia: fusion transcripts and leukemia-associated immunophenotypes. J Formos Med Assoc=Taiwan yi zhi. 2017;116(10):774–781. doi: 10.1016/j.jfma.2016.12.002
- Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–1531. doi: 10.1182/blood-2017-08-798322
- DeAngelo DJ, Stock W, Stein AS, et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017;1(15):1167–1180. doi: 10.1182/bloodadvances.2016001925
- Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783
- Pavlu J, Labopin M, Zoellner AK, et al. Allogeneic hematopoietic cell transplantation for primary refractory acute lymphoblastic leukemia: a report from the acute leukemia Working Party of the EBMT. Cancer. 2017;123(11):1965–1970. doi: 10.1002/cncr.30604