ABSTRACT
Objectives: To examine the use of splenectomy, chemotherapy, and subsequent overall survival (OS) in contemporary patients with splenic lymphomas.
Methods: We analyzed records of 6450 patients with various splenic lymphomas recorded in the National Cancer Data Base (2004–2013). Survival was compared using Mantel-Byer test to account for guarantee-time bias, stratified by age, sex, comorbidities, and lymphoma stage.
Results: Splenectomy rate was overall 58%, and varied from 49% in splenic marginal zone (SMZL) to 77% in follicular lymphoma (FL). It significantly decreased across all histologies over time (overall from 69% in 2004, to 44% in 2013). Thirty-day mortality after splenectomy was 4%. Chemotherapy use varied from 40% in FL to 76% in diffuse large B-cell lymphoma (DLBCL), but increased significantly only for SMZL and T-cell lymphomas over time. Overall, 57% of splenectomies were performed as diagnostic procedures, which was significantly less common in academic hospitals (p < 0.0001). Following a diagnostic splenectomy, chemotherapy was not administered to 29% of patients with DLBCL, 49% with mantle cell, and 42% with T-cell lymphomas. Median OS ranged from 12.4 years for FL to 1.0 year for T-cell lymphomas. We found no association between performance of splenectomy and OS across all histologies. Patients with DLBCL who did not receive chemotherapy after a diagnostic splenectomy had significantly worse OS (p = 0.001). The association between post-splenectomy chemotherapy and OS was not observed in FL or SMZL.
Conclusion: many splenic lymphomas may be treated without surgery, but a high proportion of diagnostic splenectomies indicates an ongoing need for less invasive diagnostic modalities.
Introduction
Splenic lymphomas range from indolent diseases like splenic marginal zone lymphoma (SMZL) and follicular lymphoma (FL) to aggressive entities like diffuse large B cell lymphoma (DLBCL) or hepatosplenic T-cell lymphoma [Citation1–3]. Historically, splenectomy was the main diagnostic and therapeutic intervention for splenic lymphomas, but the availability of sensitive diagnostic imaging, flow cytometry, and biomolecular analysis has largely obviated the need for an invasive surgical approach to diagnosis or staging in lymphomas [Citation2,Citation4–7]. Rare subtypes like the splenic diffuse red pulp B-cell lymphoma may still require splenectomy for unequivocal diagnosis [Citation8]. The fact that many splenic lymphomas involve the blood and bone marrow, and that they can be effectively treated with immunochemotherapy has further decreased the need for therapeutic splenectomy [Citation9–12].
Despite clinical advances, it is unknown to what extent the use of diagnostic or therapeutic splenectomy has changed in the community, whether these trends vary by histology, or are paralleled by changes in the application of chemotherapy. Most contemporary series are limited to patients with SMZL or primary splenic DLBCL [Citation12–15]. In contrast, there is paucity of data on less common histologies, except case reports and small case series [Citation16–19].
Our objective was thus to examine the current prevalence of splenectomy use for the management of splenic lymphomas, variation in the application of splenectomy and chemotherapy according to histology, practice setting, and over time, and to study potential associations between treatment modalities and survival.
Methods
Data source and study population
We used data from the National Cancer Data Base (NCDB), a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society [Citation20]. NCDB captures over 80% of newly diagnosed lymphomas in the United States (US). It contains data on patients’ demographic and socioeconomic characteristics, histology and stage of disease, treatment modalities used for the initial management (surgery, radiation therapy, and chemotherapy), and overall survival from diagnosis. All reporting facilities must gather survival data for at least 90% of cases within 5 years of diagnosis. The data are de-identified, and our study was considered exempt from human protection oversight by the Institutional Review Board at Rhode Island Hospital.
We selected patients 18 years and older who were diagnosed with a non-Hodgkin lymphoma in 2004–2013 with spleen recorded as the primary site (International Classification of Diseases in Oncology, 3rd edition, topography code C42.2). The registry manuals instruct that spleen is designated as primary site solely when it is the only site involved, when spleen is the only source of histologic confirmation, or if the treating physician states that the lymphoma originated in the spleen after complete staging. Secondary (metastatic) involvement of the spleen is not recorded. Out of 6939 cases meeting our initial criteria, we excluded 115 cases lacking histologic confirmation and 374 cases treated entirely outside of the reporting facilities, which had no available data on treatments or outcomes in the NCDB.
