ABSTRACT
Introduction: Noggin is an antagonist of bone morphogenetic proteins (BMPs) and has a strong effect on osteogenesis. Osteoporosis is a common complication of transfusion dependent beta-thalassemia (TDT) and denosumab has been recently emerged as a promising therapeutic option. This was a post hoc investigation of serum noggin levels among TDT patients with osteoporosis who participated in a randomized, placebo-control, phase 2b study.
Methods: Patients received either 60 mg denosumab (n = 32) or placebo (n = 31) every 6 months for 12 months. Noggin was measured, for the first time in thalassemia patients, at baseline and at 12 months, using a recently developed high sensitivity fluorescent immunoassay.
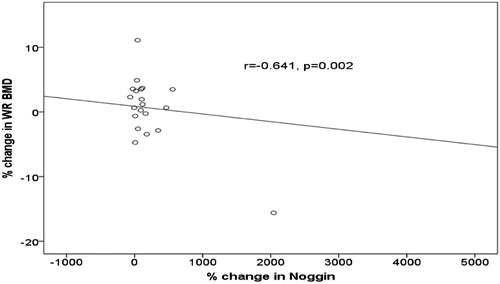
Results: Both groups showed a significant increase in noggin serum levels (denosumab p < 0.001; placebo p < 0.0001). Interestingly, the increase was higher in the placebo group. Furthermore, we observed a strong correlation between noggin and wrist bone mineral density (r = −0.641, p = 0.002) only in the denosumab group.
Conclusion: In conclusion, higher noggin levels reflected more BMP inhibition, since our assay detects free bioactive noggin, which in turn impaired bone formation in placebo group. Therefore, denosumab possibly regulates noggin and favours bone turnover in TDT patients with osteoporosis through a novel mechanism of action.
Trial registration: ClinicalTrials.gov identifier: NCT02559648.
1. Introduction
In the era of modern transfusion medicine that extends survival, osteoporosis has become a common complication affecting as many as 50% of transfusion-dependent beta-thalassemia (TDT) patients [Citation1]. The multifactorial etiology, including both genetic and disease-related variables, leads to an increased resorptive state and a subsequent reduction in bone mineral density (BMD) [Citation1]. The deregulated bone turnover remains evident regardless the treatment effects on haemoglobin levels and hormonal status [Citation2]; thus, there is a need for bone-specific agents.
Although bisphosphonates have been traditionally considered the mainstay of TDT-induced osteoporosis, there is accumulating evidence suggesting denosumab as a promising drug in the field [Citation3–5]. Denosumab is a fully human monoclonal antibody that strongly binds and inhibits receptor activator of nuclear factor kappa-B ligand (RANKL). Therefore, denosumab impairs the maturation of osteoclast precursors and prevents bone loss [Citation6]. It is currently used for the treatment of osteoporosis and bone disease both in benign and malignant disorders [Citation7–10]. Increased levels of RANKL have been also identified among thalassemia patients [Citation11]. In this context, we have evaluated the effect of denosumab on TDT-induced osteoporosis and we have found encouraging results pertaining to an improvement in BMD and a reduction in pain scores and markers of bone resorption [Citation5].
Noggin is a 64 kDa secreted homodimeric glycoprotein and is encoded by the NOG gene. It is an extracellular antagonist of bone morphogenetic proteins (BMPs) cascade; it selectively binds to BMP-2, -4, -5, -7, -13 and -14 and inhibits their interaction with BMP receptors and, subsequently, it prevents the downstream activation of BMP signalling pathway [Citation12]. Noggin is actively implicated in BMP-mediated morphogenesis of early developmental stages, as well as in ankylosing spondylitis and osteolytic bone metastases [Citation12–14]. Thus, noggin seems to have a profound impact on osteogenesis and may be influenced by anti-resorptive therapy.
Taking into consideration the need for further understanding the underlying pathophysiology in TDT-induced osteoporosis and the possible role of noggin in mediating changes in bone metabolism, we sought to evaluate noggin among patients with TDT and osteoporosis under denosumab therapy.
2. Methods
2.1. Study design
This is a post hoc analysis of a phase 2b clinical trial evaluating the efficacy and safety of denosumab among patients with TDT-induced osteoporosis (ClinicalTrials.gov identifier: NCT02559648) [Citation5]. This study was conducted at Thalassemia Reference Centre at Laiko General Hospital (Athens, Greece). The protocol was approved by the institutional review board and the independent ethics committee. The study was conducted according to the Declaration of Helsinki, International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use – Good Clinical Practice (ICH-GCP) guidelines for clinical trials, local rules and regulations of the country. All patients provided written informed consent before the inclusion in the study.
