ABSTRACT
Objective: Frequent loss of expression of platelet factor 4 (PF4) in multiple myeloma (MM) was revealed in several previous researches. The predictive analysis of serum PF4 level in newly diagnosed MM has not been well elucidated. This study is to assess if serum PF4 could be a prognostic factor in predicting treatment response and survival of MM treated with thalidomide and VAD regimens.
Methods: Sera of 122 MM were gained pre- and post-treatment of chemotherapy and oral thalidomide. Serological PF4 measurements were performed by ELISA. Kaplan–Meier method was employed for survival analysis. Log rank test was used significance analysis. Multivariate analysis of overall survival used Cox-regression.
Results: Our data showed that the median serum PF4 concentration was negatively associated with MM response and a significant correlation between serum PF4 level and unfavorable clinical features (β2-microglobulin, ISS stage, del17p and creatinine). MM with lower serum PF4 concentration at diagnosis were prone to gain complete remission and very good partial remission after two courses of chemotherapy. Besides del17p, β2-microglobulin, treatment response, the low serum PF4 concentration was an independent variable associated with a poor overall survival by univariate analysis and multivariate analysis.
Conclusions: We speculate serum PF4 is a promising response and prognostic factor in newly diagnosed MM treated with thalidomide and VAD regimens.
Introduction
Multiple myeloma (MM) is a clonal B cell disorder characterized by skeletal destruction, renal failure, anemia, susceptibility to recurrent infections and hypercalcemia [Citation1]. MM is particularly common in elderly population [Citation2]. Thus, with the aging of the population, the incidence of MM is increasing year by year and MM poses a great threat to people health.
Cytogenetic abnormalities play a crucial role in MM pathogenesis. The exchange of MM cells with bone marrow microenvironment also plays a pivotal role, especially angiogenesis. The tumor angiogenesis of MM are via autocrine or paracrine of angiogenic factors by bone marrow stromal cells and myeloma cells. Bone marrow microvessel density was significantly higher in progressive MM [Citation3]. Angiogenesis is closely related to progression and prognosis of myeloma [Citation4]. Based on this knowledge, prototypic drugs thalidomide and lenalidomide have been approved for MM by targeting both MM cells and angiogenesis [Citation5].
Previously, lots of researches were concerned with proangiogenic factors in MM angiogenesis. However, antiangiogenic factors are also correlated with MM angiogenesis. PF4 is a potent anti-angiogenesis factor and can directly inhibit the growth of cancer cell lines [Citation6]. In vivo, it is confirmed that PF4 can inhibit angiogenesis and tumor growth [Citation7].
Recently, several reports demonstrated that PF4 involved in MM pathogenesis and may be a potential new targeting agent for MM. Comparing with normal plasma cells, PF4 was downregulated in MM cells by gene expression profiling [Citation8]. PF4 gene is a tumor suppressor gene and located on the region of 4q13.3. By high-resolution allelotyping, Cheng's group showed that 4q13.3 was a common deletion region and PF4 was frequently silenced by promoter hypermethylation in MM (15/28) and MM cell lines (5/5) [Citation9]. Another study demonstrated that PF4 and its p17-70 peptide could inhibit myeloma proliferation and angiogenesis both in vitro and in vivo [Citation10]. Cheng’s further study showed that PF4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in MM [Citation11].
Several previous researches revealed that PF4 was downregulated in MM. The association of PF4 and MM treatment response as well as overall survival has not been investigated. This study is proposed to assess if serum PF4 could be a prognostic factor in predicting treatment response and survival in MM.
Methods
Study population
Ethics Committee of our hospital approved this study. One hundred and twenty two newly diagnosed MM (age range 44–73, median age 56.5 years old, male/female 64/58) were enrolled in our study. They were hospitalized in our hospital from January 2010 to December 2015. The diagnosis was performed according to the diagnostic criteria for MM from the IMWG [Citation12]. Treatment response was conducted based on IMWG uniform response criteria for MM [Citation13]. All MM cases were transplant-ineligible or bortezomib/lenalidomide-ineligible because of physical condition or economic reasons. They were treated with VAD (Vinorelbine 10 mg, d1-4; Pirarubicin 10 mg, d1-4; Dexamethasone, 10 mg, d1-4) regimen chemotherapy and oral thalidomide (100mg–200 mg/d). Subject characteristics are summarized in . Del17p test was performed by FISH. One hundred and twenty healthy cases (age range 43–70, median age 54.5 years old, male/female 33/27) were enrolled from those who came to our hospital to undergo healthy physical examination and had no any abnormal symptoms and results. All patients signed the informed consent.