Variables and endpoints
Histology was assigned by the reporting registry according to the 2008 World Health Organization classification [Citation1]. CLL, HCL, and other leukemias were not included, because the NCDB automatically assigns ‘bone marrow’ as the primary site for all leukemias. Splenectomy was identified from site-specific surgery codes and assumed to be diagnostic when it was coincident with the lymphoma diagnosis. Receipt of chemotherapy was only available as a binary indicator, without distinguishing the use of specific drugs, regimens, duration or response to treatment.
For stratification, we categorized patients’ age as <60, 60–69, 70–79, and ≥80 years. Race was recorded as white, black, or other. To measure patients’ baseline risk of mortality, we used Charlson-Deyo comorbidity index, which is a weighted score based on recorded medical comorbidities [Citation21]. Stage of the lymphoma was reported according to the Ann Arbor system. Treatment facilities were categorized as academic/research or community depending on case volume and available services.
Overall survival was the only recorded outcome, calculated from diagnosis until death or last follow-up. Survival after diagnostic splenectomy was calculated from the day of surgery until death or last follow-up.
Statistical analysis
Linearized trends were examined using a log-binomial regression model, reporting p for trend (ptrend) from the Wald test [Citation22]. Survival was compared using log-rank tests, unstratified or stratified by age group, sex, comorbidity index, and stage of the lymphoma. In order to account for guarantee-time bias, the survival analysis examining receipt of specific treatments (splenectomy or chemotherapy) was conducted using the method by Simon and Makuch, which for all subjects assigns the time-at-risk before receiving treatment to the untreated group [Citation23,Citation24]. Accordingly, survival was compared using the Mantel-Byar test [Citation25]. All reported p values are two-sided, and p < 0.05 was considered statistically significant in this exploratory study, without further adjustments for multiple testing.
Results
Patient characteristics
Among 6450 included patients (), 48% had SMZL, 27% had DLBCL, 5% had FL, 4% had mantle cell lymphoma (MCL), and 4% had T-cell lymphoma, most commonly hepatosplenic T-cell (53% of T-cell histologies), peripheral T-cell not otherwise specified (38%), or anaplastic large cell subtype (6%). The residual 725 (11%) cases included classical Hodgkin, lymphoplasmacytic, Burkitt, small lymphocytic, or unspecified histologies. Median age was lower for patients with T-cell lymphomas, who were also more frequently black (17% versus <6% for other histologies). MCL had the highest, and FL the lowest male-to-female ratio.
Table 1. Characteristics of patients with primary splenic lymphoma in the NCDB, stratified by histology, in 2004–2013.
Over 70% of SMZL, MCL, and T-cell lymphomas were disseminated at diagnosis, while over 40% of DLBCL and FL cases were diagnosed at stage I. T-cell lymphomas were more likely than others to present with B symptoms (54% versus 20–38%). The proportion of splenic lymphomas managed in academic centers was 38% across all histologies, ranging from 31% for FL to 59% for T-cell lymphomas.
Use of splenectomy and chemotherapy
Overall, 58% of patients underwent splenectomy as part of their first course of treatment (). This proportion was lowest in SMZL (49%), and highest in FL (77%). Over half of these surgeries were diagnostic (particularly in DLBCL, FL, and T-cell lymphomas). Splenectomy was the sole modality of treatment in 50% of FL and 38% of SMZL cases, but rarely (<25%) in DLBCL, MCL, or T-cell lymphomas, which were predominantly treated with chemotherapy alone or after splenectomy. Nevertheless, 29% of patients with DLBCL, 49% with MCL, and 42% with T-cell lymphomas did not receive chemotherapy as part of the first course of treatment after a diagnostic splenectomy. Chemotherapy after splenectomy was rare in SMZL or FL.
Table 2. Use of splenectomy (diagnostic or other) and chemotherapy after a diagnostic splenectomy, among patients with primary splenic lymphomas.
Splenectomy was more frequently performed for stage I (75%) compared with stage II (69%) or advanced-stage (48%) lymphomas (Supplemental Table S1). However, even among patients with advanced-stage disease, surgery was performed in 40–68% of patients, varying by histology. Among patients with stage I disease, chemotherapy was not administered to 30% of DLBCL, 62% of T-cell lymphomas, and 61% of MCL, as well as 78% of SMZL and 77% of FL.