Study design, eligibility criteria, endpoints, randomisation and masking procedures, interventions, clinical and laboratory assessments have been previously described in detail [Citation5]. In brief, this was a double-blind, placebo-controlled, single-site, randomized phase 2b clinical trial. Patients diagnosed with TDT and BMD T-score between −2.5 and −4.0 in at least one of the three examined sites (wrist bone, lumbar spine L1-L4 or femoral neck) were eligible for participation in this study. We included only skeletally mature adults above 30 years of age. Upon enrolment in the study, patients were randomly assigned in a 1:1 fashion to receive either 60 mg denosumab or placebo administered subcutaneously (sc) every six months for a total of two doses [Citation5]. Measurement of BMD of three body sites (wrist, L1-L4, femoral neck) was performed using dual-energy X-ray absorptiometry before treatment and after 12 months. Assessment of bone remodelling indices including bone resorption markers (C-terminal crosslinking telopeptide of type I collagen and tartrate-resistant acid phosphatase isoform-5b), bone formation markers (bone-specific alkaline phosphatase and osteocalcin), osteoclast regulators [sRANKL and osteoprotegerin (OPG)] and osteoblast inhibitors (dickkopf-1 and sclerostin) was made with commercially available enzyme-linked immunosorbent assays on predefined time points [Citation5]. Serum noggin was measured at baseline and after 12 months. The time points of assessments were based on our previous work in the field [Citation4,Citation15,Citation16].
2.2. Measurement of serum noggin levels
Patient serum samples were sent for noggin measurement to the laboratory of FIANOSTICS GmbH, Wiener Neustadt, Austria. Serum noggin was measured using a recently developed high sensitivity fluorescent immunoassay based on plasmonic microtiter plates that increase the signal of fluorescent dyes several hundred-fold. This assay detects free serum human noggin, which is not bound to BMPs and, thus, it is bioactive [Citation17]. This novel method has been recently described and, herein, it is presented in brief [Citation17]. The assay protocol included adsorptive coating of capture antibody in 50 mM phosphate buffer (PBS) / 150 mM NaCl pH 7.4, over-night at 4°C followed by washing with PBS containing 0.1% Triton X-100. Prevention of unspecific binding was achieved using a proprietary solution of FIANOSTICS containing synthetic polymers and mercapto-compounds. After another washing step, 20 μl duplicates of standards/samples (serum) together with 25 μl of anti-human noggin antibody labeled with AlexaFluor680 were incubated over night at room temperature in the dark. A standard fluorescence micro-plate reader was used for the measurements. Importantly, samples reading above 100 pmol/l noggin were diluted with assay buffer and re-run to check for linearity of the signal [Citation17].
2.3. Statistical analysis
Descriptive analysis was performed for describing patient and treatment profile. Univariate analyses were performed using chi-square for categorical variables and Wilcoxon test for continuous variables. Analyses of efficacy were based on the intention-to-treat principle. Correlation between two variables was assessed with Spearman rank correlation. All p-values were two-sided, the level of significance was equal to .05, and confidence intervals referred to 95% boundaries. Statistical analyses were performed using the SAS/STAT statistical package. Details on clinical trial statistical analysis have been previously described [Citation5].
3. Results
3.1. Patient characteristics
As previously reported [Citation5], sixty-three patients with TDT-induced osteoporosis were included in the present study. Patients were randomized and received either 60 mg denosumab (n = 32) or placebo (n = 31), sc, every 6 months for 12 months for a total of 2 doses. Between the two groups, no statistically significant differences were observed regarding baseline clinical and laboratory characteristics, except for a lower value of alkaline phosphatase in the placebo group (p = 0.013) and a higher value of tartrate-resistant acid phosphatase isoform-5b in the denosumab group (p = 0.026) () [Citation5].
Table 1. Baseline clinical and laboratory patient characteristics along with markers of bone turnover [Citation5].
3.2. Changes in BMD and markers of bone remodelling
At 12 months, as compared with baseline, the percentage (mean ± SD) increase of lumbar spine BMD was 5.92% ± 5.25% in the denosumab and 2.92% ± 5.56% in the placebo group (p = 0.043). Thus, the primary study endpoint was achieved. Furthermore, a significantly smaller decrease in wrist bone BMD was observed in the denosumab compared with the placebo group (−0.26% ± 5.31% and −3.92% ± 8.71%, respectively; p = 0.035). Detailed results are provided elsewhere [Citation5].