Table 1. Clinical characteristics of the 122 ND MM patients.
ELISA analysis
MM sera were gained pre- and post-treatment of chemotherapy and oral thalidomide. Fasting blood samples were collected from patients in the morning and allowed to clot at room temperature for 2 h. Sera were then separated by centrifugation at 2500 rpm for 10 min and frozen in aliquots at −80°C for subsequent analysis. Serological PF4 measurements were performed by ELISA (R&D, USA). Serum samples were diluted 1:5 and PF4 levels measured according to the manufacturer’s recommendations. Linear regression equation of the standard curve was calculated according to concentration of the standards and the corresponding optical density (OD) values. Serum sample concentration can be calculated according to the sample OD value through the regression equation. The final concentration is the actually measured concentration multipling by the dilution factor to generate values in ng/L.
Statistical analysis
All statistical procedures were performed with SPSS 17.0 (SPSS Inc., Chicago, Inc.). Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or final clinical follow-up. Kolmogorov––Smirnov test was used for assessing data normality. Data were non-normally distributed and presented as median value (interquartile range) with non-parametric analyses being employed to assess differences. Mann–Whitney test was used to evaluate differences between multiple groups. Kaplan–Meier method was employed for survival analysis. Log rank test was used significance analysis. Multivariate analysis of OS used Cox-regression. P-values < 0.05 were considered significant.
Results
PF4 and MM treatment response
Clinical characteristics of the newly diagnosed MM are shown in . PF4 serum concentration was measured in MM before and after treated with chemotherapy and oral thalidomide. The median PF4 serum concentration in healthy group is 2440.9 ± 873.2 ng/L. The median PF4 serum concentration in newly diagnosed MM (634.4 ± 30 ng/L) were significantly lower than that in healthy controls (p < 0.001). 58 MM gained complete response and very good partial response (CR&VGPR) after two courses of chemotherapy and oral thalidomide and the median PF4 serum concentration of them was 2437.9 ± 770.5 ng/L, no significance was observed between CR&VGPR and healthy group (p = 0.847). But comparing with newly diagnosed, the median PF4 serum concentration of CR&VGPR was significantly elevated (p < 0.001). Unfortunately, 64 MM didn't get CR&VGPR. The median PF4 serum concentration of this group was 629.4 ± 27 ng/L, which was lower than that of healthy (p < 0.001) and CR&VGPR group (p < 0.001). But comparing with newly diagnosed group, no significance was observed (p = 0.762).
PF4 and the unfavorable clinical features of MM
The PF4 serum concentration with respect to the clinical characteristics at diagnosis is showed in . There were 64 (52.5%) male and 58 (47.5%) female in newly diagnosed MM, no significant difference was observed regarding the PF4 serum concentration between the two groups (p = 0.927). It is found that PF4 serum concentration is associated with some unfavorable clinical features of MM at diagnosis. The PF4 serum concentration was lower in patients with elevated β2-microglobulin (p < 0.001), advanced ISS (p = 0.003), del17p (p = 0.001) and higher level of serum creatinine (p < 0.001). MM with higher PF4 serum concentration was easier gained CR&VGPR (p < 0.001). We did not identify any significant distinction in PF4 serum concentration when grouping the patients by age (p = 0.988), lactate dehydrogenase (p = 0.755), albumin (p = 0.305) or hemoglobin (p = 0.962).
Table 2. Serum PF4 concentration with respect to the clinical characteristics at diagnosis.
PF4 and survival of MM
The median follow-up duration among 122 patients was 26 months (range 6–48 months). The patients were categorized into lower (<median) and higher (≥median) group according to the serum concentration of PF4. The median survival time of newly diagnosed MM patients in lower serum concentration group is 21.00 months (95%CI: 18.38, 23.62), while in higher serum concentration group is 28.00 months (95%CI: 22.44, 33.56). Kaplan–Meier analyses of OS showed that patients with higher serum concentration of PF4 had a significantly superior outcome. Lower serum concentration of PF4 was associated with an unfavorable 3 year OS (13.3 ± 4.9% versus 23.8 ± 5.9%, P = 0.002; ). In univariate survival analysis, the presence of β2-microglobulin, del17p, treatment response (after two courses of chemotherapy) and serum creatinine were also significant predictors of survival, whereas survival was independent of gender, age, lactate dehydrogenase, ISS, albumin, hemoglobin (). In multivariate analysis with a Cox regression model, the independent variables associated with a poor OS were del17p (p = 0.0296), β2-microglobulin (p = 0.0393), treatment response (p = 0.031). In addition to these known prognostic factors, MM with lower serum PF4 concentration had a significantly inferior outcome (p = 0.006) (). Thus, the downregulated PF4 in patients at diagnosis was an independent prognostic factor in this cohort of MM.