Compared with non-academic hospitals, patients treated in academic/research centers were less likely to undergo splenectomy (56% versus 59%, χ2 p = 0.018) or diagnostic splenectomy (29% versus 35%, p < 0.0001). We observed no significant differences between the two types of hospitals in the use of chemotherapy overall (54% versus 55%, p = 0.55) or chemotherapy after a diagnostic splenectomy (44% versus 44%, p = 0.86). There was no evident variation in these differences by histology, except that the proportion of diagnostic splenectomies in SMZL was significantly lower in academic hospitals (37% versus 47%, p < 0.0001), and that T-cell lymphoma patients were more likely to receive chemotherapy in academic hospitals (77% versus 59%, p = 0.002).
Mortality at 30 days after splenectomy was 4% overall, and varied from <2% in SMZL, MCL and FL, to 6% in DLBCL, and 10% in TCL (χ2 p < 0.0001).
Trends in the use of treatment modalities
The proportion of patients undergoing splenectomy significantly decreased from 69% in 2004 to 44% in 2013 (ptrend < 0.0001). This decrease was significant in all histologies ((A)): from 61% to 34% in SMZL, from 88% to 64% in FL, from 77% to 62% in DLBCL, from 68% to 41% in MCL, and from 61% to 40% in T-cell lymphomas, respectively. Likewise, there was an overall decrease in the use of diagnostic splenectomy (from 39% to 27%, respectively) which was significant in SMZL (ptrend < 0.0001), DLBCL (ptrend = 0.013), MCL (ptrend = 0.016), and T-cell lymphoma (ptrend = 0.024), but not in FL (ptrend = 0.76).
Figure 1. Trends in the use of diagnostic or other splenectomy (A), and splenectomy with or without chemotherapy (B), stratified by histologic subtype. For clarity, bars show stacked proportions in biennial intervals; p-values are for linearized annual trends for proportions undergoing any splenectomy (A) or any chemotherapy (B).
DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; SMZL: splenic marginal zone lymphoma
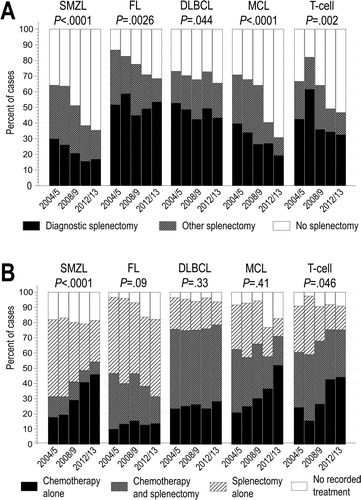
The proportion of patients with splenic lymphoma receiving chemotherapy as part of first-line treatment increased significantly only in SMZL (31% to 56%) and T-cell lymphoma (44% to 76%; (B)). Furthermore, we observed no significant change in the proportion of patients receiving chemotherapy after diagnostic splenectomy, in any histology.
Survival analysis
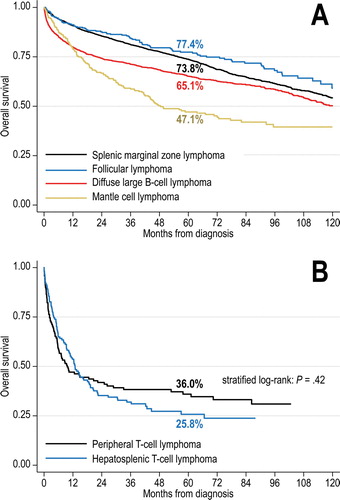
With median follow up of 6.2 years, survival markedly differed by lymphoma histology ((A)). Median OS was 11.0 years in SMZL (95%CI, 10.2 to not reached [NR]), 12.4 years in FL (95%CI, 10.9 to NR), 10.0 years in DLBCL (95%CI, 9.2–11.0), 4.0 years in MCL (95%CI, 3.5–6.5), and 1.0 years in TCL (95%CI, 0.8–1.5). In the T-cell lymphoma category, we observed no significant difference between peripheral T-cell lymphoma and hepatosplenic T-cell lymphoma ((B)). Five-year OS estimates were 73.8% for SMZL (95%CI, 72.1–75.4), 77.4% for FL (95%CI, 72.0–81.8), 65.1% for DLBCL (95%CI, 62.8–67.4), 47.1% for MCL (95%CI, 40.6–53.3), and 30.3% for T-cell lymphomas (95%CI, 24.5–36.2).
