ABSTRACT
Object: To explore the critical role of growth differentiation factor 11 (GDF11) in the pathobiology of aplastic anemia (AA). Methods: We have examined the serum GDF11 levels for 79 AA patients and 30 healthy controls. A total of 79 AA patients, which included 29 new diagnosed (untreated) cases, 14 cases with no response, 21 partial remission (PR) cases and 15 complete remission (CR) cases after immunosuppressive therapy (IST). GDF11 serum levels were assessed by an enzyme-linked immunosorbent assay. GDF11 mRNA expression in peripheral blood mononuclear (PBMNC) was detected through real time polymerase chain reaction. The correlation between GDF11 expression and erythropoietic function was evaluated. Results: The serum GDF11 levels in untreated AA patients were higher than that of the control group. The serum GDF11 levels of PR patients or CR patients after IST was decreased, compared with untreated patients, but did not recover back to the normal levels. GDF11 levels had a negative correlation with hemoglobin (Hb) levels and reticulocyte counts in AA patients. GDF11 levels did not correlate with age, sex and severity of in AA patients. Conclusion: Serum GDF11 levels were increased and negatively correlated with Hb levels and reticulocyte counts in AA patients. This suggests an impaired GDF11 response contributing to anemia in AA patients.
Introduction
Aplastic anemia (AA) is a class of disease characterized by bone marrow failure and pancytopenia. The clinical symptoms of AA patients are fatal anemia, bleeding, and infection; it is often associated with a significantly high mortality rate if left untreated [Citation1]. In the majority of cases, AA biologically behaves as an autoimmune-mediated disease. An immune response dominated by expanded cytotoxic T-cells targets hematopoietic stem (HSC) and progenitor cells (HPC), which induce HSC/HPC apoptosis [Citation2,Citation3]. Now AA can be successfully treated with either immunosuppressive therapy (IST) or hematopoietic stem cell transplantation (HSCT) [Citation4].
In hematopoiesis, cytokines are essential for the viability, proliferation and differentiation of HSC; which include granulocyte colony-stimulating factor (G-CSF), granulocyte, macrophage colony-stimulating factor (GM-CSF) and stem cell factor (SCF) [Citation5]. Erythropoiesis is a multistep process by which erythroid precursor cells proliferate and differentiate into mature red blood cells (RBCs). During the early stages of erythropoiesis, erythropoietin (EPO) regulates hematopoietic progenitor cells to differentiate into proerythroblasts [Citation6].
Growth differentiation factor 11 (GDF11) belongs to the transforming growth factor- β (TGF-β) superfamily, that also called bone morphogenetic protein 11(BMP11).
Meanwhile, the TGF-β superfamily has been shown to be negative modulators of late-stage erythropoiesis. More than 40 members of this superfamily are recognized in mammals and divided into several subcategories, including TGF-βs, GDFs, bone morphogenic proteins et al. Among them, GDF11 and GDF8 are the known major cytokines regulating erythropoiesis [Citation7].
In this study, we detected GDF11 expression in the peripheral blood of AA patients, and investigated the correlation with erythropoiesis.
Materials and methods
Patient selection
Seventy-nine patients with AA diagnosed in our center were enrolled in this study from September 2014 to June 2017, including 29 newly diagnosed patients (untreated) and 50 post- IST patients. There were 48 males and 31 females, and the median age was 31 (range, 12–74) years. According to the International AA Study Group Criteria [Citation8], the disease was considered severe if at least two of the following parameters were met: Neutrophil count <0.5 × 109/L, platelet count <20 × 109/L and reticulocyte count <20 × 109/L with hypocellular bone marrow. Very severe aplastic anemia (VSAA) was diagnosed in cases of SAA patients with a neutrophil count <0.2 × 109/L. Patients presenting with congenital AA (with chromosome fragility test) were excluded from the study. All patients were screened for paroxysmal nocturnal hemoglobinuria using anti-CD55 and anti-CD59 antibodies through the use of flow cytometry. This study included 19 patients with VSAA, 32 with SAA and 28 non severe aplastic anemia (NSAA). A complete response (CR) was defined when all of the following three criteria have been met: neutrophils counts >2.0 × 109/L; hemoglobin >110 g /L; and platelet counts >100 × 109/L. A partial response (PR) was defined as an improvement in blood counts that no longer satisfy the criteria for SAA, but are not sufficient enough to meet criteria for CR [Citation9]. The remission patients included both CR and PR patients. All AA patients had normal renal function. The characteristics of the patients with AA are shown in . Thirty healthy blood donors were selected as controls; this included 19 men and 11 women, with a median age of 29.5 (range, 18-77) years. This study was approved by the Ethics Committee of our school. Informed written consent was obtained from all patients or their guardians in accordance with the Declaration of Helsinki.