Regarding bone remodeling indices, patients of denosumab group A demonstrated a decrease in serum RANKL, serum RANKL / OPG ratio, C-terminal crosslinking telopeptide of type I collagen, tartrate-resistant acid phosphatase isoform-5b, bone-specific alkaline phosphatase between baseline and after 12 months (p < 0.01 for all comparisons), while there were no changes in dickkopf-1, SOST and osteocalcin () [Citation5]. Conversely, patients of placebo group showed an increase in serum RANKL, OPG, dickkopf-1, C-terminal crosslinking telopeptide of type I collagen, tartrate-resistant acid phosphatase isoform-5b, bone-specific alkaline phosphatase levels (p < 0.01 for all comparisons) and a slight increase of sclerostin and osteocalcin () [Citation5].
Table 2. Markers of bone turnover and pain score values in the DNM and placebo group at baseline compared to post DNM/placebo administration. Values are expressed as median (range) [Citation5].
3.3. Changes in serum noggin levels
Both groups showed an increase in noggin serum levels after 12 months compared to baseline. In the denosumab group the values (mean ± SD) were 44.2 ± 112.4 and 19.4 ± 49.1, p < 0.001, respectively, whereas in the placebo group the corresponding values were 120.3 ± 478.0 and 12.2 ± 22.1, p < 0.0001, respectively. It becomes evident that the increase was higher in the placebo group.
Importantly, we noted a strong correlation between serum noggin levels and wrist BMD. Noggin levels were negatively associated with BMD values (r = −0.641, p = 0.002) (). However, this finding was not reproduced among patients in the placebo group.
4. Discussion
To the best of our knowledge, this is the first study to evaluate noggin in patients with thalassemia. During the study period, we observed a significant increase in serum levels of noggin among patients both in the denosumab and the placebo groups; however, it was more pronounced in the latter group. Thus, we may postulate that noggin actively participates in the multifactorial process of bone disease in thalassemia and it is upregulated by pathologic factors acting in thalassemic osteopathy. Denosumab seems to counteract this effect. Noggin levels were also inversely correlated with BMD among patients receiving denosumab. Taking into consideration that our assay detects free, bioactive, serum noggin [Citation17], higher noggin levels reflect a stronger BMP inhibition, which subsequently led to less bone formation among patients in the placebo group. Thus, it could be postulated that the favourable effect of denosumab on BMD, as compared with placebo, may be partially attributed to relevant changes in noggin levels. Interestingly, this may reflect a novel mechanism of action for denosumab that has to be further elucidated. We should also underline that the increase in BMD in the denosumab compared with the placebo group could be also explained by the favourable effect of denosumab in bone formation and the reduction of bone resorption as reflected by the decreased sRANKL/OPG ratio and the fact that markers of osteoblast inhibition (sclerostin and DKK-1) did not increase significantly, differing from the placebo group.
BMPs have been demonstrated to play a key role in bone metabolism, whereas they are also implicated in the regulation of hepcidin expression in preclinical thalassemia models [Citation18,Citation19]. BMPs act through their receptors located on the osteoblasts, regulate intracellular signaling cascades such as the WNT/β-catenin pathway, and have an ultimate effect on RANKL / OPG ratio [Citation20]. Therefore, BMPs orchestrate the interactions between osteoblasts and osteoclasts and favour bone turnover. Furthermore, the downstream effectors of BMP signaling increase the expression of transcription factors that promote osteogenesis and osteoblast-related genes and, thus, they promote osteoblast differentiation [Citation20]. Overall, unopposed BMP action promotes bone formation over bone resorption during bone turnover cycles. Thus, deregulation of BMPs inhibits osteoblast function and results in impaired bone physiology leading to osteoporosis [Citation20,Citation21].
Among the inhibitors of BMP signaling, noggin binds to BMPs in the extracellular space, antagonizes their interaction to BMP receptors and prevents BMP pathway activation [Citation12]. Noggin is essential for skeletal and joint development, as it has been evident from preclinical mutational and knock-out studies [Citation21]. Under physiological circumstances, there is a negative retrograde circuit between BMPs and noggin; upregulated BMPs induce noggin expression which in turn downregulates BMPs signaling cascade. This effect protects osteoblasts from excessive BMP exposure and contributes to the homeostasis of the fine tuned bone turnover [Citation22].
