ABSTRACT
Objectives: The great majority of adult patients with immune thrombocytopenia (ITP) who fail to respond to first-line medication or who relapse following response require additional treatment. Although broad guidelines currently exist for second-line and subsequent therapies, none to date have been prescriptive. The purpose of this systematic review and network meta-analysis was to establish a clinically relevant ranking of the efficacy and safety of medications for adults (≥18 years old) with previously treated ITP.
Methods: Relevant publications from Medline, Embase, and the Cochrane database were searched from their inceptions through July 31, 2018. The primary outcome was the overall response (OR, defined as a platelet count ≥50 × 109/L at the end of treatment without rescue therapy), while the secondary endpoints included early response (ER; i.e. a platelet count ≥50 × 109/L at week 2 after initiation of treatment) and therapy-related severe adverse events (AEs).
Results: Thirteen randomized controlled trials (1,202 patients) were included in this study. According to pooled results, romiplostim appears to be the most suitable treatment in terms of OR, followed by avatrombopag, eltrombopag, fostamatinib, and rituximab. Avatrombopag produced more satisfactory outcomes than romiplostim, eltrombopag, and rituximab in terms of ER; severe AEs profiles were similar across all treatment arms.
Conclusion: Romiplostim appears to be the best option for patients who fail to respond to prior treatment or relapse thereafter, while avatrombopag and eltrombopag are reasonable alternatives. Rituximab monotherapy is not recommended, as it produces the lowest OR and ER rates.
1. Introduction
Primary immune thrombocytopenia (ITP) is an acquired autoimmune disorder characterized by isolated thrombocytopenia. Its main etiology is immune intolerance of platelet auto-antigens, which leads to an increase in autoantibody-mediated platelet destruction as well as a decrease in platelet production from megakaryocytes [Citation1,Citation2].
Although the relationship between thrombocytopenia and bleeding has been well established, there is no clear evidence of a direct correlation between the degree of thrombocytopenia and bleeding symptoms, especially in patients with a lower platelet count (PC) [Citation3]. While it is commonly held that bleeding complications are infrequent if PCs are ≥50 × 109/L, a persistent PC <30 × 109/L is also sometimes associated with severe bleeding as well as mortality [Citation4,Citation5].
First-line treatment for ITP includes corticosteroids as well as intravenous immunoglobulin. However, 60–70% of adult patients require additional treatment owing to intolerability to previously administered agents or relapse [Citation6]. Although there are multiple subsequent treatment options such as splenectomy, thrombopoietin receptor agonists (TPO-RAs), and immunosuppressive agents, the best treatment approach for overall and early recovery of PC while avoiding severe adverse events (AEs) remains unclear because it is nearly impossible to perform direct head-to-head comparisons of all possible combination therapies in randomized controlled trials (RCTs) [Citation7].
We posited that this limitation can be overcome by performing a network meta-analysis [Citation8], which unlike a pairwise meta-analysis, allows researchers to combine direct and indirect data from diverse regimens coupled with comparing results from individual trials [Citation9]. To that end, we conducted a systematic review and network meta-analysis of RCTs to compare the efficacy and safety of subsequent treatments in adult patients previously treated for ITP. We ranked the estimated efficacies of each medication to help establish evidence-based hierarchies.
2. Materials and methods
We followed the PRISMA statement guidelines during the preparation of this systematic review and network meta-analysis. The study was registered in the International Prospective Register of Systematic Review (PROSPERO 2018: CRD42018104089).
2.1. Search strategy and study selection
The electronic databases MEDLINE (via PUBMED), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for publications listed between each database’s inception date and 31 July 2018. We also searched ClinicalTrials.gov and the American Society of Hematology (2004–2018) to identify unpublished clinical trials. Only English and Chinese publications were retrieved. The following search terms combinations were used: ‘thrombocytopeni*’, ‘ITP’ and ‘Randomized Controlled Trials’. The search strategies are listed in Tables S1–S3. We also searched the references of the identified studies manually to locate additional publications.
