ABSTRACT
Objectives
The incidence of febrile neutropenia (FN) in acute leukemia patients following induction or consolidation chemotherapy is high. Several clinical practice guidelines recommend the use of a fluoroquinolone prophylaxis to prevent bacterial infection in patients being prone to prolonged profound neutropenia.
Methods
This systematic review and meta-analysis aimed to investigate the efficacy and complications of levofloxacin as a prophylaxis for FN patients following chemotherapy for acute leukemia. Two databases from MEDLINE and EMBASE were searched for published studies indexed before 10 July 2018.
Results
A total of 862 acute leukemia patients were included, with 356 in the levofloxacin prophylaxis arm and 506 in the no-prophylaxis arm. Patients receiving levofloxacin had a significantly lower FN rate than patients who did not receive the antibiotic prophylaxis (odds ratio [OR]: 0.43, 95% confidence interval [CI]: 0.32–0.58, p < .00001, I2 = 0%). The rate of microbiologically documented infection in the no-prophylaxis group was higher than that for the levofloxacin prophylaxis group (OR: 0.45, 95% CI: 0.34–0.60, p < .00001, I2 = 0%). The bacteremia rate in the levofloxacin prophylaxis group was significantly lower than that for the no-prophylaxis group (OR: 0.45, 95% CI: 0.31–0.66, p < .00001, I2 = 0%). However, the mortality rates of the two groups were quite similar between the two groups (OR: 0.67, 95% CI: 0.34–1.33, p = .26, I2 = 0%).
Conclusions
Although the levofloxacin prophylaxis for the acute leukemia patients receiving intensive chemotherapy showed advantages for infectious complications, it did not affect mortality.
Introduction
Febrile neutropenia (FN) in acute leukemia patients after receiving an induction or consolidation chemotherapy course is a matter of major concern. The incidence of FN in acute myeloid leukemia (AML) patients following such chemotherapy has been reported to range from 60% to 80%. The figures have also been high in acute lymphoblastic leukemia (ALL) patients, with reported FN rates of 40–60% [Citation1–4]. Several studies revealed that the use of ciprofloxacin, a particular fluoroquinolone prophylaxis, in patients with various types of hematologic malignancies decreased the FN rate and reduced the rate of blood stream infections [Citation5–7]. Furthermore, the clinical practice guidelines of the American Society of Clinical Oncology and the Infectious Diseases Society of America Clinical Practice Guidelines recommended (graded B-I) that an antibiotic prophylaxis with fluoroquinolone, either ciprofloxacin or levofloxacin, should be prescribed for patients who are prone to prolonged profound neutropenia (i.e. an absolute neutrophil count [ANC] of less than 0.1 × 109 neutrophils/l for 7 days) [Citation8,Citation9]. Levofloxacin is preferable to ciprofloxacin in settings of high-risk for viridans-group streptococcal infections related to oral mucositis [Citation9]. On the other hand, there have been some drawbacks in patients who received a fluoroquinolone prophylaxis, such as an increasing quinolone resistance rate in gram-negative bacilli bloodstream infections; emerging quinolone-resistant, viridans-group streptococcal infections in the oropharynx; and an increasing rate of Clostridioides difficile enterocolitis [Citation10–13]. The current systematic review and meta-analysis aimed to focus on the available studies and summarize their results in order to compare the efficacy and complications of the use of a levofloxacin prophylaxis and no-antibiotic prophylaxis during induction or consolidation chemotherapy in AML and ALL patients.
Methods
Data sources and searches
Two databases from MEDLINE and EMBASE were independently searched for published studies indexed before 10 July 2018 by two investigators (W.O. and M.C.). The search terms, which consisted of levofloxacin, neutropenia, prophylaxis, acute myeloid leukemia, and acute lymphoblastic leukemia, is available in Supplementary Data 1. The references in the included studies were examined to identify additional eligible studies. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was the reference for the performance of the meta-analysis (Supplementary Data 2) [Citation14].
Selection criteria and data extraction
To be eligible for inclusion in the meta-analysis, articles need to be randomized controlled or cohort studies (either prospective or retrospective) comparing the efficacy and complications of a levofloxacin prophylaxis and a no-antibiotic prophylaxis for either induction, consolidation, or salvage chemotherapy in AML or ALL patients. Our primary outcome of interest, which was the rate of FN after chemotherapy, needed to be described in the included studies. Our secondary outcomes of interest were also collected for analysis, but they were not part of the inclusion criteria. Those outcomes comprised the rate of microbiologically documented infection, bacteremia rate, rate of C. difficile infection, and death rate of admitted patients. Two investigators assessed each eligible study independently. When different assessments of the eligibility of included studies were made, the investigators jointly reviewed the studies concerned and reached the final decisions by consensus.