Furthermore, there is accumulating evidence suggesting an important role of noggin in osteoporosis. Transgenic mice that overexpress noggin have shown a significant reduction of BMD values and increased incidence of fractures [Citation23,Citation24]. The volume of trabecular bone and trabecular number was also decreased [Citation23,Citation24]. Although Wu et al. demonstrated a suspension of osteoblast differentiation and a decrease in osteoblast colonies [Citation24], Devlin et al. found no significant changes in osteoblast number and surface; nevertheless, the noggin-mediated effect was restricted to deregulated osteoblastic function [Citation23]. In both studies, the net effect of noggin overexpression was the inhibition of bone formation leading to bone loss. In another original study, Moffett et al. assessed the possible impact of single nucleotide polymorphisms in the noggin-encoding gene (NOG gene) in humans. No significant correlations emerged between the gene variants under investigation and BMD among Afro-Caribbean men older than 39 years [Citation25]. Interestingly, variable results have been also shown across different studies on BMP gene polymorphisms and potential associations with osteoporosis indices. These have been mainly attributed to ethnic-related genetic differences [Citation20]. Therefore, it would be tempting to conduct future studies evaluating noggin polymorphisms in other ethnicity groups.
Another extracellular BMP antagonist, gremlin, has been also implicated in osteoporosis. There have been described specific genotypes associated with decreased BMD and increased risk of osteopenia and osteoporosis [Citation26,Citation27]. Thus, its evaluation among thalassemia patients with osteoporosis would be of interest for future studies.
Interestingly, data from malignant diseases support the robust effect of noggin on bone metabolism. Noggin may determine the osteoblastic or osteolytic nature of bone metastases in prostate cancer preclinical models; whereas changes in noggin levels result in inverse alterations in bone formation by osteoblasts [Citation14,Citation28]. Thus, noggin is a promising drug target candidate, taking also into consideration the implication of BMP signaling in bone-infiltrating solid and hematological malignancies [Citation29,Citation30].
Due to the cardinal role of BMPs in osteoporosis and other bone disorders, pharmacological interference in the BMP signaling pathway would have profound therapeutic implications. Induction of bone formation through the activation of BMP cascade could be mediated by interventions at the extra- or intra-cellular level or at the BMP receptors [Citation31]. Aforementioned preclinical in vitro and in vivo data support noggin as a druggable target by small molecules, neutralising monoclonal antibodies, RNA interference or gene therapy [Citation32]. It is notable that silencing of NOG gene through siRNA interference resulted in enhanced bone formation in a preclinical model of bone repair [Citation33]. Interestingly, interventions aiming at interactions between extracellular matrix and noggin may regulate the bioavailability and functionality of the latter [Citation34]. Further research is deemed necessary in this field.
The absence of adequately sensitive techniques for noggin evaluation has necessitated the advent of alternative approaches. One of the main strengths of our study pertains to the direct fluorescence immunoassay based on metal enhanced fluorescence that was used for the determination of noggin levels. In contrast to noggin assessment with the conventional Enzyme Linked Immuno Sorbent Assay (ELISA) methodology, the novel assay minimises the background effect and produces a constant signal over time that is not degenerated. In this context, its sensitivity has been shown to be up to 300 fold higher than ELISA [Citation17]. Thus, our technique is highly sensitive and specific for free serum noggin detection and can provide valuable results.
Our study has some limitations that should be considered in the interpretation of the results. We conducted a post hoc analysis; noggin evaluation was not originally included in the primary or secondary endpoints of the clinical trial, thus, study design including sample size might not have been optimal [Citation35]. However, it is important that significant associations emerged. The baseline differences regarding tartrate-resistant acid phosphatase and alkaline phosphatase levels between the two groups may reflect dissimilar bone turnover, which should be taken also into consideration when drawing generalised conclusions. Furthermore, although our novel implemented assay present many advantages compared with ELISA, it has not been extensively validated with a gold standard method such as mass spectrometry. In addition to the above, evaluation of supplementary osteoporosis indices and at subsequent time points and accounting for potential confounders might have provided more robust results [Citation36,Citation37]. It would have been also of value to simultaneously assess BMP levels and detect possible correlations in order to provide a more integrated overview of denosumab effects on the signaling pathway.
In conclusion, our post hoc analysis revealed a favourable effect of denosumab on noggin levels and bone formation among patients with TDT and osteoporosis, whereas changes in noggin levels could determine alterations in BMD. Further mechanistic studies are deemed necessary in order to elucidate the interplay among the molecular pathways regulating bone turnover in this setting.