Table 1. The characteristics of patients with aplastic anemia (AA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of serum GDF11 were measured using a human ELISA kit (Cloud-Clone USCN, USA). The level of serum EPO was measured using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA). According to the manufacturer’s instructions, the samples were measured and read with BioTek ELx800 microplate reader (BioTek Corporation, USA) at a wavelength of 450 nm.
Real-time quantitative transcriptase-polymerase chain reaction (Q-PCR)
Peripheral blood mononuclear cells (PBMNCs) were separated from 2 mL fresh heparinized blood samples from both the AA patients and the controls. Total RNA of PBMNCs was extracted using TRIzol reagent (Invitrogen Life Technologies, USA). The reverse transcription reactions to cDNA were performed using the QIAGEN OneStep RT–PCR Kit (QIAGEN, Germany). The gene expressions were quantified by Q-PCR (SYBR Green, ABI PRISM-7500 Sequence Detection system, USA). The primer sequences were as follows: GAPDH forward 5′-GCA CCG TCT AGG CTG AGA TG-3′, reverse 5′-TGG TGA AGA CGC CAG TGG T-3′; GDF11 forward 5′-TGC GCC TAG AGA GCA TCA AGT-3′,reverse 5′-CCC AGT TAG GGG TTT CAG TCG T-3′. The relative quantification (RQ) of gene expression used 2−ΔΔCt method (ΔΔCt = (Ct target gene-Ct GAPDH) patients-(Ct target gene-Ct GAPDH)control).
Collection of clinical data
The complete blood counts (CBC) of the patients and controls were collected, including hemoglobin (Hb) level, RBC counts, reticulocytes (Ret), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular-hemoglobin concentration (MCHC), white blood cells (WBC, including neutrophils, lymphocytes and monocytes) and platelet.
Statistical analysis
All analyses were performed using SPSS Version 22.0 software (SPSS Science). Data was presented as mean ± SD. The Student t test was used for two independent groups. For more than two independent groups, One-way ANOVA was used. The Pearson correlation test was used for correlation analysis. A P value of <0.05 was considered as statistically significant.
Results
Serum GDF11 level was increased in AA patients
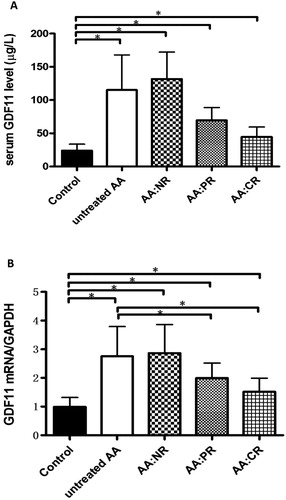
The serum GDF11 level of normal controls was 23.86 ± 1.79 μg/L. The GDF11 level of untreated AA patients was 115.0 ± 9.86 μg/L, which was higher than that of normal controls (P < 0.01), PR patients (n = 21, 69.56 ± 4.17 μg/L, P < 0.01) and CR patients (n = 15, 44.56 ± 3.82 μ/L, P < 0.01). The highest GDF11 levels were observed in 14 AA patients with no response (NR) after IST (131.8 ± 10.80 μg/L).
However, the GDF11 level decreased in remission patients but did not recover to the normal level. The GDF11 levels in PR patients and CR patients were higher than that of the controls (both P < 0.01). ((A))
Figure 1. (A) The levels of serum GDF11 in healthy controls, untreated AA patients, NR patients, PR patients and CR patients after IST. The serum GDF11 levels of all AA groups were significant higher than that of controls (all P < 0.01). GDF11 levels of AA patients in CR and PR were decreased compared with untreated AA patients (both P < 0.01). There was no significant difference found between untreated and NR group (P > 0.05). (B) The expression of GDF11 mRNA in untreated patients, NR patients, PR patients and CR patients after IST were significantly higher than that of controls (all P < 0.01). GDF11 mRNA expression of AA patients in CR and PR group were decreased compared with untreated patients (both P < 0.01). There was no significant difference between the untreated and NR group (P > 0.05). (*P < 0.01).