Outcome Definition
FN was defined as either a single oral temperature ≥38.3°C (101°F) or a temperature ≥ 38°C (100.4°F) over 1 h; the temperature must be accompanied by either an ANC of <0.5 × 109 neutrophils/l or an ANC of <1 × 109 neutrophils/l, the latter being predicted to decline to 0.5 × 109 neutrophils/l over the next 48 h [Citation9].
Quality assessment
The Jadad quality assessment scale was used to evaluate the quality of included studies which were randomized controlled studies [Citation15]. The Newcastle–Ottawa Scale, which is a 3-item scoring system examining the selection of the participants, the comparability of the groups, and the outcomes of interest for cohort studies, was used to assess the quality of the nonrandomized studies [Citation16].
Statistical analysis
All statistical analyses were calculated using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). The Mantel–Haenszel method was employed to analyze the effect estimates and the 95% confidence intervals (CI) of each included study, and to pool the data [Citation17]. Cochran’s Q test was performed and quantified, using the I2 statistic to evaluate the statistical heterogeneity among the included studies. The I2-values were classified as follows: no-, low-, moderate-, and high-heterogeneity, with the figures of ≤25%, 26–50%, 51–75%, and >75%, respectively [Citation18]. The random-effects model was used instead of the fixed-effects model because of the high likelihood of between-study heterogeneity. Funnel plots and Egger’s test were not used to assess publication bias due to the small number of included studies (less than 10). P-values less than 0.05 were considered statistically significant.
Results
From the search strategy, 547 potentially relevant studies were identified (28 from MEDLINE, and 519 from EMBASE). After excluding 25 duplicate articles, 522 articles were evaluated for relevance via a title and abstract review. Of those, 512 were eliminated. The exclusion criteria were: (1) reviews or meta-analyses, commentaries, editorials, or conference abstracts; (2) reports irrelevant to AML or ALL; (3) reports irrelevant to induction, consolidation, or salvage chemotherapy; (4) reports irrelevant to a comparison of a levofloxacin prophylaxis and a no-antibiotic prophylaxis; and/or (5) reports without primary endpoints. The remaining 10 articles underwent a full-length article review. Of those, 5 were excluded for reasons similar to the first round. Finally, 5 studies (2 randomized controlled studies and 3 retrospective cohort studies) were compatible with the inclusion criteria and were included in the meta-analysis [Citation19–23]. The literature review process is summarized at . The clinical features, types of acute leukemia, dose and duration of levofloxacin prophylaxis, duration of neutropenia, country, study period, article type, and the quality assessment score for each study are tabulated at .
Table 1. Baseline patient characteristics of each included article.
Baseline patient features
A total of 862 acute leukemia patients were included in this meta-analysis (356 in the levofloxacin prophylaxis arm, and 506 in the no-prophylaxis arm). The age range of the levofloxacin prophylaxis group was 3.9–76 years, whereas that of the no-prophylaxis group was 3–84 years. There were more males than females in both groups. Both AML and ALL patients were included in this meta-analysis, and their disease statuses consisted of newly diagnosed acute leukemia, relapse acute leukemia, and refractory acute leukemia. A variety of chemotherapy regimens were utilized for induction, reinduction, and salvage therapies. The adult patients in the levofloxacin prophylaxis arm received this medication at a dose of 500–750 mg once daily, commencing on the date of neutropenia onset [Citation19,Citation20] or on day 1 of chemotherapy [Citation21], and continuing until the resolution of the neutropenia. In the case of the levofloxacin prophylaxis arm, the neutropenia period varied from 3 to 72 days, while the duration in the no-prophylaxis arm was between 3 and 67 days [Citation19,Citation21,Citation22].