Acknowledgements
We thank all patients and their families for participation in the study.
Disclosure statement
G. Hawa, L. Sonnleitner and A. Missbichler are employees of FIANOSTICS GmbH, Wiener Neustadt, Austria. All other authors report no potential conflict of interests.
References
- De Sanctis V, Soliman AT, Elsefdy H, et al. Bone disease in β thalassemia patients: past, present and future perspectives. Metab Clin Exp. 2018;80:66–79. DOI:10.1016/j.metabol.2017.09.012. PubMed PMID: 28987275.
- Baldini M, Forti S, Marcon A, et al. Endocrine and bone disease in appropriately treated adult patients with beta-thalassemia major. Ann Hematol. 2010;89(12):1207–1213. DOI:10.1007/s00277-010-1007-0. PubMed PMID: 20582415.
- Voskaridou E, Terpos E, Spina G, et al. Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol. 2003;123(4):730–737. PubMed PMID: 14616979. doi: 10.1046/j.1365-2141.2003.04657.x
- Voskaridou E, Anagnostopoulos A, Konstantopoulos K, et al. Zoledronic acid for the treatment of osteoporosis in patients with beta-thalassemia: results from a single-center, randomized, placebo-controlled trial. Haematologica. 2006;91(9):1193–1202. PubMed PMID: 16956818.
- Voskaridou E, Ntanasis-Stathopoulos I, Papaefstathiou A, et al. Denosumab in transfusion-dependent thalassemia osteoporosis: a randomized, placebo-controlled, double-blind, phase 2b clinical trial. Blood Adv. 2018;2(21):2837–2847. PubMed PMID: 30381400. doi: 10.1182/bloodadvances.2018023085
- Anastasilakis AD, Toulis KA, Polyzos SA, et al. RANKL inhibition for the management of patients with benign metabolic bone disorders. Expert Opin Investig Drugs. 2009;18(8):1085–1102. DOI:10.1517/13543780903048929. PubMed PMID: 19558335.
- Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporos Int. 2015;26(12):2773–2783. DOI:10.1007/s00198-015-3234-7. PubMed PMID: 26202488; PubMed Central PMCID: PMCPMC4656716.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. DOI:10.1200/JCO.2010.29.7101. PubMed PMID: 21060033.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. DOI:10.1016/S0140-6736(10)62344-6. PubMed PMID: 21353695; PubMed Central PMCID: PMCPMC3090685.
- Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. DOI:10.1016/S1470-2045(18)30072-X. PubMed PMID: 29429912.
- Voskaridou E, Stoupa E, Antoniadou L, et al. Osteoporosis and osteosclerosis in sickle cell/beta-thalassemia: the role of the RANKL/osteoprotegerin axis. Haematologica. 2006;91(6):813–816. PubMed PMID: 16704959.
- Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43(4):478–481. DOI:10.1016/j.biocel.2011.01.007. PubMed PMID: 21256973.
- Xie Z, Wang P, Li Y, et al. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 2016;68(2):430–440. DOI:10.1002/art.39433. PubMed PMID: 26413886.
- Secondini C, Wetterwald A, Schwaninger R, et al. The role of the BMP signaling antagonist noggin in the development of prostate cancer osteolytic bone metastasis. PLoS One. 2011;6(1):e16078. DOI:10.1371/journal.pone.0016078. PubMed PMID: 21249149; PubMed Central PMCID: PMCPMC3020964.
- Voskaridou E, Christoulas D, Plata E, et al. High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res. 2012;44(12):909–913. DOI:10.1055/s-0032-1312618. PubMed PMID: 22581647.
- Voskaridou E, Christoulas D, Xirakia C, et al. Serum dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis. reduction post-zoledronic acid administration. Haematologica. 2009;94(5):725–728. DOI:10.3324/haema-tol.2008.000893. PubMed PMID: 19407319; PubMed Central PMCID: PMCPMC2675686. doi: 10.3324/haematol.2008.000893
- Hawa G, Sonnleitner L, Missbichler A, et al. Single step, direct fluorescence immunoassays based on metal enhanced fluorescence (MEF-FIA) applicable as micro plate-, array-, multiplexing- or point of care-format. Anal Biochem. 2018;549:39–44. DOI:10.1016/j.ab.2018.03.002. PubMed PMID: 29518350.
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24(2):218–235. DOI:10.1210/er.2002-0023. PubMed PMID: 12700180.