Figure 1. Lower serum concentration of platelet factor 4(<median serum concentration) associated with an unfavorable overall survival.
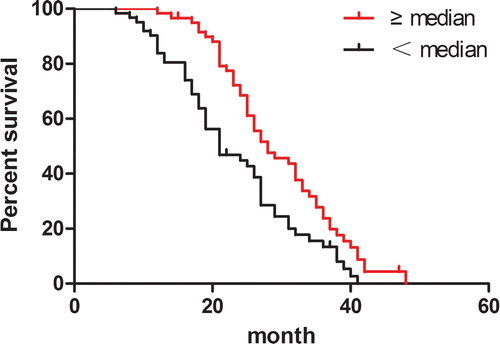
Table 3. Univariate analysis of OS in MM patients.
Table 4. Multivariate analysis of OS in MM patients.
Discussion
Frequent loss of expression of PF4 in primary MM and MM cell lines was revealed in several previous researches [Citation8,Citation9,Citation14]. PF4 may participate in MM tumorigenesis and be a promising new therapeutic target for MM [Citation9–11]. In our patient cohort, the median PF4 serum concentration in newly diagnosed MM were significantly lower than that in healthy controls, results were consistent with the previous studies [Citation8,Citation9,Citation14]. However, whether it can be a diagnostic marker, we need more clinical samples including reactive plasmacytosis and monoclonal gammopathy of undetermined significance (MGUS) for differential diagnosis. Furthermore, the median PF4 serum concentration was compared among MM before and after treatment with two courses of VAD regimens chemotherapy and oral thalidomide. Our data showed that the median PF4 serum concentration was negatively associated with MM response. PF4 is a tetrameric, lysine-rich member of the CXC chemokine family produced almost exclusively by megakaryocytes [Citation15]. Some newly diagnosed MM showed reduced platelet counts and platelet counts were elevated after CR&VGPR. Since PF4 is stored in and released by platelets, it is possible that downregulated PF4 levels merely due to reduced platelet counts. Indeed, serum PF4 levels and platelet counts were not correlated in newly diagnosed MM (r = 0.139, p = 0.127, data is shown in suppl figure 1). Hence, one can see that PF4 can be a response biomarker for MM.
Previously, the predictive analysis of PF4 level in newly diagnosed MM has not been well elucidated and studied. The principal objective of our study was to evaluate relevance of PF4 serum concentration to the unfavorable clinical features of MM at diagnosis and impact of the PF4 serum concentration on survival. Our present results showed a significant correlation between serum PF4 level and unfavorable clinical features. MM with lower serum PF4 concentration at diagnosis was prone to gain CR&VGPR. Univariate analysis and multivariate analysis of OS in MM both showed that initial response to induction chemotherapy may also be predictive of MM outcomes. It is likely that the earlier one gained CR&VGPR, the longer one lived, but there are currently limited data available to draw any conclusion. Besides, survival analysis revealed that del17p (p53) and β2-microglobulin were independent prognostic factors. Our results were consistent with the previous studies [Citation16,Citation17].
Importantly, our data demonstrate that serum PF4 level is a promising prognostic factor in newly diagnosed MM. Newly diagnosed MM patients with the low serum PF4 concentration had a significantly inferior outcome. In pancreatic ductal adenocarcinoma, a report showed that PF4 was a robust predictor of survival independent of other predictors such as stage, class, and treatment [Citation18]. Angiogenesis is a constant hallmark of MM progression and has prognostic potential [Citation4]. Aberrant angiogenesis in MM is arised from the dysregulated production of proangiogenic and antiangiogenic factors. PF4 is a major megakaryocyte-specific antiangiogenic factor. In MM, frequently hypermethylated promoter region of the PF4 gene disequilibrate the proangiogenic and antiangiogenic factors in favor of aberrant angiogenesis. Namely, PF4 was downregulated and angiostatic effects were impaired. The role that indirectly suppresses MM cell growth was weakened. Moreover, decreased PF4 means that proangiogenic factors may be relatively upregulated and further increase MM bone marrow angiogenesis. Thus MM cells obtained abundant nutrition supply and had increased proliferation and infiltration. Besides, PF4 could directly inhibit MM cell growth both in vitro and in vivo by induction of cell apoptosis, possibly mediated in part by inhibition of STAT3, via up-regulation of SOCS3 expression and an interaction with the cell surface receptor LRP1 [Citation11]. Therefore, both direct and indirect inhibitions of MM cell proliferation were decreased due to loss of expression of PF4.