Infection Outcomes after Chemotherapy
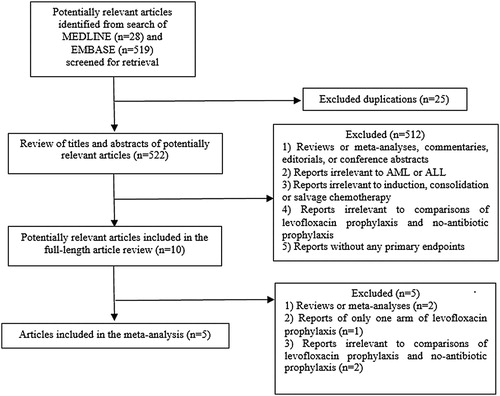
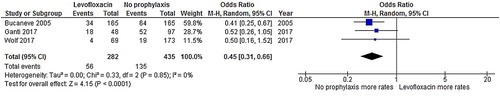
Patients who received levofloxacin had a significantly lower FN rate than patients who did not receive an antibiotic prophylaxis (odds ratio [OR]: 0.43, 95% CI: 0.32–0.58, p < .00001, I2 = 0%; ) [Citation19–23]. Additionally, the rate of microbiologically documented infection in the no-prophylaxis group was numerically higher than in the levofloxacin prophylaxis group (OR: 0.45, 95% CI: 0.34–0.60, p < .00001, I2 = 0%; ) [Citation19–23]. Likewise, the bacteremia rate in the levofloxacin prophylaxis group was significantly lower than the corresponding rate in the no-prophylaxis group (OR: 0.45, 95% CI: 0.31–0.66, p < .00001, I2 = 0%; ) [Citation19,Citation21,Citation22]. The rate of C. difficile infection in the levofloxacin prophylaxis group was also lower than in the no-prophylaxis group, although the difference did not reach statistical significance (OR: 0.37, 95% CI: 0.09–1.52, p = .17, I2 = 38%; ) [Citation21–23]. However, the death rates of the admitted patients in the levofloxacin prophylaxis group and the no-prophylaxis group were quite similar (OR: 0.67, 95% CI: 0.34–1.33, p = .26, I2 = 0%; ) [Citation19,Citation21].
Figure 2. Forest plots of the odds ratios of the levofloxacin prophylaxis and no-prophylaxis arms for the febrile neutropenia rate after chemotherapy.
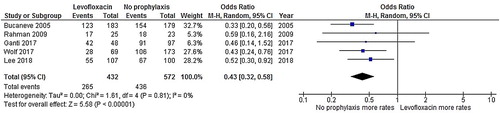
Figure 3. Forest plots of the odds ratios of the levofloxacin prophylaxis and no-prophylaxis arms for the rate of microbiologically documented infections after chemotherapy.
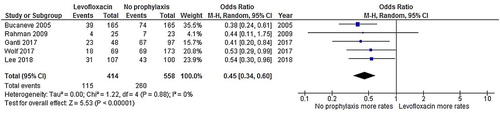
Figure 4. Forest plots of the odds ratios of the levofloxacin prophylaxis and no-prophylaxis arms for the bacteremia rate after chemotherapy.
Discussion
The results of a recent meta-analysis of the use of fluoroquinolone to prevent infections in several types of hematologic malignancies indicated that a fluoroquinolone prophylaxis decreased the FN rate and the rate of bloodstream infections [Citation24]. However, the included studies from that meta-analysis had a limited number of subjects. To highlight the efficacy of a levofloxacin prophylaxis for acute leukemia patients after receiving intensive chemotherapy, we performed the present systematic review and meta-analysis. Not only did the study reveal benefits in terms of a reduction in the FN and bacteremia rates, but the rate of microbiologically documented infections in patients receiving a levofloxacin prophylaxis also declined. Even though C. difficile infections are of concern in patients receiving a fluoroquinolone prophylaxis [Citation13], our study found quite similar rates of infection in the levofloxacin prophylaxis and no-prophylaxis groups. The mortality rates of the admitted patients in the two groups were also not different. However, apart from severe bacterial infections, there were a number of negative factors impacting on the mortality outcomes in the case of acute leukemia patients, such as fatal bleeding, disseminated thrombosis, and invasive fungal infections. Hence, we believe that the FN rate, bacteremia rate, and rate of microbiologically documented infections were more relevant outcomes to evaluate the efficacy of the levofloxacin prophylaxis.
This meta-analysis had some limitations. The relatively limited number of studies included in the meta-analysis was its major limitation. Although the primary outcome yielded enough power to show statistical significance, several secondary analyses could not reach significant results, which might be either from a lack of power or the outcomes were truly not different between the groups. In addition, the publication bias could not be assessed due to the small number of included studies. Even though many outcomes in our study provided rational support for the use of a levofloxacin prophylaxis for acute leukemia patients after chemotherapy, the antimicrobial susceptibility pattern of causative pathogens at each institute must be taken into consideration before implementing the prophylaxis. Moreover, the wide use of fluoroquinolone to prevent infections in hematological patients may inadvertently increase the rate of fluoroquinolone resistance. This meta-analysis could not show the long-term effects of levofloxacin on antimicrobial selective pressure. This may need a long-term surveillance of the antimicrobial susceptibility of causative organisms along with the use of a levofloxacin prophylaxis.