GDF11 mRNA was overexpressed in PBMNCs of AA patients
The relative expression of GDF11 mRNA in the control group was 0.99 ± 0.01. The expression of GDF11 mRNA in untreated patients, NR patients, PR patients and CR patients after IST were 2.75 ± 0.19, 2.87 ± 0.27, 2.00 ± 0.11 and 1.51 ± 0.12 respectively. The relative GDF11 mRNA expressions of all AA groups were significantly higher than that of controls (all P < 0.01). GDF11 mRNA expression of AA patients in CR and PR group was significantly decreased compared with untreated patients (both P < 0.01). But there was no significantly difference between the untreated and NR patients (P > 0.05) ((B))
GDF11 not correlated with age, sex and severity of in AA patients
The serum GDF11 level in male AA patients (86.82 ± 7.28 μg/L) had no significant difference from that in female patients (101.5 ± 8.58 μg/L, P > 0.05). We could not detect an age-related change in GDF11 serum levels (r = 0.2183, P = 0.053) in AA patients ((A)). The serum GDF11 levels in untreated patients with VSAA (n = 11), SAA (n = 9) and NSAA (n = 9) were 103.7 ± 16.18 μg/L, 110.7 ± 15.08 μg/L and 133.2 ±20.15 μg/L respectively. There was no significant difference between the three groups (all P > 0.05) ((B)).
Figure 2. (A) The serum GDF11 levels in male AA patients had no significant difference from that in female patients (P > 0.05).The GDF11 level was not correlated with age in AA patients (r = 0.2183, P > 0.05). (B) The serum GDF11 levels were tested in untreated patients with VSAA, SAA and NSAA. There was no significant difference between the three groups (all P > 0.05). (C) The serum GDF11 levels in male controls had no significant difference from that in female controls (P > 0.05). The GDF11 level was not correlated with age in the normal control groups (r = −0.3532, P > 0.05).
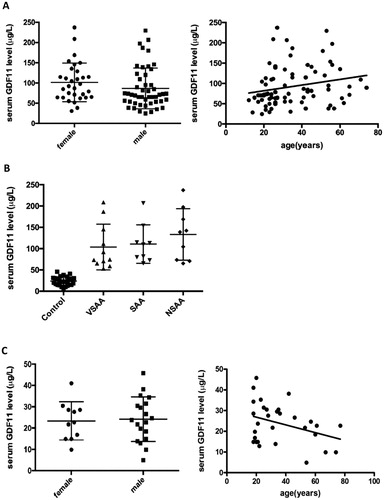
The serum GDF11 level in male healthy controls (24.15 ± 2.40 μg/L) had no significant difference from that in female controls (23.36 ± 2.71 μg/L, P > 0.05). We could not detect an age-related change in GDF11 serum levels (r = −0.3532, P = 0.0558) in normal controls ((C)).
GDF11 level was negatively correlated with Hb level and reticulocyte count, while was positively correlated with EPO level in AA patients
The GDF11 level was negatively correlated with Hb level in peripheral blood (r = −0.6906 (95% confidence interval −0.7910 to −0.5541), P < 0.0001) and with reticulocyte counts (r = −0.6487 (95% confidence interval −0.7608 to −0.4992), P < 0.0001) in AA patients ((A and B)).
Figure 3. (A) The GDF11 level was negatively correlated with Hb level in peripheral blood (r = −0.6906, P < 0.05) in AA patients. (B) The GDF11 level was negatively correlated with reticulocytes in peripheral blood (r = −0.6487, P < 0.05) in AA patients. (C) The serum EPO level in AA patients was higher than that of normal controls (P < 0.05). (D) The GDF11 level was positively correlated with EPO level in AA patients (r = 0.4016, P < 0.01).
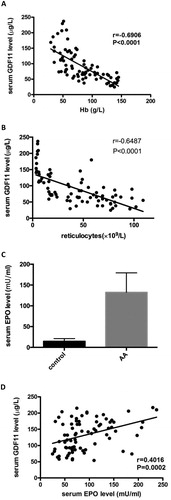
The serum EPO level in AA patients was 132.4 ± 5.27 mU/ml which higher than that of normal controls (14.63 ± 1.17 mU/ml, P < 0.05). The GDF11 level was positively correlated with EPO level in AA patients (r = 0.4016 (95% confidence interval 0.1980–0.5720), P = 0.0002) ((C and D)).