Figure 2. Kaplan-Meier curves for overall survival of patients with primary splenic B-cell (A) and T-cell (B) lymphomas in the NCDB, 2004-2013. Numbers indicate survival estimates at 5 years; the comparison in panel B was conducted using a log-rank test stratified by age, sex, stage, and comorbidity index.
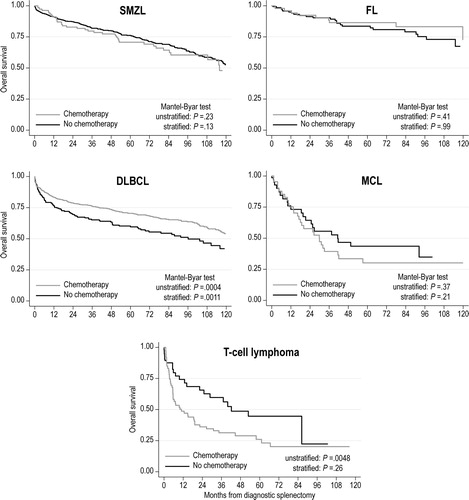
After adjusting for the guarantee-time bias, as well as differences in age, sex, stage, and comorbidity index, we observed no significant difference in OS between patients who did or did not undergo splenectomy, across all histologies (). There was also no significant association between performance of diagnostic splenectomy and OS (data not shown). DLBCL patients had a significantly worse OS if they did not receive chemotherapy after a diagnostic splenectomy (stratified Mantel-Byar test p = 0.0011, ). Conversely, no such association was evident for other histologies.
Figure 3. Simon-Makuch curves for overall survival of patients with splenic B-cell lymphomas, stratified by histology and receipt of splenectomy. p-Values are derived from Mantel-Byar tests, unstratified, or stratified by age, sex, stage, and comorbidity index.
DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; SMZL: splenic marginal zone lymphoma
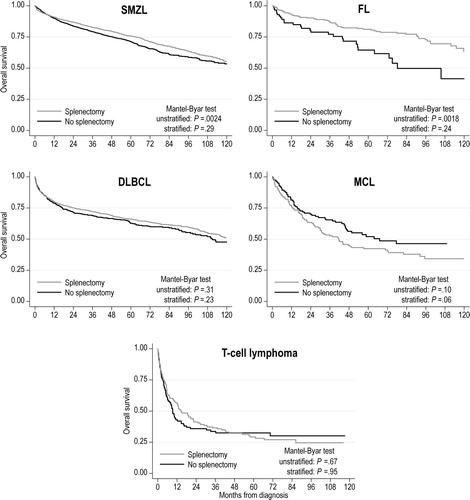
Figure 4. Simon-Makuch curves for overall survival of patients with splenic B-cell lymphomas who underwent diagnostic splenectomy, stratified by histology and receipt of subsequent chemotherapy. p-Values are derived from Mantel-Byar tests, unstratified, or stratified by age, sex, stage, and comorbidity index.
DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; SMZL: splenic marginal zone lymphoma
Discussion
In this observational study, which included most splenic lymphomas diagnosed in the US between 2004 and 2013, we have shown a significant decrease in the use of splenectomy. Nevertheless, a substantial proportion of patients (varying from 49% in SMZL to 77% in FL) still undergo surgery, including 48% of those with advanced-stage disease. More than half of surgeries were performed for diagnosis, and from 29% (in DLBCL) to 85% (in SMZL) of patients do not receive chemotherapy after a diagnostic splenectomy. Short-term mortality after splenectomy was low (4%), but higher in more aggressive histologies which require systemic therapy. After adjusting for baseline characteristics, we found no association between splenectomy and OS across histologies. However, patients with DLBCL who did not receive chemotherapy after a diagnostic splenectomy had significantly worse survival. This association was not observed in other histologies, like mantle cell and T-cell lymphomas, even though they are usually disseminated and treated with chemotherapy.