- Parrow NL, Gardenghi S, Ramos P, et al. Decreased hepcidin expression in murine beta-thalassemia is associated with suppression of Bmp/Smad signaling. Blood. 2012;119(13):3187–3189. DOI:10.1182/blood-2012-01-405563. PubMed PMID: 22461476.
- Biver E, Hardouin P, Caverzasio J. The “bone morphogenic proteins” pathways in bone and joint diseases: translational perspectives from physiopathology to therapeutic targets. Cytokine Growth Factor Rev. 2013;24(1):69–81. DOI:10.1016/j.cytogfr.2012.06.003. PubMed PMID: 22749766.
- Bandyopadhyay A, Yadav PS, Prashar P. BMP signaling in development and diseases: a pharmacological perspective. Biochem Pharmacol. 2013;85(7):857–864. DOI:10.1016/j.bcp.2013.01.004. PubMed PMID: 23333766.
- Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102(12):2106–2114. DOI:10.1172/JCI3459. PubMed PMID: 9854046; PubMed Central PMCID: PMCPMC509165.
- Devlin RD, Du Z, Pereira RC, et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144(5):1972–1978. DOI:10.1210/en.2002-220918. PubMed PMID: 12697704.
- Wu XB, Li Y, Schneider A, et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112(6):924–934. DOI:10.1172/JCI15543. PubMed PMID: 12975477; PubMed Central PMCID: PMCPMC193662.
- Moffett SP, Dillon KA, Yerges LM, et al. Identification and association analysis of single nucleotide polymorphisms in the human noggin (NOG) gene and osteoporosis phenotypes. Bone. 2009;44(5):999–1002. DOI:10.1016/j.bone.2008.12.024. PubMed PMID: 19167531.
- Cheung CL, Lau KS, Sham PC, et al. Genetic variants in GREM2 are associated with bone mineral density in a southern Chinese population. J Clin Endocrinol Metab. 2013;98(9):E1557–E1561. DOI:10.1210/jc.2013-1983. PubMed PMID: 23902946.
- Kaminski A, Bogacz A, Uzar I, et al. Association betweenGREM2 gene polymorphism with osteoporosis and osteopenia in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2018;228:238–242. DOI:10.1016/j.ejogrb.2018.07.009. PubMed PMID: 30014930.
- Schwaninger R, Rentsch CA, Wetterwald A, et al. Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am J Pathol. 2007;170(1):160–175. DOI:10.2353/ajpath.2007.051276. PubMed PMID: 17200191; PubMed Central PMCID: PMCPMC1762703.
- Buijs JT, Petersen M, van der Horst G, et al. Bone morphogenetic proteins and its receptors; therapeutic targets in cancer progression and bone metastasis? Curr Pharm Des. 2010;16(11):1291–1300. PubMed PMID: 20166979. doi: 10.2174/138161210791033987
- Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8(1):7. DOI:10.1038/s41408-017-0037-4. PubMed PMID: 29330358; PubMed Central PMCID: PMCPMC5802524.
- Lowery JW, Rosen V. Bone morphogenetic protein-based therapeutic approaches. Cold Spring Harb Perspect Biol. 2018;10(4):a022327. DOI:10.1101/cshperspect.a022327. PubMed PMID: 28389444.
- Tsialogiannis E, Polyzois I, Oak Tang Q, et al. Targeting bone morphogenetic protein antagonists: in vitro and in vivo evidence of their role in bone metabolism. Expert Opin Ther Targets. 2009;13(1):123–137. DOI:10.1517/14728220802637725. PubMed PMID: 19063711.
- Takayama K, Suzuki A, Manaka T, et al. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab. 2009;27(4):402–411. DOI:10.1007/s00774-009-0054-x. PubMed PMID: 19252814.
- Paine-Saunders S, Viviano BL, Economides AN, et al. Heparan sulfate proteoglycans retain noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277(3):2089–2096. DOI:10.1074/jbc.M109151200. PubMed PMID: 11706034.
- Srinivas TR, Ho B, Kang J, et al. Post hoc analyses: after the facts. Transplantation. 2015;99(1):17–20. DOI:10.1097/TP.0000000000000581. PubMed PMID: 25525920.
- Seeman E, Delmas PD. Bone quality–the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–2261. DOI:10.1056/NEJMra053077. PubMed PMID: 16723616.
- Cefalu CA. Is bone mineral density predictive of fracture risk reduction? Curr Med Res Opin. 2004;20(3):341–349. DOI:10.1185/030079903125003062. PubMed PMID: 15025843.