However, given the relatively low incidence of MM, our single-center sample size was small. Karyotype abnormality including G-banding and/or FISH analysis and treatment regimen such as bortezomib, lenalidomide or high dose chemotherapy followed by autologous stem cell transplantation can all influence overall survival of MM. We need more data available to support our results. In addition, patients need ongoing follow-up to draw a more precise conclusion.
Conclusions
In conclusion, the median PF4 serum concentration was negatively associated with MM response. The serum level of PF4 was correlated with some unfavorable clinical features such as β2-microglobulin, ISS stage, del17p, creatinine. MM with lower serum PF4 concentration at diagnosis were prone to gain CR&VGPR after two courses of chemotherapy. Besides del17p, β2-microglobulin, treatment response, the lower serum PF4 concentration was an independent variable associated with a poor overall survival by univariate analysis and multivariate analysis. Serum PF4 level is a promising response and prognostic factor in newly diagnosed MM.
Supplemental Material
Download JPEG Image (34.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clinic Proc. 2016;91(1):101–119. doi: 10.1016/j.mayocp.2015.11.007
- Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016; 91(7):719–734. doi: 10.1002/ajh.24402
- Scavelli C, Nico B, Cirulli T, et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27(5):663–674. doi: 10.1038/sj.onc.1210691
- Vacca A, Ria R, Reale A, et al. Angiogenesis in multiple myeloma. Chem Immunol Allergy. 2014;99:180–196. doi: 10.1159/000353312
- Yabu T, Tomimoto H, Taguchi Y, et al. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood. 2005;106(1):125–134. doi: 10.1182/blood-2004-09-3679
- Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin Thromb Hemost. 2004;30(3):379–385. doi: 10.1055/s-2004-831051
- Yamaguchi K, Ogawa K, Katsube T, et al. Platelet factor 4 gene transfection into tumor cells inhibits angiogenesis, tumor growth and metastasis. Anticancer Res. 2005;25(2A):847–851.
- Davies FE, Dring AM, Li C, et al. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102(13):4504–4511. doi: 10.1182/blood-2003-01-0016
- Cheng SH, Ng MH, Lau KM, et al. 4q loss is potentially an important genetic event in MM tumorigenesis: identification of a tumor suppressor gene regulated by promoter methylation at 4q13. 3, platelet factor 4. Blood. 2007; 109(5):2089–2099. doi: 10.1182/blood-2006-04-018770
- Yang L, Du J, Hou J, et al. Platelet factor-4 and its p17-70 peptide inhibit myeloma proliferation and angiogenesis in vivo. BMC Cancer. 2011;11:261. doi: 10.1186/1471-2407-11-261
- Liang P, Cheng SH, Cheng CK, et al. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98(2):288–295. doi: 10.3324/haematol.2012.065607
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5
- Durie B, Harousseau J, Miguel J, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284
- Hose D, Moreaux J, Meissner T, et al. Induction of angiogenesis by normal and malignant plasma cells. Blood. 2009;114(1):128–143. doi: 10.1182/blood-2008-10-184226
- Cervi D, Yip T-T, Bhattacharya N, et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111(3):1201–1207. doi: 10.1182/blood-2007-04-084798
- Chang H, Qi C, Yi Q-L, et al. P53 gene deletion detected by fluorescence in situ hybridization is an adverse prognostic factor for patients with multiple myeloma following autologous stem cell transplantation. Blood. 2005;105(1):358–360. doi: 10.1182/blood-2004-04-1363
- Kowalska M, Kaminska J, Fuksiewicz M, et al. A survey of prognostic value of serum factors in multiple myeloma patients before treatment: macrophage-colony stimulating factor (M-CSF) is a powerful predictor of survival. Medical Oncol. 2011;28(1):194–198. doi: 10.1007/s12032-009-9403-9
- Poruk KE, Firpo MA, Huerter LM, et al. Serum platelet factor 4 is an independent predictor of survival and venous thromboembolism in patients with pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prevent. 2010;19(10):2605–2610. doi: 10.1158/1055-9965.EPI-10-0178