We observed a 49% rate of splenectomy in SMZL, similar to a previous study from 1995 to 2009 (52%) [Citation26]. Higher OS in our cohort (74% versus 68% in prior studies) may reflect advances in the management of SMZL, particularly rituximab [Citation12,Citation27–29]. Other series confirm no association between splenectomy and OS in SMZL [Citation30]. In the largest series of 87 patients with splenic DLBCL, 61% had stage I/II disease, 45% underwent a diagnostic splenectomy, and nearly all received chemotherapy [Citation14]. The authors found no significant OS difference between splenectomized and non-splenectomized patients. Although OS and progression-free survival (PFS) appeared better after splenectomy in stage I/II lymphoma, this observation is confounded by lack of adjustment for guarantee-time bias. In another case series of 66 splenic DLBCL diagnosed by splenectomy, 87% were treated with chemotherapy, but outcomes without chemotherapy were not reported [Citation15]. Our results indicate that up to 29% of splenectomized patients with DLBCL may not receive chemotherapy, which was associated with worse survival, though we could not discern the reasons for omitting chemotherapy. Additionally, chemotherapy administered at progression would not be captured by the NCDB, potentially explaining observed >50% 5-year OS in this group. Our findings highlight the importance of administering immunochemotherapy for splenic DLBCL even when it is resected, and question the need for surgery when diagnosis can be obtained by alternative means.
In contrast, we observed no association between post-splenectomy chemotherapy and OS in indolent lymphomas like SMZL or FL. This observation is reassuring, despite prior suggestions of poor outcomes with surgery alone in splenic FL from small case series without adjustment for baseline characteristics [Citation31,Citation32]. A larger found no survival advantage of post-splenectomy ‘adjuvant’ chemotherapy in SMZL [Citation33]. Therefore, both SMZL and FL may thus be safely be managed with ‘watchful waiting’ after a splenectomy, deferring additional therapy. MCL is more complex because of its heterogeneity. Splenic MCL not expressing SOX11 is an exceptionally indolent entity with prolonged remissions after splenectomy, despite involvement of the blood and marrow [Citation18,Citation34–36]. At the same time, aggressive MCL, including the blastoid subtype, may present with massive splenomegaly [Citation35,Citation37,Citation38]. In our analysis MCL exhibited the worst OS among B-cell lymphomas, suggesting that many cases of ‘splenic MCL’ may have been a disseminated nodal MCL with prominent splenomegaly. Nevertheless, we found no association between OS and either splenectomy or post-splenectomy chemotherapy in MCL. Similarly, T-cell lymphomas were histologically heterogeneous, usually advanced-stage, and had poor outcomes regardless of therapy [Citation19,Citation39,Citation40]. The significantly lower rate of chemotherapy for T-cell lymphomas in non-academic hospitals is concerning, particularly because it was not observed for other histologies, underscoring the importance of expertise in the management of rare T-cell lymphomas.
The main limitations of our analysis result from general drawbacks of the NCDB: reliance on local histology assignment without expert review, lack of data on PFS, specific chemotherapy regimens, or response to treatment. Central review of histology, while not preformed routinely in clinical practice or by the registries, is important because splenic lymphomas exhibit significant clinical heterogeneity even within broad categories like DLBCL, MCL, or SMZL. We could not verify that lymphomas were truly ‘primary splenic’, rather than having prominent splenic involvement, particularly in stage III/IV disease. Pathologies like CLL, HCL, or splenic diffuse red pulp B-cell lymphoma could not be included because of the limitations of registry coding. We could not discern whether splenectomy was performed for emergency or elective reasons. Furthermore, the NCDB does not include many important prognostic factors for lymphomas: the International Prognostic Index for DLBCL or its FL- or MCL-specific versions, direct values of laboratory tests, or performance status. Despite the large sample size, numbers within histology-specific subgroups were still too small to build more complex multivariable survival models. Considering the retrospective nature of this study and pervasive indication bias in treatment assignments, our findings should be considered descriptive or hypothesis-generating, and would need prospective confirmation.
In conclusion, although the use of splenectomy has declined, nearly half of patients with splenic lymphomas in the US undergo surgery, highlighting the ongoing need for reliable, non-invasive diagnostic modalities. Considering frequent disseminated stage at presentation and lack of association with survival, the value of splenectomy can be questioned when less morbid alternatives are available. Clinicians should administer standard chemotherapy in splenic DLBCL regardless of whether surgery was performed. Conversely, diagnostic splenectomy, if already performed, may be sufficient for the initial management of indolent lymphomas like SMZL or FL. Splenic MCL and T-cell lymphomas have worse prognosis, which does not appear to be influenced by splenectomy, and further research is needed to meet therapeutic needs in these subtypes.