Studies that met all of the following criteria were included: (1) patients were adults (≥18 years old) with primary ITP; (2) patients failed to respond to prior treatment or relapsed, and had PCs < 30 × 109/L; (3) the studies revealed at least 1 of the following 3 outcomes: overall response (OR [PC ≥50 × 109/L at the end of treatment without rescue therapy]), early response (ER [PC ≥50 × 109/L at week 2 after the initiation of therapy]), and/or severe AEs (grade 3 or more, based on the Common Terminology Criteria for Adverse Events version 4.0); and (4) the studies were RCTs. Patients receiving concomitant medications for ITP were eligible if the doses were stable for at least 2 weeks prior to randomization. Rescue medication was defined as an increased dose of concurrent ITP therapy or the use of any new drug to increase platelet counts. Studies on patients with secondary ITP and those involving both children and adults in which adult data were not extractable separately were excluded. Two independent reviewers (Yang and Lin) searched the literature and scanned study titles and abstracts to assess their applicability to our investigation.
2.2. Data extraction and quality assessment
Two independent researchers (Yang and Yao) were responsible for extracting data from the included trials. Disagreements were solved via discussion or consultation with the senior authors. The following data were extracted: (1) general information (author, publication year); (2) study characteristic (design, country, randomization, number of each group); (3) patient characteristics (sex, age, classification, baseline PC, duration of ITP, splenectomy status, concomitant medication); (4) components of intervention (dosage, duration, interval, and total amounts); and (5) outcome (OR, ER, and severe AEs).
In accordance with the Cochrane Collaboration Reviewers’ Handbook (Version 5.1.0) [Citation10], 2 authors (Yang and Lin) independently assessed all relevant studies for bias. We considered the following aspects: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and any other biases. Any divergences were discussed with the senior authors to achieve a consensus. Qualitative information was then synthesized using Review Manager, version 5.3 (The Cochrane Collaboration, London, UK).
2.3. Data synthesis and analysis
A network meta-analysis on random effects was performed virtually using the STATA13 software (StataCorp, College Station, TX, USA). Evidence from both direct (head-to-head trials) and indirect (using common comparators without actual head-to-head trials) comparisons was combined. The primary endpoint was the incidence of OR, while the secondary endpoints were the incidences of ER and severe AEs. Moreover, all treatment effects were measured as dichotomous data and presented as the summary of risk ratios with 95% confidence intervals. We also calculated the surface under the cumulative ranking curve (SUCRA) to determine the hierarchy of the efficacy and risk of severe AEs, where SUCRA values of 100% indicated the most effective treatment or the treatment with the highest risk of AEs, and values of 0% indicated the least effective or least risky treatment. Publication bias was assessed using a funnel plot, Egger's test, and Begg's test.
3. Results
3.1. Study selection and characteristics
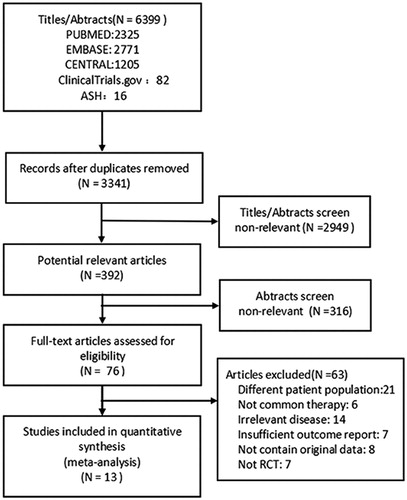
Our search identified 6,399 citations from the literature; the selection flowchart is presented in . The systematic review included 13 studies (1,202 participants) with 5 interventions, including 6 with eltrombopag (ELT) [Citation11–16], 3 with romiplostim (ROM) [Citation17–19], 2 with rituximab (RTX) [Citation20,Citation21], 1 with avatrombopag (AVA) [Citation22], and 1 with fostamatinib (FOS) [Citation23] trials. lists the characteristics of the included studies. All studies were randomized, double-blind, and placebo-controlled trials published between 2006 and 2018 and performed in various countries encompassing Asia, Africa, Europe, and North America. The duration of treatment ranged from 4 to 24 weeks. When different drug doses were used in a particular study, the dose arm with the best therapeutic effect was selected, for example, the 20 mg AVA and 75 mg ELT arms. The characteristics of the patients, whose ages ranged from 25 to 54 years, are shown in .