GDF11 is not a prognostic factor of response
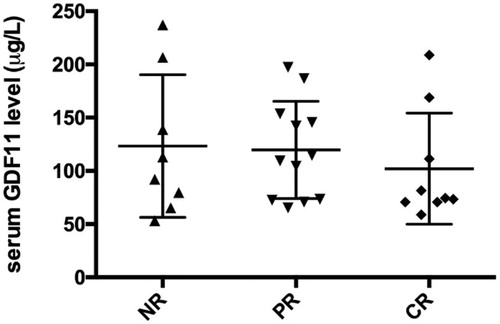
After 6 months, a follow-up was done, which showed that from 29 untreated AA patients, 9 cases achieved CR, 12 cases achieved PR and 8 cases had no response after IST. The pretreatment serum GDF11 levels in these patients with NR, PR and CR were 123.3 ± 23.67 μ/L, 119.7 ± 13.18 μ/L and 102.1 ± 17.39 μ/L respectively. There was no significant difference between the three groups (P > 0.05) ().
Discussion
Anemia is a common complication in hematopoietic diseases; including AA, myelodysplastic syndrome (MDS), thalassemia, iron deficiency, et al. Endogenous EPO and its receptor are critical for the proliferation and differentiation of erythroid progenitors during early-stage erythropoiesis. However, late-stage erythroid cells are not dependent on EPO. Anemia in MDS is characterized by excess apoptosis of premature RBCs and ineffective erythropoiesis with increased levels of endogenous EPO [Citation5,Citation10–13].
Previous studies have demonstrated that GDF11 is a modulator in late-stage erythropoiesis. Suragani et al. [Citation14] reported that GDF11-ActRIIB-Smad2/3 signaling plays a key role in regulating proliferation and differentiation of late-stage erythroid cells. GDF11 bonding with ActR IIA/IIB activates and links to a type I receptor, ALK4 or ALK5. This recombinant protein initiates phosphorylated Smad 2/3 which inhibits terminal erythroid differentiation. In anemia, increased GDF11 expression promotes proliferation of erythroid precursors and blocks erythroid maturation.
Our previous studies have showed that GDF11 might involve in the pathology of MDS. In MDS patients, high GDF11 levels in high-risk MDS patients was accompanied by a low levels of RBC, hemoglobin and HCT, which implies that GDF11 negatively correlated with late-stage erythropoiesis [Citation15]. Yamagishi et al [Citation16] found that serum GDF11 levels in hemodialysis (HD) patients were significantly higher than those in age-matched healthy controls. Dussiot et al. [Citation17] found that GDF11 expression was overexpressed in β-thalassemia and inhibits erythropoiesis.
In this study, our results showed that the serum GDF11 levels and GDF11 expression in PBMNCs in AA patients are significantly higher than those in normal controls. The levels of GDF11 were negatively correlated with hemoglobin levels. This indicates that the expression of GDF11 was associated with the differing degree of anemia. Therefore, we postulated that the overexpression of GDF11 might be involved in the pathogenesis of anemia in AA patients.
Several researchers have reported that GDF11 can be blocked by activin-receptor traps; including sotatercept ACE-011 and ACE-536. ACE-536 and its mouse equivalent RAP-536 are ligand trapping fusion proteins containing the extracellular domain of ActR IIB modified to reduce activin binding. ACE-536 increases the number of RBCs and Hb level by promoting maturation of late-stage erythroblasts [Citation14]. Both ACE-011 and its mouse equivalent RAP-011 are ligand traps made up of the extracellular domain of ActRIIA. ACE-011 treatment could increase the RBCs counts and MCH levels by decreasing the ineffective erythropoiesis [Citation18]. RAP-011 could treat anemic patients by decreasing the ineffective erythropoiesis, iron overload in thalassemic mice model. Besides, inactivation of GDF11 can induce apoptosis of immature erythroblasts through Fas-FasL pathway [Citation17]. Thus, GDF11 might be a novel target to treat anemia of AA.