Acknowledgements
This study was presented in part at the 58th American Society of Hematology Annual Meeting & Exposition, 3–6 December 2016, San Diego, CA. This study used a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigators. AJO reports research funding for the institution from Genentech, TG Therapeutics, and Spectrum Pharmaceuticals, and consulting for Spectrum Pharmaceuticals.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Jaleh Fallah http://orcid.org/0000-0001-7365-2104
Adam J. Olszewski http://orcid.org/0000-0002-6472-6658
Additional information
Funding
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press; 2008.
- Iannitto E, Tripodo C. How I diagnose and treat splenic lymphomas. Blood. 2011;117(9):2585–2595. doi: 10.1182/blood-2010-09-271437
- Shimizu-Kohno K, Kimura Y, Kiyasu J, et al. Malignant lymphoma of the spleen in Japan: a clinicopathological analysis of 115 cases. Pathol Int. 2012;62(9):577–582. doi: 10.1111/j.1440-1827.2012.02844.x
- Behdad A, Bailey NG. Diagnosis of splenic B-cell lymphomas in the bone marrow: a review of histopathologic, immunophenotypic, and genetic findings. Arch Pathol Lab Med. 2014;138(10):1295–1301. doi: 10.5858/arpa.2014-0291-CC
- Sreedharanunni S, Sachdeva MU, Malhotra P, et al. Role of blood and bone marrow examination in the diagnosis of mature lymphoid neoplasms in patients presenting with isolated splenomegaly. Hematology. 2015;20(9):530–537. doi: 10.1179/1607845415Y.0000000005
- Falk S, Stutte HJ. Primary malignant lymphomas of the spleen. A morphologic and immunohistochemical analysis of 17 cases. Cancer. 1990;66(12):2612–2619. doi: 10.1002/1097-0142(19901215)66:12<2612::AID-CNCR2820661225>3.0.CO;2-Q
- Kehoe J, Straus DJ. Primary lymphoma of the spleen. clinical features and outcome after splenectomy. Cancer. 1988;62(7):1433–1438. doi: 10.1002/1097-0142(19881001)62:7<1433::AID-CNCR2820620731>3.0.CO;2-V
- Traverse-Glehen A, Baseggio L, Salles G, et al. Splenic diffuse red pulp small-B cell lymphoma: toward the emergence of a new lymphoma entity. Discov Med. 2012;13(71):253–265.
- Ruchlemer R, Wotherspoon AC, Thompson JN, et al. Splenectomy in mantle cell lymphoma with leukaemia: a comparison with chronic lymphocytic leukaemia. Br J Haematol. 2002;118(4):952–958. doi: 10.1046/j.1365-2141.2002.03716.x
- Seymour JF, Cusack JD, Lerner SA, et al. Case/control study of the role of splenectomy in chronic lymphocytic leukemia. J Clin Oncol. 1997;15(1):52–60. doi: 10.1200/JCO.1997.15.1.52
- Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. Treatment of splenic marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30(1–2):139–148. doi: 10.1016/j.beha.2016.07.004
- Olszewski AJ, Ali S. Comparative outcomes of rituximab-based systemic therapy and splenectomy in splenic marginal zone lymphoma. Ann Hematol. 2014;93(3):449–458. doi: 10.1007/s00277-013-1900-4
- Xing KH, Kahlon A, Skinnider BF, et al. Outcomes in splenic marginal zone lymphoma: analysis of 107 patients treated in British Columbia. Br J Haematol. 2015;169(4):520–527. doi: 10.1111/bjh.13320
- Bairey O, Shvidel L, Perry C, et al. Characteristics of primary splenic diffuse large B-cell lymphoma and role of splenectomy in improving survival. Cancer. 2015;121(17):2909–2916. doi: 10.1002/cncr.29487
- Shimono J, Miyoshi H, Kiyasu J, et al. Clinicopathological analysis of primary splenic diffuse large B-cell lymphoma. Br J Haematol. 2017;178(5):719–727. doi: 10.1111/bjh.14736
- Howard MT, Dufresne S, Swerdlow SH, et al. Follicular lymphoma of the spleen: multiparameter analysis of 16 cases. Am J Clin Pathol. 2009;131(5):656–662. doi: 10.1309/AJCPF9V8XRDYWTIR