Table 1. Characteristics of the included studies.
Table 2. Patients’ characteristics.
3.2. Quality assessment
The risk of bias is summarized in . Information on random sequence generation and allocation concealment was unclear or not well-described in some studies (classified as ‘unclear’). Two studies [Citation19,Citation22] were classified as having a ‘high risk’ of reporting bias because of their small placebo arm sample sizes (n = 4 and n = 5, respectively). Only 1 study [Citation16] was classified as having ‘no other bias’ because it received no pharmaceutical industry sponsorship.
3.3. OR
All investigated studies reported the OR (). The numbers of all patients and of those who achieved an OR, along with the definition of OR in each RCT, are shown in Table S4. The pooled results revealed that (1) AVA, ELT, FOS, and ROM produced significantly better responses than placebo although the ORs of RTX- and placebo-treated patients were not significantly different; (2) ROM was significantly superior to FOS and RTX, although ROM- and ELT-treated patients did not have significantly different ORs, nor did ROM- and AVA-treated patients; and (3) AVA was significantly superior to RTX, while the ORs of AVA- and ELT-treated patients were not significantly different, nor were the ORs of AVA- and FOS-treated patients; (4) ELT was significantly superior to RTX with the ORs of ELT- and FOS-treated patients not found to be significantly different; and (5) the ORs of RTX- and FOS-treated patients were not significantly different.
Figure 3. Network analysis comparing overall responses. (a) The network of comparisons included in the study for overall response (OR; i.e. a platelet count ≥50 × 109/L at the end of treatment). The line width is proportional to the number of trials performed to compare the treatment groups. (b) The summary effect estimate (risk ratio of OR) for each combination of treatments. Risk ratios are indicated by dots and 95% confidence intervals by bars. (c) The surface under the cumulative ranking curve (SUCRA) is shown for each treatment. (d) Ranking of each arm according to the SUCRA values of OR. AVA, avatrombopag; ELT, eltrombopag; FOS, fostamatinib; ROM, romiplostim; RTX, rituximab.
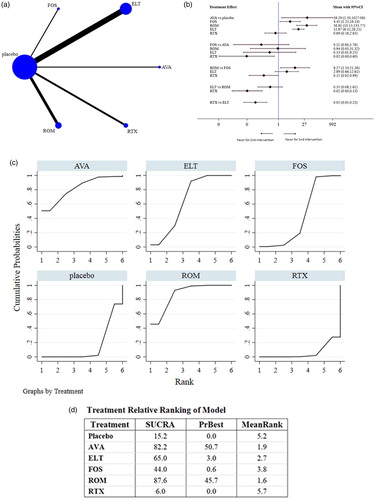
ROM (87.6%) showed the highest SUCRA value for OR, followed by AVA (82.2%), ELT (65.0%), and FOS (44.0%); RTX (6.0%) had the lowest value. These data indicate that patients had the highest probability of achieving an OR when treated with ROM. The small size of the AVA arm should be noted (1 study, n = 20) when drawing conclusions from these findings.