GDF11 is also known as a rejuvenation factor in many other systems [Citation19]. However, different studies showed entirely different results. In mice models, some researchers found that the blood of young mice appears to bring new life to ageing organs in the brain and muscles [Citation20,Citation21]. Poggioli et al. [Citation22] showed circulating GDF11/8 levels decreases in line with the age. Meanwhile, Egerman et al. [Citation23] reported that the levels of GDF11 shows an age-related increase and inhibits myogenesis or muscle regeneration. In our study, we showed that GDF11 levels were not correlated with age gender or the severity of AA for both AA patients and controls. Further studies are required to explore the GDF11 function in different pathogenesis processes.
In conclusion, our data has showed that GDF11 levels are significantly increased in AA patients and are negatively correlated with Hb levels. This suggests an impaired GDF11 response contributing to anemia in AA. Treatments targeting GDF11 might help to improve anemia in patients with AA.
Acknowledgements
This project is partly supported by The National Natural Science Foundation of China (No. 81170472, 81400088, 81400085, 81570111, 81770118, 81700117), Application Bases and Advanced Technology Research Program of Tianjin (No. 14JCYBJC27200, 09JCYBJC11200). This project is partly supported by Project of Tianjin Education Committee (No. 20140118).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878
- Gargiulo L, Zaimoku Y, Scappini B, et al. Glycosylphosphatidylinositol-specific T cells, IFN-γ-producing T cells, and pathogenesis of idiopathic aplastic anemia. Blood. 2017;129:388–392. doi: 10.1182/blood-2016-09-740845
- Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76
- Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428–1436. doi: 10.1182/blood-2016-08-693481
- Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96:602–606. doi: 10.3324/haematol.2010.030536
- Liang R, Ghaffari S. Advances in understanding the mechanisms of erythropoiesis in homeostasis and disease. Br J Haematol. 2016;174:661–673. doi: 10.1111/bjh.14194
- Rochette L, Zeller M, Cottin Y, et al. Growth and differentiation factor 11 (GDF11): Functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol Ther. 2015;156:26–33. doi: 10.1016/j.pharmthera.2015.10.006
- Marsh JC, Ball SE, Cavenagh J, et al. British Committee for Standards in Haematology. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x
- Di Bona E, Rodeghiero F, Bruno B, et al. Rabbit antithymocyte globulin (r-ATG) plus cyclosporine and granulocyte colony stimulating factor is an effective treatment for aplastic anaemia patients unresponsive to a first course of intensive immunosuppressive therapy. Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Br J Haematol. 1999;107:330–334. doi: 10.1046/j.1365-2141.1999.01693.x
- Hattangadi SM, Wong P, Zhang L, et al. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–6268. doi: 10.1182/blood-2011-07-356006
- Grover A, Mancini E, Moore S, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J Exp Med. 2014;211:181–188. doi: 10.1084/jem.20131189
- Wu H, Liu X, Jaenisch R, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1
- Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nat Med. 2015;21:221–230. doi: 10.1038/nm.3814
- Suragani RN, Cadena SM, Cawley SM, et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late- stage erythropoiesis. Nat Med. 2014;20:408–414. doi: 10.1038/nm.3512
- Han Y, Zhang GC, Wang HQ, et al. GDF11 is increased in patients with myelodysplastic syndrome. Int J Clin Exp Pathol. 2016;9:6031–6038.
- Yamagishi S, Matsui T, Kurokawa Y, et al. Serum levels of growth differentiation factor 11 are Independently associated with low hemoglobin values in hemodialysis patients. Biores Open Access. 2016;5:155–158. doi: 10.1089/biores.2016.0015
- Dussiot M, Maciel TT, Fricot A, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat Med. 2014;20:398–407. doi: 10.1038/nm.3468
- Iancu-Rubin C, Mosoyan G, Wang J, et al. Stromal cell- mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol. 2013;41:155–166. doi: 10.1016/j.exphem.2012.12.002
- Laviano A. Young blood. N Engl J Med. 2014;371:573–575. doi: 10.1056/NEJMcibr1407158
- Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141
- Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152
- Poggioli T, Vujic A, Yang P, et al. Circulating growth differentiation factor 11/8 levels decline with age. Circ Res. 2016;118:29–37. doi: 10.1161/CIRCRESAHA.115.307521
- Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits Skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010