- Koiso H, Yokohama A, Mitsui T, et al. Follicular lymphoma presenting with marked splenomegaly: report of three cases. Acta Haematol. 2012;128(1):47–52. doi: 10.1159/000337037
- Angelopoulou MK, Siakantariz MP, Vassilakopoulos TP, et al. The splenic form of mantle cell lymphoma. Eur J Haematol. 2002;68(1):12–21. doi: 10.1034/j.1600-0609.2002.00551.x
- Lu CL, Tang Y, Yang QP, et al. Hepatosplenic T-cell lymphoma: clinicopathologic, immunophenotypic, and molecular characterization of 17 Chinese cases. Hum Pathol. 2011;42(12):1965–1978. doi: 10.1016/j.humpath.2011.01.034
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8
- McClintock S, Ma Z-q, Rieger RH. Incorrect inference in prevalence trend analysis due to misuse of the odds ratio. Ann Epidemiol. 2016;26(2):136–140. doi: 10.1016/j.annepidem.2015.12.009
- Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106
- Ollila TA, Olszewski AJ. Radiation therapy in primary testicular lymphoma: does practice match the standard of care? Leuk Lymphoma. 2018: Epub ahead of print; DOI:10.1080/10428194.10422018.11480776. PMID: 29966467.
- Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81–86. doi: 10.1080/01621459.1974.10480131
- Olszewski AJ. Survival outcomes with and without splenectomy in splenic marginal zone lymphoma. Am J Hematol. 2012;87(11):E119–E122. doi: 10.1002/ajh.23314
- Else M, Marin-Niebla A, de la Cruz F, et al. Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol. 2012;159(3):322–328. doi: 10.1111/bjh.12036
- Kalpadakis C, Pangalis GA, Sachanas S, et al. Rituximab monotherapy in splenic marginal zone lymphoma: prolonged responses and potential benefit from maintenance. Blood. 2018;132(6):666–670. doi: 10.1182/blood-2018-02-833608
- Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the surveillance, Epidemiology, and end results database. Cancer. 2013;119(3):629–638. doi: 10.1002/cncr.27773
- Starr AG, Caimi PF, Fu P, et al. Splenic marginal zone lymphoma: excellent outcomes in 64 patients treated in the rituximab era. Hematology. 2017;22(7):405–411. doi: 10.1080/10245332.2017.1279842
- Shimono J, Miyoshi H, Kamimura T, et al. Clinicopathological features of primary splenic follicular lymphoma. Ann Hematol. 2017;96(12):2063–2070. doi: 10.1007/s00277-017-3139-y
- Mollejo M, Rodriguez-Pinilla MS, Montes-Moreno S, et al. Splenic follicular lymphoma: clinicopathologic characteristics of a series of 32 cases. Am J Surg Pathol. 2009;33(5):730–738. doi: 10.1097/PAS.0b013e318193fcef
- Lenglet J, Traulle C, Mounier N, et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk Lymphoma. 2014;55(8):1854–1860. doi: 10.3109/10428194.2013.861067
- Royo C, Navarro A, Clot G, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia. 2012;26(8):1895–1898. doi: 10.1038/leu.2012.72
- Yoong Y, Kurtin PJ, Allmer C, et al. Efficacy of splenectomy for patients with mantle cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2001;42(6):1235–1241. doi: 10.1080/10428190127511
- Clot G, Jares P, Gine E, et al. A gene signature that distinguishes conventional and leukemic nonnodal mantle cell lymphoma helps predict outcome. Blood. 2018;132(4):413–422. doi: 10.1182/blood-2018-03-838136
- Bjorck E, Landgren O, Schoumans J, et al. Molecular cytogenetic approach to the diagnosis of splenic lymphoma: a case report of blastoid mantle cell lymphoma. Leuk Lymphoma. 2003;44(7):1229–1234. doi: 10.1080/1042819031000077061
- Lunning MA, Stetler-Stevenson M, Silberstein PT, et al. Spontaneous (pathological) splenic rupture in a blastic variant of mantle cell lymphoma: a case report and literature review. Clin Lymphoma. 2002;3(2):117–120. doi: 10.3816/CLM.2002.n.018
- Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080–1085. doi: 10.1093/annonc/mdn751
- Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558