3.4. ER
We extracted ER data from 7 studies [Citation12,Citation13,Citation15,Citation17,Citation20–22] that collectively comprised 494 patients (). Because of the lack of data available for statistical analysis, ER as related to FOS was not analyzed. The numbers of patients overall and of those who obtained an ER in each RCT are presented in Table S5. The pooled results were as follows: (1) The AVA, ELT, and ROM arm produced responses that were significantly superior to those of the placebo or RTX arms, while the ERs of RTX- and placebo-treated patients were not significantly different; (2) the ERs of ROM- and AVA-treated patients were not significantly different, nor were the ERs of ROM- and ELT-treated patients of those between ELT- and AVA-treated patients.
Figure 4. Network analysis comparing early responses. (a) The network of comparisons included in the study for early response (ER; i.e. platelet count ≥50 × 109/L at week 2 after the initiation of treatment). The line width is proportional to the number of trials comparing the treatment groups. (b) The summary effect estimate (risk ratio of ER) for each combination of treatments. Risk ratios are indicated by dots and 95% confidence intervals by bars. (c) The surface under the cumulative ranking curve (SUCRA) is shown for each treatment. (d) Ranking of each arm according to the SUCRA values of ER. AVA, avatrombopag; ELT, eltrombopag; FOS, fostamatinib; ROM, romiplostim; RTX, rituximab.
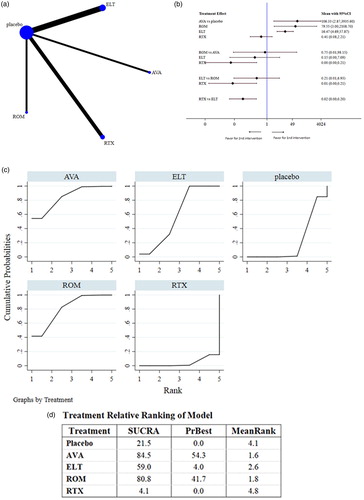
AVA (84.5%) had the highest SUCRA value for ER, followed by ROM (80.8%) and ELT (59.0%); RTX (4.1%) showed the lowest SUCRA value. These data indicate that AVA may be the optimal choice for obtaining an ER in patients with previously treated ITP. However, the small size of the AVA arm and unavailable FOS data should be noted.
3.5. Safety
Ten studies comprising 1,057 patients provided data regarding severe AEs related to each intervention (Figure S1); because of the lack of sufficient data for statistical analysis, severe AEs caused by AVA were not analyzed. The numbers of patients who experienced severe AEs are shown in Table S6. The pooled data showed no significant differences in severe AEs between patients receiving the 4 types of intervention. SUCRA rankings revealed that RTX carries the least severe AE risk (20.4%), while FOS carries the highest (74.0%). Additionally, ROM (32.1%) and ELT (63.5) were associated with a modest risk. All AEs were successfully managed.
3.6. Publication bias
The evaluation of publication bias was performed virtually using a network funnel plot for OR. As shown in Figure S2, a few included studies were not symmetrically distributed around the vertical line, indicating that a certain level of publication bias may exit in this network analysis. A quantitative analysis for publication bias was conducted; both Egger’s test (P = 0.448) and Begg’s test (P = 0.371) suggested a lack of such bias in the included studies.
4. Discussion
Our systematic review and network meta-analysis on the efficacy and safety of therapeutic options for patients previously treated for ITP included 13 trials with 1,202 randomly assigned participants. The main findings of our study are as follows: (1) ROM appears to be the most suitable treatment in terms of OR, followed by AVA, ELT, FOS, and RTX; (2) AVA produces more satisfactory outcomes than ROM, ELT, or RTX in terms of ER; and (3) therapy-related severe AE profiles are similar across treatments, with RTX the least likely to cause severe AEs.