Conclusions
The use of a levofloxacin prophylaxis for acute leukemia patients after receiving intensive chemotherapy showed several advantages in terms of infectious outcomes, but it did not improve the death rate.
Supplemental Material
Download MS Word (63.5 KB)Supplemental Material
Download MS Word (13.6 KB)Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Weerapat Owattanapanich http://orcid.org/0000-0002-1262-2005
Methee Chayakulkeeree http://orcid.org/0000-0002-4582-4914
References
- Kato H, Fujita H, Akiyama N, et al. Infectious complications in adults undergoing intensive chemotherapy for acute myeloid leukemia in 2001–2005 using the Japan Adult Leukemia Study Group AML201 protocols. Support Care Cancer. 2018; doi:10.1007/s00520-018-4292-0.
- Kapoor R, Simalti AK, Roy S, et al. Clinicohematological profile of febrile neutropenia in childhood acute leukemia and Utility of serum procalcitonin levels in neutropenic patients. Indian J Crit Care Med. 2018;22:336–339. doi: 10.4103/ijccm.IJCCM_516_17
- Di Blasi R, Cattaneo C, Lewis RE, et al. Febrile events in acute lymphoblastic leukemia: a prospective observational multicentric SEIFEM study (SEIFEM-2012/B ALL). Ann Hematol. 2018;97:791–798. doi: 10.1007/s00277-018-3252-6
- De Rosa FG, Motta I, Audisio E, et al. Epidemiology of bloodstream infections in patients with acute myeloid leukemia undergoing levofloxacin prophylaxis. BMC Infect Dis. 2013; doi:10.1186/1471-2334-13-563.
- Saito T, Yoshioka S, Iinuma Y, et al. Effects on spectrum and susceptibility patterns of isolates causing bloodstream infection by restriction of fluoroquinolone prophylaxis in a hematology-oncology unit. Eur J Clin Microbiol Infect Dis. 2008;27:209–216. doi: 10.1007/s10096-007-0428-8
- Chong Y, Yakushiji H, Ito Y, et al. Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int J Infect Dis. 2011;15:e277–e281. doi: 10.1016/j.ijid.2010.12.010
- Sohn BS, Yoon DH, Kim S, et al. The role of prophylactic antimicrobials during autologous stem cell transplantation: a single-center experience. Eur J Clin Microbiol Infect Dis. 2012;31:1653–1661. doi: 10.1007/s10096-011-1489-2
- Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy. J Clin Oncol. 2013;31:794–810. doi: 10.1200/JCO.2012.45.8661
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073
- Harris AD, Perencevich EN, Johnson JK, et al. Patient-to-patient transmission is important in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae acquisition. Clin Infect Dis. 2007;45:1347–1350. doi: 10.1086/522657
- Lautenbach E, Metlay JP, Bilker WB, et al. Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections: the role of inadequate empirical antimicrobial therapy. Clin Infect Dis. 2005;41:923–929. doi: 10.1086/432065
- Prabhu RM, Piper KE, Litzow MR, et al. Emergence of quinolone resistance among viridans group streptococci isolated from the oropharynx of neutropenic peripheral blood stem cell transplant patients receiving quinolone antimicrobial prophylaxis. Eur J Clin Microbiol Infect Dis. 2005;24:832–838. doi: 10.1007/s10096-005-0037-3
- Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;doi: 10.1136/bmj.b2535
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4
- Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z
- Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. West Sussex: John Wiley & Sons; 2009.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557
- Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–987. doi: 10.1056/NEJMoa044097
- Rahman MM, Khan MA. Levofloxacin prophylaxis to prevent bacterial infection in chemotherapy-induced neutropenia in acute leukemia. Bangladesh Med Res Counc Bull. 2009;35:91–94. doi: 10.3329/bmrcb.v35i3.4130
- Ganti BR, Marini BL, Nagel J, et al. Impact of antibacterial prophylaxis during reinduction chemotherapy for relapse/refractory acute myeloid leukemia. Support Care Cancer. 2017;25:541–547. doi: 10.1007/s00520-016-3436-3
- Wolf J, Tang L, Flynn PM, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65:1790–1798. doi: 10.1093/cid/cix644
- Lee SSF, Fulford AE, Quinn MA, et al. Levofloxacin for febrile neutropenia prophylaxis in acute myeloid leukemia patients associated with reduction in hospital admissions. Support Care Cancer. 2018;26:1499–1504.
- Mikulsa M, Averbuch D, Tissot F, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J Infect. 2018;76:20–37. doi: 10.1016/j.jinf.2017.10.009