ROM (AMG-531, Nplate), which yielded the best OR, has been approved by the US Food and Drug Administration (FDA) for adults with chronic ITP [Citation24]. Previous data have shown durable responses with ongoing ROM treatment in 38–52% of patients [Citation18]. Outcomes of real-world use in adults are similar to those reported in clinical trials, although response patterns are more varied [Citation24]. ROM has the advantage of not suppressing the immune system and is easy to wean off, while its disadvantages include its high cost and regular monitoring (i.e. weekly injections at a physician’s office) [Citation25]. The other commonly used TPO-RA, ELT (Revolade, Promacta), has been used for treating ITP for 10 years. Zhang et al. [Citation26] demonstrated that ROM and ELT produce similar overall platelet responses in adults with ITP; in contrast, Cooper and colleagues [Citation27] found that ROM produces better results. After analyzing additional studies using the network meta-analysis method, we came to the same conclusion as Cooper et al.
AVA (E5501, YM477, AKR 501, Doptelet) is a novel, orally-active, non-peptide TPO-RA. Although it was temporarily approved by the FDA for thrombocytopenia in adults with chronic liver disease [Citation22], our data suggested that AVA increases PC and that it has a higher SUCRA ranking than ELT, FOS, and RTX. AVA is taken orally once a day, and its lack of food interactions may render it preferable to ROM and ELT [Citation22]. Nevertheless, it is not possible to draw definite conclusions since our findings are based on 20 subjects taking AVA plus 5 in the placebo group; additional RCTs are required to fully characterize the effect of AVA.
FOS (R935788, R788), is an oral, relatively selective spleen tyrosine kinase (Syk) inhibitor approved by the FDA in April 2018 for treating ITP in adults who received multiple prior treatments. In the phase 3 FIT1 and FIT2 trials that comprised 75 patients each, nearly 50% of patients exhibited an overall platelet response to FOS, including those who did not respond after splenectomy or treatment with TPO-RAs and/or RTX [Citation23]. While patient-related factors may explain the low FOS ranking in this study, another reason may be related to the single-drug mechanism. Thrombocytopenia is caused by anti-platelet antibodies, damaged megakaryocyte production, and sputum cell-mediated platelet destruction; these etiologies vary among patients [Citation3]. While platelet destruction mediated by autoantibodies is more sensitive to FOS than to other agents [Citation28–30], FOS-related data were less abundant owing to the drug’s more recent introduction; hence, its therapeutic effect ought to be verified in additional clinical trials.
Lastly, RTX received the lowest SUCRA ranking; the OR rates of RTX and placebo were not significantly different. Although RTX was meant to be an alternative to splenectomy (which has a remission rate of 77% [range, 58–91%]) [Citation31], its response rate was markedly lower (60–70%) [Citation32]. Our data do not support using RTX for ITP; however, a number of RCTs showed higher response rates when RTX was combined with other drugs such as dexamethasone [Citation33] or recombinant human thrombopoietin (rhTPO) [Citation34]. We hypothesized that combined therapy integrates the advantages related to time windows and mechanisms of action. As such, RTX monotherapy may not be the optimal choice for patients with previously treated ITP, especially those who are corticosteroid-resistant [Citation20,Citation21].
A rapid increase in PC is desirable for ITP patients with bleeding risks [Citation35]; hence, ER was analyzed as a secondary endpoint. TPO-RAs showed higher SUCRA values than RTX in terms of EA; the drugs’ rankings may be related to their different mechanisms of action. Recent studies have found that TPO-RAs not only promote platelet production but also reduce the damage of mononuclear macrophages to platelets by regulating the proportion of Fc receptor subtypes [Citation30]. This synergistic effect may be responsible for the rapid elevation of platelets by TPO-RAs. AVA could be the most proper treatment in terms of achieving ER. As we showed, most responses to AVA occurred by day 7, which was consistent with the results from the latest phase 3 study [Citation36]. Previous data showed that ROM has a slightly delayed response of 1–4 weeks, depending on the starting dose and weekly dose increases [Citation24]; this was consistent with our findings that ROM ranks slightly lower for ER. Moreover, RTX was ranked last in terms of ER; this agent produced a delayed response (at both standard and low doses) in a previous study in which the PC of the RTX arm began to rise 6–8 weeks after commencing treatment and peaked rapidly. This may be related to the reduction of platelet autoantibodies after the elimination of B-cell clones [Citation37]. A systematic review concluded that the median response time of RTX was 5.5 weeks after treatment initiation [Citation38]; our data demonstrated that RTX was not an optimal approach for rapidly increasing the PC.
Given the requirement of long-term administration, severe AEs were also a secondary endpoint in our study (although this endpoint was not investigated for AVA owing to insufficient data for statistical power). Our data suggested no significant differences in severe AEs among the investigated agents. Most AEs appeared to be mild-to-moderate and resolved either spontaneously or after medical intervention. In contrast to OR and ER, the SUCRA rankings revealed that RTX had the best (safest) score. Although RTX (an immunosuppressive agent) may increase the risk of infection, our data suggest that the incidences of serious infections were rare, which may be related to the short treatment time and active prevention before medication [Citation39]. This makes RTX more suitable for combination therapy, which can increase its curative effect while minimizing the risk of AE. Of note, patients with a high risk of thromboembolism should choose the appropriate medication carefully; low incidences of thromboembolic events were observed with all drugs except for FOS.
To our knowledge, ours is the first study that estimated the efficacy and safety of various medications in adult patients with previously treated ITP. However, the study had some limitations. First, only a few RCTs were included for some treatment arms; for example, only 1 RCT was available for FOS (although the number of participants was relatively large). This limitation generally resulted in a higher β error (lower power to detect differences) and publication bias [Citation7]. Second, the endpoints were determined only for OR and ER; other outcomes such as sustained response and clinically significant bleeding should be further investigated as they were not well-reported in the included RCTs. Besides, detailed information on ER and severe AEs was obtained from 7 and 10 RCTs, respectively, which may lead to unreliable conclusions. Third, rhTPO, which is one of the most widely used TPO-RAs in China, was not included in our analysis. Two RCTs dealing with rhTPO were excluded: one [Citation31] included patients 12–82 years in age among whom data for adults could not be extracted separately; the other [Citation40] compared rhTPO plus danazol to rhTPO alone without referring to other treatment arms, resulting in a node unconnected to the other treatments’ network. Lastly and quite notably, there were no suitable splenectomy RCTs identified; therefore, we were unable to compare the efficacy and safety of our investigated medications to those of splenectomy.
In summary, our systematic review and network meta-analysis data suggest that suitable therapeutic strategies are available for adults with previously treated ITP. ROM appears to be the best treatment for patients who fail to respond to first-line ITP medication or relapse thereafter, avatrombopag and eltrombopag are reasonable alternatives, while RTX monotherapy is not recommended because it produces the lowest OR and ER rates. Future head-to-head trials of our tested regimens are critical to provide more evidence supporting the optimal therapy for previously treated adult ITP patients.
Geolocation information:
The study was conducted in Nanjing, Jiangsu province, China.
Data availability:
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplemental Material
Download Zip (654 KB)Acknowledgements
We thank Yuyang Ma and Qijun Huang for providing methodology and statistical analysis support for this review.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ran Yang http://orcid.org/0000-0002-1097-3573
Additional information
Funding
References
- British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120(1):574–596.
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984
- Neunert C. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv. 2017;2017(1):400–405.
- Cohen YC, Djulbegovic B, Shamai-Lubovitz O, et al. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–1638. doi: 10.1001/archinte.160.11.1630
- Portielje JE, Westendorp RG, Kluin-Nelemans HC, et al. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97(9):2549–2554. doi: 10.1182/blood.V97.9.2549
- Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annu Rev Med. 2005;56:425–442. doi: 10.1146/annurev.med.56.082103.104644
- Arai Y, Jo T, Matsui H, et al. Comparison of up-front treatments for newly diagnosed immune thrombocytopenia - a systematic review and network meta-analysis. Haematologica. 2018;103(1):163–171. doi: 10.3324/haematol.2017.174615
- Caldwell DM, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Br Med J. 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. doi: 10.1016/S0895-4356(97)00049-8
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1. 0. [Internet] March 2011. The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org.
- Yang R, Li J, Jin J, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 2017;176(1):101–110. doi: 10.1111/bjh.14380
- Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799–806. doi: 10.1111/j.1538-7836.2012.04695.x
- Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2
- Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–648. doi: 10.1016/S0140-6736(09)60402-5
- Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Eng J Med. 2007;357(22):2237–2247. doi: 10.1056/NEJMoa073275
- Huang Y, Liu X, Chen Y, et al. The efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Chin J Hematol. 2018;39(1):32–36.
- Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized phase III clinical trial. Int J Hematol. 2011;94(1):71–80. doi: 10.1007/s12185-011-0886-8
- Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2
- Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Eng J Med. 2006;355(16):1672–1681. doi: 10.1056/NEJMoa054626
- Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9978):1653–1661. doi: 10.1016/S0140-6736(14)61495-1
- Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. doi: 10.1182/blood-2011-08-374777
- Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. 2014;123(25):3887–3894. doi: 10.1182/blood-2013-07-514398
- Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921–930. doi: 10.1002/ajh.25125
- Grace RF, Neunert C. Second-line therapies in immune thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):698–706. doi: 10.1182/asheducation-2016.1.698
- Dolph M, Roy A, Bhor M, et al. A decision framework for treating chronic immune thrombocytopenia with thrombopoietin receptor agonists. J Comp Eff Res. 2018. DOI:10.2217/cer-2018-0034.
- Zhang J, Liang Y, Ai Y, et al. Eltrombopag versus romiplostim in treatment of adult patients with immune thrombocytopenia: a systematic review incorporating an indirect-comparison meta-analysis. PloS one. 2018;13(6):e0198504. doi: 10.1371/journal.pone.0198504
- Cooper K, Matcham J, Helme K, et al. Update on romiplostim and eltrombopag indirect comparison. Int J Technol Assess Health Care. 2014;30(1):129–130. doi: 10.1017/S0266462313000767
- Newland A, Lee EJ, McDonald V, et al. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy. 2018;10(1):9–25. doi: 10.2217/imt-2017-0097
- Podolanczuk A, Lazarus AH, Crow AR, et al. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154–3160. doi: 10.1182/blood-2008-07-166439
- Liu XG, Liu S, Feng Q, et al. Thrombopoietin receptor agonists shift the balance of Fcγ receptors towards inhibitory receptor IIb on monocytes in ITP. Blood. 2016;128(6):852–861. doi: 10.1182/blood-2016-01-690727
- Neunert CE, Cooper N. Evidence-based management of immune thrombocytopenia: ASH guideline update. Hematology Am Soc Hematol Educ Program. 2018;2018(1):568–575.
- Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91(4):E267–E272. doi: 10.1002/ajh.24310
- Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91–98. doi: 10.1007/s12185-010-0753-z
- Zhou H, Xu M, Qin P, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541–1547. doi: 10.1182/blood-2014-06-581868
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi: 10.1182/blood-2009-06-225565
- Jurczak W, Chojnowski K, Mayer J, et al. Avatrombopag, a novel oral thrombopoietin receptor agonist, demonstrates superiority to placebo for the treatment of chronic immune thrombocytopenic purpura in a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Blood. 2017;130(Suppl 1):17.
- Kelly K, Gleeson M, Murphy PT. Slow responses to standard dose rituximab in immune thrombocytopenic purpura. Haematologica. 2009;94(3):443–444. doi: 10.3324/haematol.2008.001396
- Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25–33. doi: 10.7326/0003-4819-146-1-200701020-00006
- Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. doi: 10.1182/blood-2011-11-393975
- Wang S, Yang R, Zou P, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222–228. doi: 10.1007/s12185-012-1124-8